Abstract
There is mounting evidence that zinc release from glutamatergic nerve terminals serves as a neuromodulator at synaptic sites within the retina and CNS. However, it has not been possible to reliably measure the concentration of zinc co-released with glutamate in the confines of the synaptic cleft. Thus, much of the evidence supporting this view derives from electrophysiological studies showing the modulatory effects of exogenous zinc on the membrane currents of ligand- and voltage-gated channels. In the present study, we took advantage of the unique properties of the glutamatergic photoreceptor terminal to demonstrate a feedback signal mediated by endogenous zinc at the synaptic sites from which it is discharged. Through its ability to block voltage-gated calcium channels in the photoreceptor terminal, zinc suppresses the radial dark current of the visual cell, and reduces its release of glutamate. It follows that chelation of extracellular zinc, e.g., with histidine, will lead to an increase both in the dark current and in the release of glutamate, changes that result in an enhancement of the light-evoked a-wave of the ERG and can account for the b-wave enhancement observed previously after zinc chelation when inner retinal responses were not blocked by aspartate.
Keywords: skate, retina, rod photoreceptors, zinc, feedback
Zinc is unquestionably one of the most ubiquitous trace elements in biological systems, and it is now universally acknowledged that zinc is indispensable to all living organisms: (i) zinc serves as an integral and essential component of scores of enzymes, (ii) it participates in a wide variety of metabolic functions, and (iii) it plays a significant role in translation and transcription of the genetic message. Moreover, zinc is indispensable to the growth and development of all forms of life (cf. reviews by Vallee, 1988; O’Halloran, 1993), and the serious consequences of zinc deficiency have been well documented (cf. Smith et al., 1973; Leopold, 1978; Hambidge, 1981; Krebs et al., 2000; Di Cello et al., 2005; Olmez et al., 2007). With the advent of a range of sensitive detection methods (cf. Danscher et al., 1985; Frederickson et al., 1982, 1987; Christensen et al., 1992; Simons, 1993; Thompson et al., 2002), zinc was shown to be present in virtually every tissue of the body where it exists primarily complexed with proteins that serve both metabolic reactions and structural functions. Although there is evidence for the presence of free or loosely bound Zn2+ ions within the cytoplasm and body fluids (Frederickson, 1989; Reyes, 1996; Simons, 1991; Zalewski et al., 2006), labile (‘chelatable’) zinc is localized mainly within the synaptic vesicles of glutamatergic nerve terminals throughout the retina and CNS (Beaulieu et al., 1992; Wu et al., 1993; Frederickson and Moncrief, 1994). Imaging techniques have also proven useful in demonstrating the stimulation-induced release and uptake of zinc in neural tissues (Assaf and Chung, 1984; Howell et al., 1984; Charton et al., 1985; Brown and Dyck, 2002; Redenti and Chappell, 2005; Redenti et al., 2007), and there is reason to suggest that zinc serves as a neuromodulator at synaptic sites in these regions. However, there are no available methods to reliably measure the concentration of zinc co-released with glutamate within the confines of the synaptic cleft. Thus, much of the evidence supporting this view is indirect, and derives from electrophysiological studies showing the modulatory effects of exogenous zinc on the membrane currents of ligand- and voltage-gated channels (cf. Christine and Choi, 1990; Hollmann et al., 1993; Wu et al., 1993; Harrison and Gibbons, 1994; Hirasawa et al., 1998; Han and Wu, 1999). In such circumstances, there can be no assurance that similar effects would be realized when zinc is released from presynaptic sites under physiological conditions. Progress in this direction was made using histidine to chelate zinc in the intact retina, resulting in an enhancement of the b-wave component of the electroretinogram (ERG), although a site of action could not be resolved (Redenti and Chappell, 2002; Rosenstein and Chappell, 2003). In the present study, we took advantage of the unique properties of the glutamatergic photoreceptor terminal, specifically, the release of neurotransmitter in darkness and its suppression by light, to demonstrate a feedback signal mediated by endogenous zinc at the synaptic sites from which it is discharged while responses from postsynaptic cells were blocked by aspartate.
All surgical and animal handling procedures were conducted in accordance with methods approved by the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eyes of dark-adapted skates (Raja erinacea) that had been deeply anesthetized with MS222 (Tricaine, ethyl 3-aminobenzoate methanesulfonate) and pithed anteriorly and posteriorly, were enucleated under dim red light, and the globe hemisected just anterior to the equator. After removing most of the vitreous humor with absorbent wicks, the posterior eyecup was placed on a Ringer-soaked pad in contact with a chlorided silver disc that served as the reference electrode. The ERG from this all-rod retina was picked up by a chlorided silver wire electrode in contact with the vitreal surface of the eyecup that led to the input stage of high-gain DC-coupled differential amplifier (model DP-301, Warner Instruments, Hamden, CT). Light-evoked responses were elicited by achromatic stimuli (duration = 1 sec), displayed on an oscilloscope and recorded with pClamp software (Molecular Devices Corporation, Sunnyvale, CA); data analysis was performed in Microcal Origin 6.0 (OriginLab Corporation, Northampton, MA). Light stimuli were delivered by a photostimulator with a 12V 100W tungsten-halogen lamp operated at 6.5A. The test beam had an unattenuated retinal illuminance of 390 μW/cm2 (defined as Log I = 0), and was attenuated by a series of neutral density filters allowing intensity to be changed in half log unit increments. The retina was continuously superfused with a control Ringer solution, which contained (in mM): NaCl (250), KCl (6), CaCl2 (4), urea (360), d-glucose (10), NaHCO3 (20), MgCl2 (4), NaH2PO4 (0.2), HEPES (5); the solution was oxygenated and titrated with sodium hydroxide to pH 7.5. A continuous stream of superfusate from a gravity-fed series of test solutions was delivered across the surface of the retina via a glass capillary on one side of the eyecup and removed by gentle suction through a similar capillary on the opposite side; electronically controlled valves were used to switch between test solutions. ERG intensity-response series were obtained in half log unit increments from Log I = - 7.0 to - 0.5, first in normal Ringer and again after 30 minutes in Ringer to which Na L-aspartate (75 mM) had been substituted for equimolar amounts of NaCl. Aspartate has no detectable effect on rhodopsin kinetics (Brin and Ripps, 1977), but it has been shown to block responses from post-receptoral retinal neurons, thereby isolating the photoreceptor-generated a-wave response from inner retinal components of the ERG (Dowling and Ripps, 1972). Following 40 minutes in aspartate, the superfusate was switched to test solutions in which histidine (100 μM), zinc (50 μM), or zinc plus histidine had been added to the aspartate-Ringer solution. In each instance, the retina was bathed in the test solution for 30 minutes prior to obtaining the ERG intensity-response series.
The full field ERG response elicited by a brief (1 sec) stimulus (Fig. 1A, upper trace) shows the summated response of the various underlying potentials that contribute to this transretinal voltage, i.e., the initial a-wave derived largely from the light-evoked closure of the cyclic GMP-gated photoreceptor channels, the vitreal-positive b-wave reflecting primarily the depolarizing response of ON bipolar cells, and the slowly developing c-wave that reflects the radial currents generated by the positive and negative components that contribute to the response, i.e., the cornea-positive potential of the RPE and the negative slow PIII of the Müller cell (cf. Sarthy and Ripps, 2001). Each of the components increases with increasing flash intensity, and reaches its maximum (i.e., saturates) at Log I ≈ -2.5, which corresponds to an intensity of ~1.23 μW/cm2. Using this saturating flash intensity as control, the middle trace of Fig. 1A shows that 75 mM Na L-aspartate, a concentration sufficient to block glutamate-gated channels on post-synaptic horizontal and bipolar cells (Dowling and Ripps, 1972), effectively eliminated the b-wave (and to a great extent the c-wave of the ERG), and served to isolate the photoreceptor potential (a-wave). Because in normal Ringer the rapid onset of the b-wave interrupts the a-wave before reaching its peak, the amplitude of the latter appears much greater after exposure to aspartate (Dowling and Ripps, 1972). However, it is noteworthy that the addition of 100 μM histidine, a zinc-chelating agent, produced a further enhancement of the a-wave potential (Fig. 1A, lower trace). Figure 1B shows recordings over a 4 log unit range of intensities of the aspartate-isolated responses of the skate retina before and after the addition of 100 μM histidine. Note that the receptor potential in response to the lowest intensity flash (log I = - 6.5) was not visible in the aspartate-treated preparation, but it was clearly evident after the addition of histidine. At all other intensities, zinc chelation produced an increase in the photoreceptor response.
Figure 1.
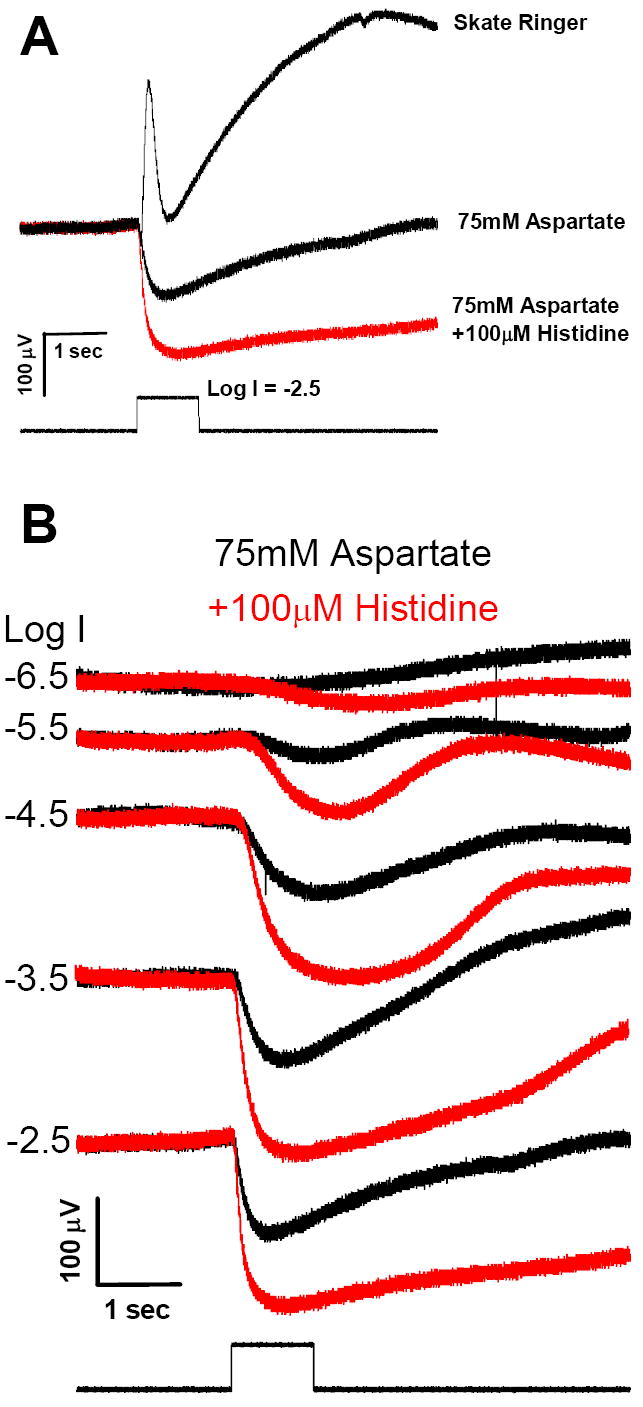
Effect of zinc chelation on the aspartate-isolated a-wave. A. Electroretinograms (ERGs) recorded from a single skate eyecup preparation in response to a 1 second flash of light plotted with pre-flash baselines superposed. The ERG recorded in normal skate Ringer (upper trace) exhibits a small a-wave at light onset that is quickly obscured by a prominent, vitreal-positive transient b-wave, and followed by the slowly developing c-wave. After 30 minute superfusion with 75mM Na L-aspartate the b-wave and c-waves are absent, but the amplitude of the a-wave (the photoreceptor-generated ERG component) appears significantly greater (middle trace). Adding the zinc chelator histidine (100μM) to the aspartate solution for an additional 30 minutes (following 40 minutes in aspartate) produced a 2-fold increase in the a-wave amplitude (lower trace, in red). B. ERG intensity-response series recorded from one eyecup showing histidine enhancement of the isolated a-wave response. The ERG recorded at the Log intensity (Log I) indicated on the left after 30 minutes in 75mM Na L-aspartate is shown in black. Responses obtained after adding 100μM histidine to the aspartate solution for an additional 30 min (following 40 minutes in aspartate) are superposed in red. Records at each intensity are shifted vertically for clarity of presentation.
Graphical representation of the averaged data recorded over the full range of test intensities are illustrated in Fig. 2. The data from each preparation (n =5) were normalized to the peak response amplitude obtained after 30 minutes in 75 mM Na L-aspartate to a flash of intensity of Log I = -2.5. The peak amplitudes of the initial recordings of the aspartate isolated a-wave (squares) and subsequent recordings after the addition of histidine (circles) were well fit by the Hill equation of the form V/Vmax = In / (In + I50) where V is the response amplitude evoked by light of intensity I, Vmax is the maximal response at saturation, n is the Hill coefficient, and I50 is the intensity that gives rise to a half-maximal response. For both sets of recordings, the I50 value was relatively constant; log I = -4.4 in aspartate and -5.0 after addition of histidine. Although the value of Vmax increased by 53% in histidine, the Hill coefficients were n=1.0 for both fitted curves. These data provide good evidence that zinc chelation by histidine significantly enhances the ERG a-wave, and that in the absence of histidine, the release of endogenous zinc from the glutamatergic photoreceptor terminal serves as a feedback signal to suppress the light-evoked photocurrent.
Figure 2.
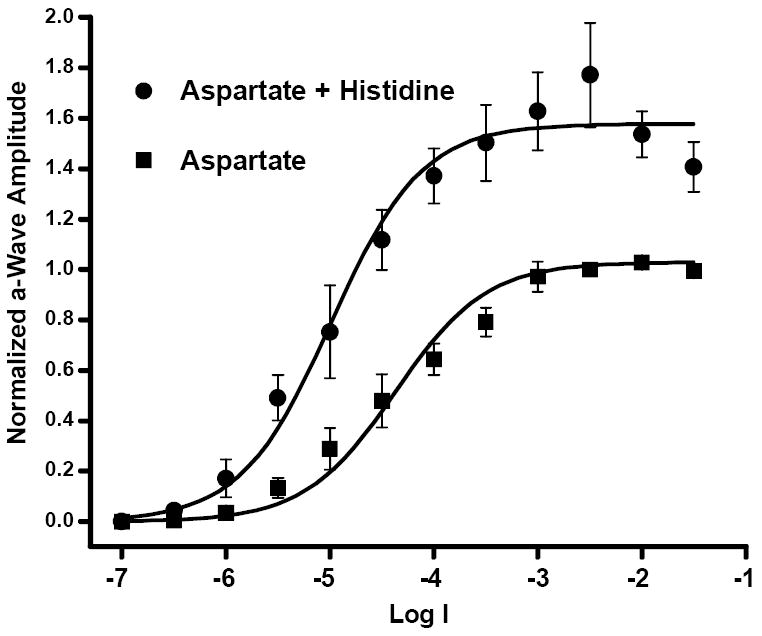
Intensity-response data of ERG a-wave peak amplitudes recorded from five preparations confirm a histidine enhancement effect. Responses from each preparation were normalized to the response amplitude at Log I = -2.5 after 30 minutes in 75mM Na L-aspartate. The mean of responses at each intensity obtained from all five preparations after 30 minutes in aspartate (filled squares, ±SEM, N=5)) were fitted (lower curve) by the equation V/Vmax = In/(In + I50) where I50 is the intensity at which the amplitude of the response is ½Vmax. Following addition of 100μM histidine to the aspartate superfusate for 30 minutes, there was a significant enhancement of the a-wave amplitude (circles, ±SEM, N=5) having a Vmax (upper curve) over 50% greater than control.
The specificity of the histidine effect is further illustrated by the bar graphs in Fig. 3, which compares these results with another series of aspartate isolated a-wave recordings. Here, the ΔVmax for each individual experiment was determined and averaged. As described above, introducing histidine (100 μM) to the aspartate Ringer solution enhanced the averaged photoreceptor response by more than 50 % (58% ±12.9%). In contrast, adding zinc (50μM) to the aspartate Ringer solution led to a reduction of over 25% (-27.3% ±5.2%) in the a-wave response and the zinc effect was reduced (-15.2% ±7.8%) when histidine (100μM) was added to the zinc-containing solution. Using the Student’s t-test the one-tailed probability of the histidine enhancement and the zinc attenuation were significant at the p = 0.0053 and p = 0.0068 levels, respectively. The smaller attenuation observed when histidine was added to the zinc solution, however, was not significantly different from aspartate alone (p = 0.075).
Figure 3.
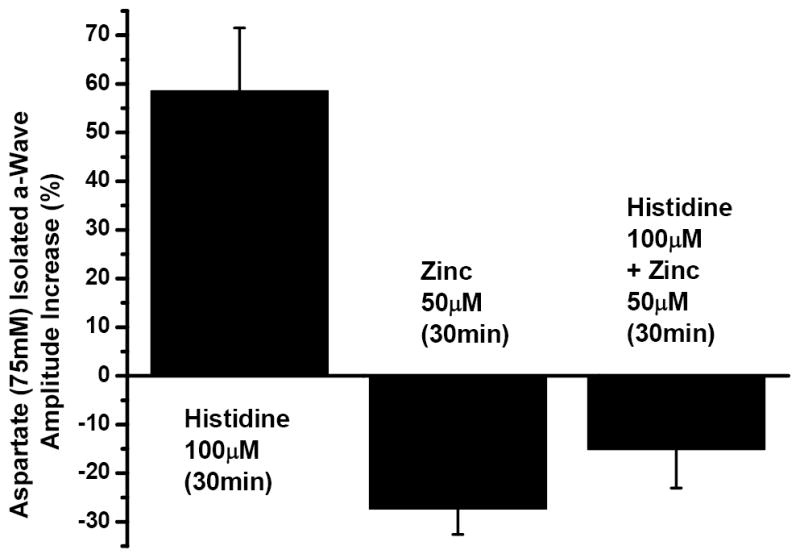
Zinc attenuation of ERG a-wave response amplitude is reduced by histidine. The mean ΔVmax obtained from the relationship above fitted to each dataset for 75mM Na L-aspartate to which 100μM histidine had been added for 30 minutes is shown as a percent increase from the a-wave amplitude after 30 minutes in aspartate alone (left column, ±SEM, N=5). The mean ΔVmax of similar curves fitted to normalized responses recorded after adding 50μM zinc (middle column, ±SEM, N=4) or 50μM zinc plus 100μM histidine (right column, ±SEM, N=4) for the final 30 minutes following 40 minutes in aspartate show that the a-wave amplitude attenuation induced by zinc is reduced in the presence of histidine.
The pioneering work by Wu et al. (1993) demonstrating the presence of labile zinc in the synaptic terminals of salamander photoreceptors has been confirmed and extended in studies of other vertebrate species (cf. Qian et al., 1997; Ugarte and Osborne, 1999), and there is evidence for the presence of a vesicular zinc transporter (ZnT-3) in the region of the outer limiting membrane and photoreceptor inner segments of the mouse retina (Redenti and Chappell, 2004). Moreover, the depolarization-induced zinc release seen in the rat retinal slice (Redenti and Chappell, 2005) and from isolated zebrafish rods (Redenti et al., 2007) are further indications of a role for zinc at the level of the outer plexiform layer of the vertebrate retina. It has long been known that histidine is a major amino acid ligand of zinc (Giroux and Henkin, 1972; Evans et al., 1979), and recent studies had shown that its ability to chelate zinc resulted in enhancement of the ERG b-wave (Redenti and Chappell, 2002; Rosenstein and Chappell, 2003). However, there was uncertainty as to whether the effect was due to the relief of Zn2+ inhibition at the bipolar cell, or through reduced feedback inhibition at the photoreceptor terminal. In this connection it is useful to recall that concentrations of zinc as low as 5 μM are capable of suppressing the voltage-dependent calcium current in the photoreceptor terminal (Wu et al., 1993), and that a reduction in calcium influx results in a decrease in both the a- and b-waves of the ERG (Gosbell et al., 1996). These results and those of the present study are consistent with a zinc-mediated negative feedback loop which reduces glutamate release, and is most effective in darkness when the co-release of zinc and glutamate are maximal (Wu et al., 1993). Enhancing the permeability of the calcium channels at the rod terminals will give rise to a greater photoreceptor dark current and an increase in the light-evoked a-wave. On the other hand, it seems likely that the histidine-induced increase in the ERG b-wave previously observed (Redenti and Chappell, 2002), results form the concomitant increase in glutamate release from the photoreceptor terminals.
In sum, we applied aspartate to isolate ERG responses of the distal retina, enabling us to examine the changes induced by removal (chelation) of extracellular zinc on the photoreceptor currents generating the a-wave. We found that zinc chelation enhanced the a-wave response, an effect mediated presumably by relieving the block of calcium channels at the photoreceptor terminal. Clearly, zinc is an important element in visual information processing in the distal retina, and by its ability to suppress transmitter release, may protect from the excitotoxic effect of glutamate. While the presence of zinc released at photoreceptor terminals may reduce absolute sensitivity of the retina to light (Redenti and Chappell, 2003), its reduction of glutamate release may reduce saturation and desensitization of glutamate receptors on postsynaptic retinal neurons (Bowie and Lange, 2002; Shen et al., 2004) and thereby increase incremental sensitivity at low light intensities through an appropriate shift of the intensity-response relationship (Chappell and Naka, 1991).
Acknowledgments
We are grateful to Jane Zakevicius, M.Sc. for her untiring assistance throughout the course of this work and to Dr. James Gordon for his assistance with statistical analysis. This study was supported by National Science Foundation Grant #0615987, PSC-CUNY #68490-0037, and NCRR/NIH #RR-03037 (RLC) research grants (EY-06516, EY-12028) and a Core Grant (EY-01792) from the National Eye Institute, an unrestricted award to the Department of Ophthalmology from Research to Prevent Blindness, Inc., and a Senior Scientific Investigator Award (to HR) from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Dyck R, Cynader M. Enrichment of glutamate in zinc-containing terminals of the cat visual cortex. Neuroreport. 1992;3:861–864. doi: 10.1097/00001756-199210000-00010. [DOI] [PubMed] [Google Scholar]
- Bowie D, Lange CD. Functional stoichiometry of glutamate receptor desensitization. J Neurosci. 2002;22:3392–3403. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brin KP, Ripps H. Rhodopsin photoproducts and rod sensitivity in the skate retina. J Gen Physiol. 1977;69:97–129. doi: 10.1085/jgp.69.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Dyck RH. Rapid, experience-dependent changes in levels of synaptic zinc in primary somatosensory cortex of the adult mouse. J Neurosci. 2002;22:2617–2625. doi: 10.1523/JNEUROSCI.22-07-02617.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell RL, Naka K-I. Sensitivity transformation for vertebrate vision. Visual Neurosci. 1991;6:371–374. doi: 10.1017/s0952523800006593. [DOI] [PubMed] [Google Scholar]
- Charton G, Rovira C, Ben-Ari Y, Leviel V. Spontaneous and evoked release of endogenous Zn2+ in the hippocampal mossy fiber zone of the rat in situ. Exp Brain Res. 1985;58:202–205. doi: 10.1007/BF00238969. [DOI] [PubMed] [Google Scholar]
- Christensen MK, Frederickson CJ, Danscher G. Retrograde tracing of zinc-containing neurons by selenide ions: a survey of seven selenium compounds. J Histochem Cytochem. 1992;40:575–579. doi: 10.1177/40.4.1313065. [DOI] [PubMed] [Google Scholar]
- Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10:108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danscher G, Howell G, Perez-Clausell J, Hertel N. The dithizone, Timm’s sulphide silver and the selenium methods demonstrate a chelatable pool of zinc in CNS. A proton activation (PIXE) analysis of carbon tetrachloride extracts from rat brains and spinal cords intravitally treated with dithizone. Histochemistry. 1985;83:419–422. doi: 10.1007/BF00509203. [DOI] [PubMed] [Google Scholar]
- Di Cello F, Siddharthan V, Paul-Satyaseela M, Kim KS. Divergent effects of zinc depletion in brain vs non-brain endothelial cells. Biochem Biophys Res Commun. 2005;335:373–376. doi: 10.1016/j.bbrc.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972;60:698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Johnson PE, Brushmiller JG, Ames RW. Detection of labile zinc-binding ligands in biological fluids by modified gel filtration chromatography. Anal Chem. 1979;51:839–843. doi: 10.1021/ac50043a016. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987;20:91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Manton WI, Frederickson MH, Howell GA, Mallory MA. Stable-isotope dilution measurement of zinc and lead in rat hippocampus and spinal cord. Brain Res. 1982;246:338–341. doi: 10.1016/0006-8993(82)91188-x. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Moncrieff DW. Zinc-containing neurons. Biol Signals. 1994;3:127–139. doi: 10.1159/000109536. [DOI] [PubMed] [Google Scholar]
- Giroux EL, Henkin RI. Competition for zinc among serum albumin and amino acids. Biochim Biophys Acta. 1972;273:64–72. doi: 10.1016/0304-4165(72)90191-2. [DOI] [PubMed] [Google Scholar]
- Gosbell A, Favilla I, Jablonski P. The effects of insulin on the electroretinogram of bovine retina in vitro. Curr Eye Res. 1996;15:1132–1137. doi: 10.3109/02713689608995145. [DOI] [PubMed] [Google Scholar]
- Hambidge KM. Zinc deficiency in man: its origins and effects. Philos Trans Roy Soc Lond B Biol Sci. 1981;294:129–144. doi: 10.1098/rstb.1981.0094. [DOI] [PubMed] [Google Scholar]
- Han Y, Wu SM. Modulation of glycine receptors in retinal ganglion cells by zinc. Proc Natl Acad Sci USA. 1999;96:3234–3238. doi: 10.1073/pnas.96.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacol. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Kudo Y, Tokimasa T. Actions of zinc on rapidly inactivating A-type and non-inactivating M-type potassium currents in bullfrog sympathetic neurons. Neurosci Lett. 1998;255:5–8. doi: 10.1016/s0304-3940(98)00683-1. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Krebs NF, Westcott JE, Arnold TD, Kluger BM, Accurso FJ, Miller LV, Hambidge KM. Abnormalities in zinc homeostasis in young infants with cystic fibrosis. Pediatr Res. 2000;48:256–261. doi: 10.1203/00006450-200008000-00022. [DOI] [PubMed] [Google Scholar]
- Leopold IH. Zinc deficiency and visual impairment? Am J Ophthalmol. 1978;85:871–875. doi: 10.1016/s0002-9394(14)78122-x. [DOI] [PubMed] [Google Scholar]
- O’Halloran TV. Transition metals in control of gene expression. Science. 1993;261:715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- Olmez A, Yalcin SS, Yurdakok K, Coskun T. Serum zinc levels in children with acute gastroenteritis. Pediatr Int. 2007;49:314–317. doi: 10.1111/j.1442-200X.2007.02371.x. [DOI] [PubMed] [Google Scholar]
- Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol. 1997;78:2402–2412. doi: 10.1152/jn.1997.78.5.2402. [DOI] [PubMed] [Google Scholar]
- Redenti S, Chappell RL. Zinc chelation enhances the zebrafish retinal ERG b-wave. Biol Bull. 2002;203:200–202. doi: 10.2307/1543396. [DOI] [PubMed] [Google Scholar]
- Redenti S, Chappell RL. Zinc chelation enhances the sensitivity of the ERG b-wave in dark-adapted skate retina. Biological Bulletin. 2003;205:213–214. doi: 10.2307/1543258. [DOI] [PubMed] [Google Scholar]
- Redenti S, Chappell RL. Localization of zinc transporter-3 (ZnT-3) in mouse retina. Vis Res. 2004;44:3317–3321. doi: 10.1016/j.visres.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Redenti S, Chappell RL. Neuroimaging of zinc released by depolarization of rat retinal cells. Vision Res. 2005;45:3520–3525. doi: 10.1016/j.visres.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Redenti S, Ripps H, Chappell RL. Zinc release at the synaptic terminals of rod photoreceptors. Exp Eye Res. 2007;85:580–584. doi: 10.1016/j.exer.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Reyes JG. Zinc transport in mammalian cells. Am J Physiol. 1996;270:C401–C410. doi: 10.1152/ajpcell.1996.270.2.C401. [DOI] [PubMed] [Google Scholar]
- Rosenstein FJ, Chappell RL. Endogenous zinc as a retinal neuromodulator: evidence from the skate (Raja erinacea) Neurosci Lett. 2003;345:81–84. doi: 10.1016/s0304-3940(03)00472-5. [DOI] [PubMed] [Google Scholar]
- Sarthy V, Ripps H. The Müller cell of the vertebrate retina: structure and function. Kluwer Academic/Plenum Publishers; NY: 2001. [Google Scholar]
- Shen W, Finnegan SG, Slaughter MM. Glutamate receptor subtypes in human retinal horizontal cells. Vis Neurosci. 2004;21:89–95. doi: 10.1017/s0952523804041094. [DOI] [PubMed] [Google Scholar]
- Simons TJB. Calcium-dependent zinc efflux in human red blood cells. J Membr Biol. 1991;123:73–82. doi: 10.1007/BF01993965. [DOI] [PubMed] [Google Scholar]
- Simons TJB. Measurement of free Zn2+ ion concentration with the fluorescent probe mag-fura-2 (furaptra) J Biochem Biophys Methods. 1993;27:25–37. doi: 10.1016/0165-022x(93)90065-v. [DOI] [PubMed] [Google Scholar]
- Smith JC, Jr, McDaniel EG, Fan FF, Halsted JA. Zinc: a trace element essential in vitamin A metabolism. Science. 1973;181:954–955. doi: 10.1126/science.181.4103.954. [DOI] [PubMed] [Google Scholar]
- Thompson RB, Peterson D, Mahoney W, Cramer M, Maliwal BP, Suh SW, Frederickson C, Fierke C, Herman P. Fluorescent zinc indicators for neurobiology. J Neurosci Methods. 2002;118:63–75. doi: 10.1016/s0165-0270(02)00144-9. [DOI] [PubMed] [Google Scholar]
- Ugarte M, Osborne NN. The localization of free zinc varies in rat photoreceptors during light and dark adaptation. Exp Eye Res. 1999;69:459–461. doi: 10.1006/exer.1999.0727. [DOI] [PubMed] [Google Scholar]
- Vallee BL. Zinc: biochemistry, physiology, toxicology and clinical pathology. Biofactors. 1988;1:31–36. [PubMed] [Google Scholar]
- Wu SM, Qiao X, Noebels JL, Yang XL. Localization and modulatory actions of zinc in vertebrate retina. Vision Res. 1993;33:2611–2616. doi: 10.1016/0042-6989(93)90219-m. [DOI] [PubMed] [Google Scholar]
- Zalewski P, Truong-Tran A, Lincoln S, Ward D, Shankar A, Coyle P, Jayaram L, Copley A, Grosser D, Murgia C, Lang C, Ruffin R. Use of a zinc fluorophore to measure labile pools of zinc in body fluids and cell-conditioned media. Biotechniques. 2006;40:509–520. doi: 10.2144/06404RR02. [DOI] [PubMed] [Google Scholar]


