Abstract
Background & Aims
Pediatric acute liver failure (PALF) results in death or need for liver transplantation (LT) in up to 50% of patients. A scoring system for predicting death or LT [Liver Injury Units (LIU) score] in PALF was previously derived by our group, and used peak values during hospital admission of total bilirubin, prothrombin time/international normalized ratio, and ammonia as significant predictors of outcome. The aims of this study were to test the predictive value of the LIU score in a subsequent validation set of patients and to derive a hospital admission score (aLIU) predictive of outcome.
Methods
Data were obtained from 53 children admitted with PALF from 2002–2006. Outcome was defined at sixteen weeks as alive without LT, death, or LT.
Results
Survival without LT at sixteen weeks for each LIU score quartile was 92%, 44%, 60%, and 12 % respectively (p < 0.001). The ROC C index for predicting death or LT by 4 weeks was 86.3. An admission LIU score was derived using admission total bilirubin and prothrombin time/international normalized ratio. Survival without LT at sixteen weeks for each quartile using the aLIU score was 85%, 77%, 69%, and 31% (p = 0.001). The ROC C index for predicting death or LT by four weeks was 83.7.
Conclusions
The original LIU score is a valid predictor of outcome in PALF. The aLIU score is promising and needs to be validated in subsequent patients.
Keywords: fulminant hepatic failure, children, survival, mortality, transplantation
Pediatric acute liver failure (PALF) is a life threatening illness in which a previously healthy child rapidly progresses to severe hepatic dysfunction and coagulopathy within eight weeks of onset of symptoms (1). Although criteria for ALF in adults includes the presence of hepatic encephalopathy, assessment of encephalopathy in younger children is difficult; thus pediatric criteria do not require this feature (1–3). Etiology is indeterminate in almost half of PALF cases, which differs from adults in the United States where leading etiologies are acetaminophen ingestion (40%), idiosyncratic drug reactions, and hepatotrophic viruses (1,4,5). PALF results in death or the need for liver transplantation (LT) nearly 50% of the time (1). The ability to predict which children will progress to death or LT would be a useful tool in order to expedite consideration of patients for LT early in their course, affording them the best opportunity to undergo LT. Moreover, a scoring system that could stratify patients into low, moderate, and high risk of death or need for LT would be useful to stratify patients for clinical trials and as a surrogate marker of outcome. Prognostic factors that have been found to predict outcome in PALF have included elevated serum total bilirubin, prothrombin time (PT), blood ammonia, and white blood cell count; low alanine aminotransferase; and delayed onset of hepatic encephalopathy (6–9). The PALF Study Group identified total bilirubin ≥ 5 mg/dL, INR ≥ 2.55, and the presence of hepatic encephalopathy as risk factors on admission that predicted death or LT (1). Smaller case series have investigated the prognostic role of serum hepatocyte growth factor and serum para-aminobenzoic acid in PALF (10,11).
We have previously derived a severity of illness score for PALF utilizing objective laboratory measures that are routinely available. In a multivariate analysis of patients from 1993–2001, peak laboratory values during hospitalization of serum total bilirubin, PT/international normalized ratio (INR), and ammonia were significant predictors of transplant-free survival in PALF (12). Applying these variables, we then derived a risk of death or need for LT staging model (low, moderate, high risk) called the Liver Injury Units (LIU) score. The following formula predicted outcome: LIU = 3.584*peak total bilirubin (mg/dl) + 1.809*peak PT (seconds) + 0.307*peak ammonia (µmol/l). Substituting INR for PT, the formula was LIU = 3.507*peak total bilirubin + 45.51*peak INR + 0.254*peak ammonia. In the current study, we applied this derived LIU score to a subsequent group of children with PALF to determine if this model was predictive of death or need for LT in an independent cohort of PALF patients. In addition, we derived a new model [admission LIU (aLIU)] based on laboratory values at admission to the hospital and tested its value in estimating risk of death or need for LT in PALF.
Methods
Demographics and clinical course of all children admitted to The Children’s Hospital in Denver that met criteria for PALF from January 2002 – December 2006 were entered prospectively into a database. This group is referred to as the validation set. The training set refers to the original data set from which the LIU score was derived. This study was reviewed and approved by the Colorado Multiple Institutional Review Board. PALF was defined as acute onset of severe liver dysfunction plus the presence of liver-derived coagulopathy (PT > 20 seconds or INR > 2.0, uncorrectable by parenteral administration of vitamin K in the absence of encephalopathy, or INR > 1.5 or PT > 15 seconds in the presence of hepatic encephalopathy) within eight weeks of onset of liver injury without prior known existing liver disease. Each year, PALF criteria were reviewed with the neonatal and pediatric intensive care unit staff to ensure complete identification of PALF patients and enrollment in the database. Patients were treated by a standardized protocol including intensive care, intracranial pressure monitoring for grade III or IV encephalopathy, and listing for orthotopic liver transplantation for persistent marked coagulopathy (PT > 40 seconds) or grade III or IV encephalopathy if there were no contraindications to LT. The LIU score was not used in treatment decisions. Data recorded included admission serum total bilirubin, sodium, ammonia, PT/INR and peak total bilirubin, ammonia, PT/INR, and lowest serum sodium during the hospital admission. LIU scores were calculating using overall peak laboratory values, on the day each laboratory value peaked, and on admission laboratory values. Patients were classified as primary referrals if they presented directly to or were already admitted to our hospital in PALF versus outside referrals if PALF developed at an outside institution. Admission was defined as when patients first presented or were transferred to our facility and met the diagnostic criteria for PALF. Daily laboratory studies were obtained until PALF resolved, death, or LT. In our laboratory, INR is not reported at a value greater than 6.0. Etiology of PALF was categorized as indeterminate, metabolic disease, acetaminophen toxicity, infections, complications of hematology/oncology disease, sepsis or ischemia-reperfusion injury, or other defined etiology. Outcome was defined at sixteen weeks as alive without LT, death without LT, or LT and time to death or LT was used for estimating survival probabilities.
Statistical analysis
Validation of the LIU score
LIU scores were calculated based on peak values during hospitalization in the validation set. Kaplan-Meier survival curves were constructed based on the previously derived quartiles of the LIU score. Proportion of patients surviving without LT was stratified by the LIU quartiles and plotted to sixteen weeks. Individual receiver operating characteristics (ROC) curves were generated to predict death or LT at four weeks following admission in order to validate the LIU score. An ROC curve provides a graphical representation of the relationship between the true-positive and false-positive prediction of the model. The C-index (area under the ROC curve) demonstrates the accuracy of the test, or how well the test separates the group being tested into those with and without the outcome in question. A C-index > 85 is highly predictive. A logistic regression model was used to obtain the C-index and also the probability of death or liver transplantation at four weeks for given values of the index, based on the final model derived using admission values. All variables have been reported as median (range). All analyses were performed using Version 9.1 of SAS. Statistical significance was set at a p < 0.05.
Admission LIU (aLIU) score derivation
A risk of death or LT score based on admission laboratory values was derived using Cox’s proportional hazards regression model. Variables that were significant for p value < 0.05 were included in the final multivariate model. The coefficients (log hazard ratios) estimated using the multivariate regression modeling were used to determine prognostic scores, whereby estimated coefficients in the model become weights in the score. For ease of use, we multiplied the coefficients by 100. The derived admission formula (aLIU) was then tested against the outcomes at four weeks and an ROC curve was generated. Patient characteristics and laboratory values in the training and validation sets were compared by a chi-square test (categorical variables) and a Wilcoxon rank sum test (continuous variables).
Results
Clinical and outcome data
53 patients (31 males, 22 females) were identified with PALF over five years. Etiology of PALF was indeterminate (n = 14), sepsis/ischemia-reperfusion injury (n = 14), viral infection (n = 8), metabolic disease (n = 5), acetaminophen toxicity (n = 4), hematology/oncology-related (n = 4), and other etiologies (n = 4). Thirteen percent (n = 7) underwent liver transplantation, 23% (n = 12) died without liver transplantation, with an overall transplant free survival of 64 % at sixteen weeks. There were no significant differences in gender, age, diagnosis, laboratory values, or outcome between the training set of PALF patients in which the LIU score was derived and this validation set (Table 1 and Table 2). There were mild differences in diagnoses, and a trend towards higher admission ammonia level in outside referrals compared to primary referrals (Table 3).
Table 1.
Demographics for the training set and validation set
| Training Set (1993–2001) | Validation Set (2002–2006) | P value | |
|---|---|---|---|
| Gender | 0.53 | ||
| Male | 32 | 30 | |
| Female | 31 | 23 | |
| Median age in years (range) | 4.5 (0–21.6) | 3.6 (0–17.1) | 0.55 |
| 0 – <6 months (number) | 14 | 13 | |
| 6 months – <3 years (number) | 11 | 9 | |
| ≥3 years (number) | 38 | 31 | |
| Diagnosis | 0.32 | ||
| Indeterminate | 13 | 14 | |
| Sepsis/Ischemia | 9 | 14 | |
| Infection | 6 | 8 | |
| Metabolic | 9 | 5 | |
| Acetaminophen Overdose | 9 | 4 | |
| Heme-Oncology | 10 | 4 | |
| Other | 7 | 4 | |
| Outcome | |||
| Alive w/o liver transplantation | 32 | 30 | 0.48 |
| Liver transplantation | 11 | 7 | |
| Death w/o liver transplantation | 20 | 16 | |
Table 2.
Comparison of admission and peak laboratory values for the training and validation set.
| Training Set (1993–2001) | Validation Set (2002–2006) | p-value | |
|---|---|---|---|
| Admission Total Bilirubin - mg/dl | 4.0 (0.5–42.4) | 3.5 (0.1–31.9) | 0.44 |
| Admission Prothrombin Time - secs | 26.9 (20.1–100.0) | 26.4 (19.7–100.0) | 0.67 |
| Admission INR | Not available | 2.8 (1.7–6.0) | NA |
| Admission Ammonia - umol/l | 57 (5–952) | 58 (9–468) | 0.84 |
| Peak Total Bilirubin - mg/dl | 14 (0.8–73.1) | 8.7 (0.3–84.5) | 0.25 |
| Peak Prothrombin Time - secs | 35.2 (21.0–128.0) | 35.5 (19.7–100.0) | 0.81 |
| Peak INR | Not available | 3.4 (1.7–6.0) | NA |
| Peak Ammonia - umol/l | 107 (10–1398) | 88 (24–468) | 0.41 |
Values expressed as median (range)
Table 3.
Comparison of diagnosis, admission laboratory values, and peak laboratory values between primary referrals (patients who presented or were already admitted with PALF to our hospital) versus outside referrals.
| Primary Referrals | Outside Referrals | p-value | ||
|---|---|---|---|---|
| Diagnosis | 0.06 | |||
| Indeterminate | 4 | 10 | ||
| Sepsis/Ischemia | 10 | 4 | ||
| Infection | 4 | 4 | ||
| Metabolic | 1 | 4 | ||
| Acetaminophen Overdose | 1 | 3 | ||
| Heme-Oncology | 4 | 0 | ||
| Other | 2 | 2 | ||
| Admission Total Bilirubin - mg/dl | 4 (0.1–31.9) | 2.3 (0.1–28.2) | 0.70 | |
| Admission Prothrombin Time - secs | 30.4 (19.8–100) | 25.5 (19.7–66.3) | 0.18 | |
| Admission INR | 2.8 (1.65–6) | 2.77 (1.69–6) | 0.67 | |
| Admission Ammonia - umol/l | 47 (9–116) | 61 (25–468) | 0.06 | |
| Peak Total Bilirubin - mg/dl | 9.4 (0.3–84.5) | 6.1 (0.6–48.6) | 0.28 | |
| Peak Prothrombin Time - secs | 41.6 (20.5–100) | 32.3 (19.7–100) | 0.25 | |
| Peak INR | 3.48 (1.78–6) | 3.28 (1.72–6) | 0.74 | |
| Peak Ammonia - umol/l | 86 (24–184) | 97 (25–468) | 0.25 | |
Values expressed as median (range)
Validation of the LIU score
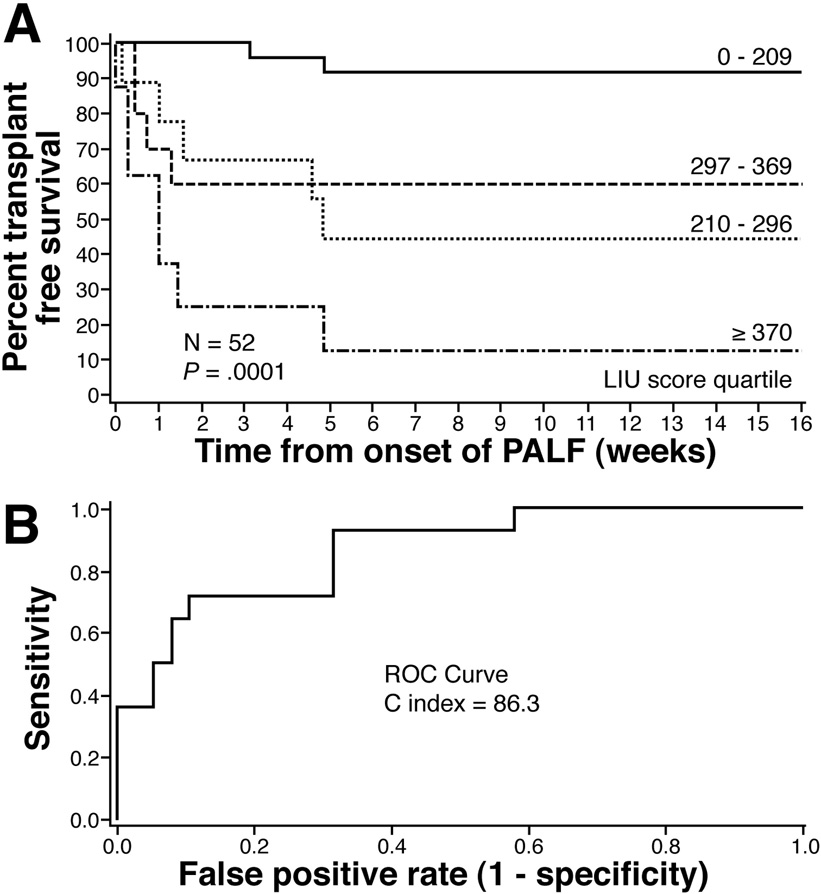
Figure 1 shows the proportion of patients alive without liver transplantation plotted in a survival analysis stratified by the previously defined quartiles for the LIU score using INR (12). Survival without LT at sixteen weeks for each quartile using the LIU score calculated with INR was 92%, 44%, 60%, and 12% respectively (p=0.0001). The area under the ROC curve (C-index) to predict death or need for LT at 4 weeks was 86.3 (95% CI 75.6 – 97.4). Survival without LT at sixteen weeks for each quartile using the LIU score with PT (Figure 2) was 79%, 83%, 60%, and 18%, respectively (p=0.0001). The C index at 4 weeks was 80.6 (95% CI 65.7 – 95.3).
Figure 1. Validation of the Liver Injury Units (LIU) score using INR.
(A) Survival without liver transplantation stratified by quartiles developed on the previously defined training set. LIU = 3.507 * peak total bilirubin (mg/dl) + 45.51 * peak INR + 0.254 * peak ammonia (umol/L). A low risk of death or liver transplantation (LIU score < 209) and a high risk of death or liver transplantation (LIU score ≥370) can be defined. (B) ROC curves for predicting death or liver transplantation at 4 weeks using the LIU score. A C index > 85 is considered predictive. The C index was 86.3 (95% CI 75.6 – 97.4).
Figure 2. Validation of the Liver Injury Units (LIU) score using PT.
(A) Survival without liver transplantation stratified by quartiles developed on the previously defined training set. LIU = 3.584 * peak total bilirubin (mg/dl) + 1.809 * peak PT (seconds) + 0.307 * peak ammonia (umol/L). A low risk of death or liver transplantation (LIU score < 152) and a high risk of death or liver transplantation (LIU score ≥228) can be defined. (B) ROC curve for predicting death or liver transplantation at 4 weeks using the LIU score. The C index was 80.6 (95% CI 65.7 – 95.3).
Application of the LIU Score to Individual Peak Laboratory Values
To determine if there were differences in predictive value of the LIU score if calculated on the days that each of the three laboratory values peaked, we calculated LIU scores on the day when total bilirubin, ammonia and INR peaked. The C index for the LIU scores using INR calculated when each variable peaked were 75.6 (95% CI 58.2 – 92.8) for total bilirubin, 77.7 (95% CI 62.5 – 92.7) for ammonia, and 86.2 (95% CI 73.9 – 99.0) for INR respectively.
Application of the LIU Score to Admission Data
To determine if the LIU score was applicable to admission data as opposed to overall peak laboratory values, a LIU score using admission laboratory values was calculated. The admission LIU scores were based on 43 patients due to missing admission ammonia values. Survival without LT at sixteen weeks for each quartile using the LIU score applied to admission INR, bilirubin, and ammonia was 73%, 60% and 25% for the first three quartiles respectively (p=0.02). No patients had LIU scores in the highest-risk quartile when using admission laboratory values. The C-index at 4 weeks was 79.3 (95% CI 64.9 – 94.1). Survival without LT at sixteen weeks for each quartile using the LIU score applied to admission PT, bilirubin, and ammonia was 73%, 71% and 29% for the first three quartiles respectively (p=0.01). The C-index at 4 weeks was 77.7 (95% CI 60.7 – 94.7).
Derivation of the Admission LIU (aLIU) Score
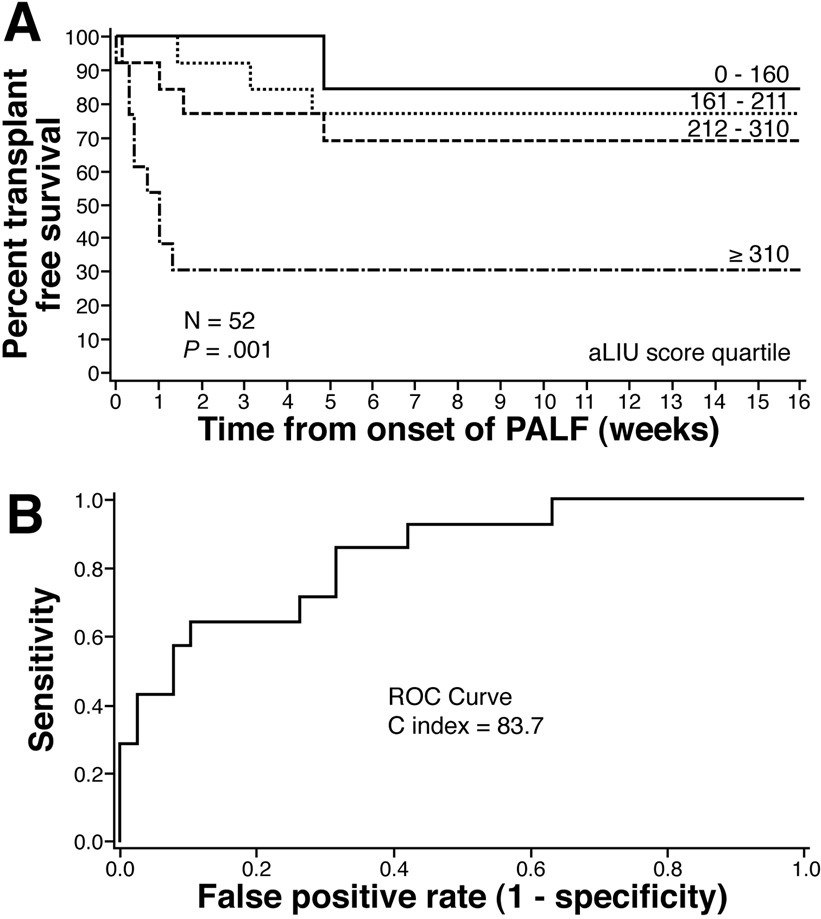
Since the application of the LIU score to admission laboratory values had a lower C index, we derived a new admission LIU (aLIU) score. Forward and backward stepwise Cox regression models were run to determine correlation of admission serum sodium, ammonia, total bilirubin, and INR/PT with outcomes of LT or death. Total bilirubin and INR/PT were determined to be independently associated with death or need for liver transplantation (p < 0.05) (Table 4). The unadjusted relative risks are those obtained by univariate analysis, for each variable shown in Table 4. Admission total bilirubin, PT, and INR were significant by univariate analysis, whereas admission ammonia was not. A final model using both forward and backward stepwise algorithms was derived and using Cox’s regression modeling, we developed a model of PALF risk of death or LT staging based on admission laboratory values. Table 4 also shows the relative risk for each variable when adjusted for other variables entered into the model. The derived aLIU formula was aLIU = 8.4*admission total bilirubin (mg/dl) + 50.0*admission INR. Substituting PT for INR, the aLIU = 6.9*admission total bilirubin (mg/dl) + 4.0*admission PT (seconds). The proportion of patients surviving without transplantation was plotted in a survival analysis stratified by quartiles of the aLIU score (Figure 3). Survival without LT at sixteen weeks for each quartile when INR was used was 85%, 77%, 69%, and 31% (p=0.001). The C index calculated for survival at four weeks using INR was 83.7 (95% CI 71.8 – 95.9). Figure 4 shows the survival data expressed in quartiles of the aLIU score using PT. Survival without LT at sixteen weeks for each quartile was 77%, 69%, 69%, and 43% (p=0.08). The C index at four weeks using PT was 78.9 (95% CI 64.3 – 93.6). The three lowest quartiles of the aLIU score (< 310 for INR; < 233 for PT) were associated with a relatively low risk of death or LT and the highest quartile (≥310 for INR; ≥233 for PT) was associated with a high risk of death or LT. Table 5 provides the risk of death or LT at four weeks based on the aLIU score using INR.
Table 4.
Analysis of factors at hospital admission associated with death or liver transplantation by 16 weeks.
| Risk Factor | Relative Risk | (95% CI) | p-value |
|---|---|---|---|
| Unadjusted | |||
| Admission total bilirubin | 1.05 | (1.00–1.10) | 0.05 |
| Admission PT | 1.03 | (1.00–1.05) | 0.04 |
| Admission INR | 1.35 | (0.99–1.84) | 0.06 |
| Admission NH3 | 1.00 | (0.99–1.01) | 0.65 |
| PT model, adjusted relative risks | |||
| Admission total bilirubin | 1.07 | (1.01–1.13) | 0.02 |
| Admission PT | 1.05 | (1.00–1.10) | 0.03 |
| Admission NH3 | 1.00 | (0.99–1.01) | 0.72 |
| INR model, adjusted relative risks | |||
| Admission total bilirubin | 1.10 | (1.03–1.17) | 0.003 |
| Admission INR | 2.09 | (1.25–3.47) | 0.005 |
| Admission NH3 | 1.00 | (0.99–1.01) | 0.50 |
| Final PT model | |||
| Admission total bilirubin | 1.07 | (1.02–1.13) | 0.009 |
| Admission PT | 1.04 | (1.01–1.07) | 0.004 |
| Final INR model | |||
| Admission total bilirubin | 1.09 | (1.03–1.15) | 0.003 |
| Admission INR | 1.65 | (1.16–2.34) | 0.005 |
Cox’s regression analysis was used to identify significant factors. PT = prothrombin time; INR = International Normalized Ratio, NH3 = ammonia.
Figure 3. Derivation of the admission Liver Injury Units (aLIU) score using INR.
(A) Survival without liver transplantation stratified by quartiles developed using the newly derived aLIU score. aLIU = 8.4 * admission bilirubin (mg/dl) + 50.0 * admission INR. A low risk of death or liver transplantation (aLIU < 310) and a high risk of death or liver transplantation (aLIU ≥ 310) can be defined based on admission values. (B) ROC curve for predicting death or liver transplantation at 4 weeks using the aLIU score. The C index was 83.7 (95% CI 71.8 – 95.9).
Figure 4. Derivation of the admission Liver Injury Units (aLIU) score using PT.
(A) Survival without liver transplantation stratified by quartiles developed using the newly derived aLIU score. aLIU = 6.9 * admission bilirubin (mg/dl) + 4.0 * admission PT. A low risk of death or liver transplantation (aLIU < 233) and a high risk of death or liver transplantation (aLIU ≥ 233) can be defined based on admission values. (B) ROC curve for predicting death or liver transplantation at 4 weeks using the aLIU score. The C index was 78.9 (95% CI 64.3 – 93.6).
Table 5.
Risk of death or liver transplantation (%) based on admission LIU score using INR. aLIU = 8.4 × admission total bilirubin (mg/dl) + 50.0 × admission INR
| Admission LIU Score (INR) | Risk of death or liver transplantation by 4 weeks (95% confidence interval) |
|---|---|
| 173 | 0.1 (0.03–0.26) |
| 222 | 0.2 (0.10–0.35) |
| 255 | 0.3 (0.18–0.45) |
| 282 | 0.4 (0.26–0.56) |
| 306 | 0.5 (0.33–0.67) |
| 331 | 0.6 (0.39–0.78) |
| 358 | 0.7 (0.45–0.87) |
| 390 | 0.8 (0.52–0.94) |
| 439 | 0.9 (0.61–0.98) |
| 485 | 0.95 (0.69–0.99) |
| 585 | 0.99(0.82–1.00) |
Discussion
In this study, using a second independent validation cohort of children with PALF at a single institution, the previously derived LIU score was shown to be strongly predictive of death or LT (C index 86.3). In the validation set, the second and third quartiles showed overlap when compared to the training set of data, however the lowest and highest quartiles clearly reflected patients with low and high likelihood of death or LT. The predictive value was better for the LIU score using INR rather than PT, which may be explained by the manner in which the INR is derived. INR is a mathematical manipulation of the PT meant to standardize this test across different laboratories taking into account different clotting reagents and techniques. Because the PT measured on a day to day basis can vary and techniques used in different countries vary considerably, the INR standardizes this measurement and thus limits variation in the calculated score.
We also performed analysis by calculating LIU scores on the days that each variable peaked, using the corresponding daily values for the other variables, as opposed to using the cumulative peak values. The LIU scores using INR calculated on these individual variable peak days had only a slightly lower C index (75 – 86) than the overall peak LIU score. This suggests that the LIU score has the potential to be used on a daily basis, reflecting the risk for death or need for liver transplantation at any given point in time during hospitalization.
Both the King’s College Hospital Criteria and the Model for End-Stage Liver Disease (MELD) score have been evaluated as prognostic indices in adult ALF with mixed results (13–18). Several other laboratory variables not included in these have been studied in adult ALF including alpha-fetoprotein (19,20), phosphorus (21), ammonia (22), lactate (23), and the APACHE II score (24). Adding serum sodium values to the MELD score has also received attention for predicting survival in chronic liver failure (25–28). We analyzed admission sodium values and did not find a correlation to outcome in PALF.
Following validation of the LIU score, we applied the LIU score to admission values to see if this was as predictive of outcome. We found a lower C index (79.3) when the LIU score was applied to admission values. We then derived a new admission LIU score that used only total bilirubin and PT/INR(C index 83.7), which appears promising although is slightly less predictive of outcome than the peak value LIU score. The poorer performance of the aLIU was believed to be due to variability in the duration and severity of PALF at the time of admission or transfer of patients to our hospital. Thus, admission laboratory values may be reflective of a later time point in the disease process in certain patients, most likely those transferred from other hospitals.
Our study included children with ALF caused by acetaminophen overdose, oncologic disorders, and sepsis/ischemia-reperfusion injury. As shown in our previous study, the LIU score appears to be valid in these patients. Acetaminophen overdose patients often have a better survival than other PALF patients; however, this was reflected by lower quartile LIU scores in acetaminophen survivors. Analysis of the LIU score excluding oncology and sepsis/ischemia-reperfusion injury patients did not significantly change the C index (data not shown).
The distribution of diagnoses we report differ slightly than those reported by the PALF Study Group, in which we have less indeterminate causes and more sepsis-ischemia reperfusion injury, infectious, and hematologic-oncologic etiologies (1). This is likely due to our balance of primary versus outside referrals as shown in Table 3, in which we would expect primary referrals to have a slightly higher rate of sepsis-ischemia reperfusion injury and hematologic-oncologic etiologies than outside referrals. Other studies likely included patients that were primarily referred to centers for liver transplantation evaluation and thus may represent a more severely ill and selected population skewed toward indeterminate causes of PALF.
In conclusion, despite the limitations of a single center study, the current study validates the LIU score in a second set of patients with PALF and lends merit to this scoring system. This score could be useful as a dynamic biomarker to predict outcome and need for liver transplantation, for stratification of patients for clinical trials, and as a surrogate marker of outcome. The aLIU score was less predictive of outcome, which was not surprising given the variable clinical status of patients at the time of admission or transfer to our hospital. Further prospective analysis of the predictive value of the LIU score in additional independent sets of PALF patients will provide guidance for the clinical and research value of these two scoring systems in the clinical care and research setting of PALF.
Acknowledgments
Financial Support: B. Lu is supported by the NIH training grant 5T32DK067009. There are no financial or other conflicts of interest to be disclosed by any authors in this manuscript.
List of Abbreviations
- PALF
pediatric acute liver failure
- LT
liver transplantation
- LIU
Liver Injury Units
- aLIU
admission Liver Injury Units
- PT
prothrombin time
- INR
international normalized ration
- ROC
receiver operating characteristics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brandy R Lu, Email: lu.brandy@tchden.org.
Jane Gralla, Email: gralla.jane@tchden.org.
Edwin Liu, Email: liu.edwin@tchden.org.
Emily L. Dobyns, Email: dobyns.emily@tchden.org.
Michael R. Narkewicz, Email: narkewicz.michael@tchden.org.
Ronald J. Sokol, Email: sokol.ronald@tchden.org.
References
- 1.Squires RH, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J Pediatri. 2006;148(5):652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker A, Alonso ME, Aw MM, et al. Hepatic failure and liver transplant: Working group report of the Second Work Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. JPGN. 2004;39:S632–S639. doi: 10.1097/00005176-200406002-00009. [DOI] [PubMed] [Google Scholar]
- 3.Durand P, Debray D, Mandel R, et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139:871–876. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 4.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 5.Schiødt FV, Atillasoy E, Shakil AO, et al. Etiology and outome for the 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5(1):29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 6.Lee WS, McKiernan P, Kelly DA. Etiology, outcome and prognostic indicators of childhood fulminant hepatic failure in the United Kingdom. J Pediatr Gastroenterol Nutr. 2005;40:575–581. doi: 10.1097/01.mpg.0000158524.30294.e2. [DOI] [PubMed] [Google Scholar]
- 7.Dhawan A, Cheeseman P, Mieli-Vergani G. Approaches to acute liver failure in children. Pediatr Transplantation. 2004;8:584–588. doi: 10.1111/j.1399-3046.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 8.Ciocca M, Ramonet M, Cuarterolo M, et al. Prognostic factors in pediatric acute liver failure. Arch Dis Child. 2008;93(1):48–51. doi: 10.1136/adc.2006.115113. [DOI] [PubMed] [Google Scholar]
- 9.Nicolette L, Billmire D, Faulkenstein K, et al. Transplantation for acute hepatic failure in children. J Pediatr Surg. 1998;33(7):998–1003. doi: 10.1016/s0022-3468(98)90521-8. [DOI] [PubMed] [Google Scholar]
- 10.Aw M, Mitry RR, Huges RD, et al. Serum hepatocyte growth factor and vascular endothelial growth factor in children with acute liver failure. J Pediatr Gastroenterol Nutr. 2007;44:224–227. doi: 10.1097/MPG.0b013e31802c686b. [DOI] [PubMed] [Google Scholar]
- 11.Lebel S, Nakamachi Y, Hemming A, et al. Glycine conjugation of para-aminobenzoic acid (PABA): A pilot study of a novel prognostic test in acute liver failure in children. J Pediatr Gastroenterol Nutr. 2003;36:62–71. doi: 10.1097/00005176-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Liu E, MacKenzie T, Dobyns EL, et al. Characterization of acute liver failure and development of a continuous risk of death staging system in children. J Hepatol. 2006;44:134–141. doi: 10.1016/j.jhep.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Yantorno SE, Kremers WK, Ruf AE, et al. MELD is superior to King’s College and Clichy’s Criteria to assess prognosis in fulminant hepatic failure. Liver Transpl. 2007;13:822–828. doi: 10.1002/lt.21104. [DOI] [PubMed] [Google Scholar]
- 14.Katoonizadeh A, Decaestecker J, Wilmer A, et al. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007;27(3):329–334. doi: 10.1111/j.1478-3231.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 15.Dhiman RK, Jain S, Maheshwari U, et al. Early indicators of prognosis in fulminant hepatic failure: An assessment of the Model for End-Stage Liver Disease (MELD) and King’s College Hospital Criteria. Liver Transpl. 2007;13:814–821. doi: 10.1002/lt.21050. [DOI] [PubMed] [Google Scholar]
- 16.Zaman MB, Hoti E, Qasim A, et al. MELD score as a prognostic model for listing acute liver failure patients for liver transplantation. Transplant Proc. 2006;38:2097–2098. doi: 10.1016/j.transproceed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Kremers WK, van IJperen M, Kim WR, et al. MELD score as a predictor of pretransplant and posttransplant survival in OPTN/UNOS status 1 patients. Hepatology. 2004;39(3):764–764. doi: 10.1002/hep.20083. [DOI] [PubMed] [Google Scholar]
- 18.Shakil AO, Kramer D, Mazariegos GV, et al. Acute liver failure: Clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6(2):163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 2005;41(1):26–31. doi: 10.1002/hep.20511. [DOI] [PubMed] [Google Scholar]
- 20.Schiødt FV, Ostapowicz G, Murray N, et al. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl. 2006;12(12):1776–1781. doi: 10.1002/lt.20886. [DOI] [PubMed] [Google Scholar]
- 21.Baquerizo A, Anselmo D, Shackleton C, et al. Phosphorus as an early predictive factor in patients with acute liver failure. Transplantation. 2003;75(12):2007–2014. doi: 10.1097/01.TP.0000063219.21313.32. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia V, Singh R, Acharya AK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut. 2006;55(1):98–104. doi: 10.1136/gut.2004.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacQuillan GC, Seyam MS, Nightingale P, et al. Blood lactate but not serum phosphate levels can predict patient outcome in fulminant hepatic failure. Liver Transpl. 2005;11(9):1073–1079. doi: 10.1002/lt.20427. [DOI] [PubMed] [Google Scholar]
- 24.Peláez-Luna M, Martinez-Salgado J, Olivera-Martinez MA. Utility of the MAYO End-Stage Liver Disease score, King’s College Criteria, and a new in-hospital mortality score in the prognosis of in-hospital mortality in acute liver failure. Transplant Proc. 2006;38:927–929. doi: 10.1016/j.transproceed.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Selcuk H, Uruc I, Temel MA, et al. Factors prognostic of survival in patients awaiting liver transplantation for end-stage liver disease. Dig Dis Sci. 2007;52(11):3217–3223. doi: 10.1007/s10620-007-9742-3. [DOI] [PubMed] [Google Scholar]
- 26.Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Biggins SW, Rodriguez HJ, Bacchetti P, Bass , et al. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41(1):32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 28.Ruf AE, Kremers WK, Chavez LL, et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11(3):336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]