Abstract
Background:
Approximately 5% of patients administered gentamicin (GM), an aminoglycoside antibiotic, experience vestibular ototoxicity resulting in balance dysfunction. In the present study, we sought to identify susceptibility genes associated with GM-induced vestibular dysfunction using a case/control design.
Methods:
White cases (n = 137; 55 men, 82 women) were recruited based on physician-confirmed unilateral or bilateral vestibular dysfunction attributed to GM administration. Controls (n = 126; 54 men, 72 women) were healthy, age-matched individuals without vestibular dysfunction or balance impairment. Buccal cell samples were obtained from all subjects and DNA was genotyped for 15 polymorphisms in 9 genes. Candidate genes were identified primarily for their roles in oxidative stress based on predicted mechanisms of gentamicin-induced ototoxicity. Statistical analyses included the multi-dimensionality reduction (MDR) method for identifying gene × gene interactions across multiple candidate genes.
Results:
Both single gene and MDR analyses revealed the NOS3 (ENOS) p.Glu298Asp polymorphism as significantly associated with GM-induced vestibular dysfunction (both p ≤ 0.03). MDR analysis revealed a three-gene combination, consisting of NOS3 (p.Glu298Asp), GSTZ1 (p.Lys32Glu), and GSTP1 (p.Ile105Val), that provided the highest predictive model for GM-induced vestibular dysfunction (64% accuracy; p = 0.009).
Conclusions:
The results indicate that carriers of risk alleles at three oxidative stress-related genes have increased susceptibility to GM-induced vestibular dysfunction.
Keywords: Aminoglycoside antibiotic, balance, genetic susceptibility, ototoxicity, pharmacogenomics
1. Introduction
The irreparable ototoxic potential of chemotherapy agents and aminoglycoside antibiotics, such as gentamicin (GM), is well known [23]. A significant fraction (6–16%) of individuals who receive treatment with such drugs every year experience hearing loss or vestibular dysfunction do not also experience hearing loss, indicating different susceptibilities [9,15,20]. For example, of 53 patients with bilateral vestibular failure, nine were the result of drug-induced ototoxicity, but only one patient presented with hearing impairment resulting from ototoxicity [20]. Similarly, Lerner et al. [15] observed auditory dysfunction in three of 33 GM-treated patients and vestibular dysfunction in three different patients, with no patients exhibiting both deficits. Moreover, intra-tympanic administration of GM is used specifically for the treatment of vertigo for Meniere's disease, and hearing loss, although not unexpected, does not occur in some patients following this treatment [8,24].
While specific genetic susceptibility has not been observed for drug-induced vestibular dysfunction, genetic susceptibility to hearing loss associated with drug-induced ototoxicity has been established [3]. The maternal heritability of hearing loss in response to various aminoglycoside antibiotics has been established in multiple families, including the identification of mutations in the mitochondrially encoded 12S RNA (MTRNR1) gene [9]. The m. 1555A>G mutation in the 12S rRNA gene has been shown to explain up to 30% of hearing loss cases in relation to drug therapy [9]. Remarkably, this same mutation is not associated with loss of vestibular function [6], although it has not been examined in a large cohort. Importantly, the only specific environmental risk factors known to contribute to GM-induced ototoxicity are age (i.e., higher risk for infants and children) and dose/duration of treatment [2].
GM and other aminoglycosides are not metabolized by the body, so all effects resulting from GM are specific to the drug itself [2], rather than to a drug metabolite. Following drug entry into the inner ear, several studies now show that the production of nitric oxide (NO) and related reactive oxygen species (ROS) is an important factor in GM-related ototoxicity [23,28,31,39]. The presence of ROS scavengers and NO synthase inhibitors has been shown to reduce GM toxicity [26,28,30]. Moreover, the use of neurotrophic factors (important for cell survival and regeneration in the inner ear) alone [16,41] and in combination with ROS scavengers [28,30] also reduces GM-related ototoxicity. Based on this large body of evidence, Takumida et al. [28] and others [1] have proposed a model of GM-induced ototoxicity centered on NO and ROS. Most recently, isosorbide was shown to delay GM-induced vestibular hair cell death by inhibiting NO and ROS production [29].
In the present case/control study, we sought to identify genetic variants that contribute to susceptibility to GM-induced vestibular dysfunction. Candidate genes were primarily selected based on the proposed oxidative stress model of GM-induced ototoxicity (e.g., brain-derived neurotrophic factor; endothelial nitric oxide synthase; glutathione S-transferases). We also examined myosins VI, VIIA and XVA as candidate genes, as they have all been indicated as candidate genes for vestibular dysfunction given their importance for hair cell structure and function [10,17] and myosin VIIA has been shown to be required for GM accumulation in mouse hair cells [19]. Thus, polymorphisms in each of these three myosin genes were included in the present study. The complete list of candidate genes is shown in Table 1.
Table 1.
Candidate genes selected for genotyping, including gene symbol, chromosome position, polymorphisms genotyped, and the corresponding reference SNP numbers
| Name | Symbol | Location | Reference sequence |
Polymorphism (nucleotide) |
Polymorphism (amino acid) |
Reference SNP number (rs#) |
|---|---|---|---|---|---|---|
| Mitochondrially encoded 12S RNA |
MT-RNR1 | Mito. | NC_001807.4 | m.1555A>G | — | |
| Myosin VI | MYO6 | 6q13 | NM_004999.3 | c.2417-87A>C | rs13211391 | |
| Myosin VIIA | MYO7A | 11q13 | NM_000260.2 | c.46C>T | p.Ser16Leu | rs1052030 |
| c.5859A>C | p.Ile1954Leu | rs948962 | ||||
| Myosin XVA | MYO15A | 8q24 | NM_016239.2 | c.5928C>T | p.Arg1977Lys | rs854777 |
| BDNF | BDNF | 11p13 | NM_001709.3 | c.195A>G | p.Val66Met | rs6265 |
| Nitric oxide | NOS3 | 7q36 | NM_000603.3 | c.893G>T | p.Glu298Asp | rs1799983 |
| synthase 3 (ENOS) | c.-813T>C* | rs2070744 | ||||
| c.582+250N27 (4_5)** | — | |||||
| GST Pi | GSTP1 | 11q13 | NM_000852.2 | c.312A>G | p.Ile105Val | rs1695 |
| c.340C>T | p.Ala114Val | rs1138272 | ||||
| GST M3 | GSTM3 | 1p13 | NM_000849.3 | c.468+21_22insAGG | rs1799735 | |
| GST Zeta 1 | GSTZ1 | 14q24 | NM_145870.1 | c.94A>G | p.Lys32Glu | rs3177427 |
| c.124A>G | p.Arg42Gly | rs7972 |
Abbreviations: SNP, single nucleotide polymorphism; BDNF, brain-derived neurotrophic factor; ENOS; endothelial nitric oxide synthase; GST, glutathione S-transferase.
often referred to as T-786C.
often referred to as the intron 4 variable nucleotide tandem repeat.
2. Methods
2.1. Subjects
Subjects were recruited for participation in a case/control study. Cases were recruited from various vestibular dysfunction support groups through advertisements and direct mailings. Cases were included if they could document profound unilateral or bilateral vestibular loss due specifically to GM administration after the age of 18 yr. Approximately age-matched control subjects were included if they did not have vestibular dysfunction, with or without previous GM therapy. Normal vestibular function was determined with the use of a clinical balance function questionnaire and medical history information. There are no apparent sex or racial differences in susceptibility to GM-induced vestibular dysfunction, so no inclusion limitations were made on those parameters, although the vast majority of recruited subjects were white. Because allele frequenties for many polymorphisms differ by race and only 10 non-white subjects completed the study, all results are presented for the 263 white participants. Children have higher risk for GM-induced ototoxicity, so only individuals > 18 yr. and who were > 18 yr. at the time of GM therapy were included. All subjects provided written informed consent and all procedures were approved by the University of Maryland Institutional Review Board.
2.2. Procedures
Potential subjects completed medical history and balance function questionnaires, and patients specifically provided medical record release authorizations. The questionnaires addressed the date of GM therapy (age of administration), reason for GM therapy, onset of vestibular symptoms following GM therapy, changes in vestibular function over time, and family history of vestibular dysfunction regardless of origin. Vestibular loss was confirmed through medical records obtained from each patient's physician, particularly focusing on vestibulo-ocular reflex (VOR) gains less than 3 standard deviations below the mean and abnormal phase and time constants in pseudorandom sum of sines rotation from 0.01–1.5 Hz., or electronystagmographic verification of absent responses to caloric irrigation. GM must have been indicated as the most likely cause of the vestibular dysfunction, according to the medical records, for inclusion in the study. Controls completed a similar medical history questionnaire and were specifically asked about GM administration.
2.3. Genotyping
DNA was collected using the Epicentre BuccalAmp DNA Extraction Kit, which relies on buccal swabs. Extraction was performed following the manufacturer's instructions and DNA was refrigerated. When extractions failed, new DNA samples were collected where possible and the extraction was repeated. All subjects were genotyped for the candidate polymorphisms shown in Table 1, with genotyping performed randomly on case and control samples and blinded to group status. Restriction-digest and gel electrophoresis methods were used for all polymorphisms, relying either on previously published methods or on de novo designs. Primers, annealing temperatures and specific enzymes are provided in Table 2. Multiple sequence-verified control samples were used in all genotyping assays to verify accuracy of the genotyping results. Uncertain genotypes were repeated with sequence verified control samples. Sequence confirmation was performed for 20 samples across all polymorphisms with 99% consistency.
Table 2.
Primers, annealing temperatures and enzymes used in the genotyping assays for each of the candidate single nucleotide polymorphisms (SNPs)
| Gene | Reference SNP number (rs#) |
Forward primer Reverse primer |
Annealing temp (°C) |
Enzyme |
|---|---|---|---|---|
| MT-RNR1 | — | 5′ GGGTCGAAGGTGGATTTAGC 3′ | 54 | BsmAI |
| 3′ ACTCTGGTTCGTCCAAGTGC 5′ | ||||
| MYO6 | rs13211391 | 5′ GGGAGCAAGCTTTATTCGTT 3′ | 49 | SspI |
| 3′ CTATGTTGCCCAGGCTGACT 5′ | ||||
| MYO7A | rs948962 | 5′ TCTTTCCTGAGAAGGAGCAG 3′ | 57 | EcoO109I |
| 3′ ATGGGCCGAGCTTTCTTTAT 5′ | ||||
| rs1052030 | 5′ CTTCTCTTCCCCCTTGTGTG 3′ | 51 | DpnII | |
| 3′ CAGAGTCGCAGAGCTTCACC 5′ | ||||
| MYO15 | rs854777 | 5′ CACTCCCCAACCTGACATCT 3′ | 57 | SfoI |
| 3′ GCTCAGCTCCTAGAGGGACA 5′ | ||||
| BDNF | rs6265 | 5′ GAGGCTTGACATCATTGGCT 3′ | 60 | Eco72I |
| 3′ CGTGTACAAGTCTGCGTCCT 5′ | ||||
| NOS3 | rs1799983 | 5′GACCCTGGAGATGAAGGCAGGAG3′ | 60 | BanII |
| 3′ACCTCCAGGATGTTCTAGCGGTGA5′ | ||||
| rs10952298 | 5′ CCAGGCCCACCCCAACCTTAT 3′ | 53 | MspI | |
| 3′ TCATTCAGTGACGCACGCTT 5′ | ||||
| Intron 4 | 5′ CCTGGTTATCAGGCCCTATG 3′ | 59 | N/A | |
| VNTR | 3′ AGGCTGCTCCTGCTACTGAC 5′ | |||
| GSTP1 | rs1695 | 5′CTCTATGGGAAGGACCAGCAGGA3′ | 65 | Alw26I |
| 3′ CAAGCCACCTGAGGGGTAAGG 5′ | ||||
| rs1138272 | 5′ TTGACAGGATTTGGTACTAGCC 3′ | 52 | AciI | |
| 3′ TGGTCTCCCACAATGAAGGT 5′ | ||||
| GSTM3 | rs1799735 | 5′ CCTCAGTACTTGGAAGAGCT 3′ | 52 | MnlI |
| 3′ CACATGAAAGCCTTCAGGTT 5′ | ||||
| GSTZ1 | rs7972 | 5′ TGACCACCCAGAAGTGGTAG 3′ | 52 | FokI |
| 3′ AGTCCACAAGACACAGGTTC 5′ | ||||
| rs3177427 | 5′ TGACCACCCAGAAGTGGTAG 3′ | 52 | Alw26I | |
| 3′ AGTCCACAAGACACAGGTTC 5′ |
2.4. Statistics
T-tests and chi square tests were performed to compare demographic and clinical measurements between cases and controls using SAS software. Allele frequency differences were compared between cases and controls, using the software PowerMarker (www.powermarker.net [40]). Hardy-Weinberg equilibrium (HWE) was tested in both cases and controls using Powermarker and the inbreeding coefficients were calculated for the two groups independently. In addition, the Armitage trend test was used to compare genotype distributions between cases and controls as this test is not dependent on assumptions about HWE [22]. Haplotype frequencies in cases and controls were also estimated with PowerMarker. The haplotype trend analysis in Power Marker was performed to test for haplotype frequency differences between cases and controls. The relative effects of specific haplotypes were tested using the estimated haplotype frequencies from PowerMarker and calculating the number of chromo-somes of each type and comparing their distributions relative to all other haplotypes in cases and controls. An odds ratio was calculated using the generated 2 × 2 tables using SAS. As this was the first analysis of these genes for vestibular ototoxicity susceptibility, correction for multiple testing (e.g., Bonferroni, etc.) was not performed; however, all analyses that were performed are presented in the results section.
Multilocus analysis was performed using the multifactor dimensionality reduction (MDR) method (http://sourceforge.net/projects/mdr/ [21]). Briefly, MDR is nonparametric and model free, making it a unique tool for identifying gene × gene interactions. MDR collapses all of the genetic data into two categories, high and low risk, by comparing all single locus and all multilocus combinations and then categorizing each genotype into either high risk or low risk on the basis of the ratio of cases to controls who have that genotype. MDR ultimately selects one genetic model, either single or multilocus, that most successfully predicts phenotype or disease status. The prediction error of the model is estimated using 10-fold cross validation. The 10-fold cross validation is repeated 10 times to ensure that results are not due to chance divisions of the data. The average number of times that the same best model comes up is given as the cross-validation consistency and is represented as a continuous value from 1–10. Cross validation consistency and prediction error minimization are both used to choose the single best model. Statistical significance is determined empirically by permuting the case and control labels 1,000 times. Generating the p values using permutations eliminates the problem of multiple testing.
Multilocus models generatedby MDR were subjected to dendrogram analysis as described by Moore et al. [18]. The dendrograms allow visualization of the nature of the interactions between variables and to assess the statistical nature of the relationship between markers (i.e., redundant, additive, or synergistic). The determination of the nature of the interactions is based on the information gain associated with variable (genotype) interactions, using the algorithm of Jakulin and colleagues [13], as implemented in Moore et al. [18]. Interaction dendrograms were created using the MDR software.
3. Results
A total of 383 subjects were initially recruited for the study, with 273 subjects successfully matching all inclusion criteria. Of the 110 excluded subjects, 45 were excluded due to lack of qualifying evidence of GM-induced ototoxicity, while all other subjects failed to complete various aspects of the study. Subject characteristics for the 263 white subjects are shown in Table 3. More women than men were recruited for both the case and control groups, and the cases (both men and women) were significantly older than controls. Cases had significantly higher rates of hearing and renal complications, which were attributed to their GM therapy.
Table 3.
Subject characteristics
| Cases | Controls | |
|---|---|---|
| N men, N women | 55, 82 | 54, 72 |
| Age, yr. (SD) | 61.4 (12.7) | 56.1 (13.4)* |
| Age of GM admin, yr. (SD) | 55.9 (12.9) | NA** |
| N Unilateral, N Bilateral | 70, 67 | NA |
| Hearing complicationsa, N (%) | 47 (34%) | 4 (3%)* |
| Renal complications, N (%) | 20 (14%) | 0* |
| Family history of vertigo, N (%) | 20 (15%) | 11 (9%) |
| Family history of balance problems, N (%) | 12 (9%) | 16 (13%) |
P <0.05 vs. cases.
Two controls received GM therapy.
Does not distinguish between hearing loss, tinnitus, or other GM-related hearing complications.
Analysis of genotype frequencies for deviations from HWE was performed independently in cases and controls. In cases, 4 of the 15 SNPs deviated from HWE (GSTP1 p.Ala114Val, GSTZ1 p.Lys32Glu, MTRNR1 m.l555A>G, MY07A p.Ser16Leu) with p values between 0.001 and 0.046. In controls 6 of the 15 SNPs deviated from HWE (GSTM3 c.468+21_2insAGG, GSTP1 p.Alal 14Val, GSTZ1 p.Lys32Glu, NOS3 p.Glu298Asp, MY07A p.Ser16Leu, MY06 c.2417-87A>C) with p values ranging from 0.001 to 0.013) with the exception of GSTZ1 p.Lys32Glu with p < 0.0001. Repeat genotyping of these SNPs was performed in the entire sample and showed 97% replication.
Statistical analysis first proceeded by examining each polymorphism individually in relation to case/control status. The first set of analyses examined allele frequency with GM-induced vestibular dysfunction and revealed three genes significantly associated (NOS3 p.Glu298Asp, p = 0.03; GSTZ1 p.Arg42Gly, p = 0.02; GSTM3 c.468+21_2insAGG, p=0.03). Analysis of genotype frequency with the Armitage trend test revealed that the NOS3 p.Glu298Asp polymorphism was significantly associated with GM-induced vestibular dysfunction (p = 0.03) as was GSTZ1 p.Arg42Gly (p = 0.03). GSTM3 was marginally significant for the trend test (p = 0.055). The minor allele frequency for NOS3 p.Glu298Asp (Asp allele) was 37% in cases compared to 27% in controls.
Haplotype analyses were performed for polymorphisms within particular genes or biological systems (i.e., a pseudo-haplotype across related genes). The pseudo-haplotype analysis was used to determine whether inherited variants in different genes that may have similar or related functions cluster differently in cases vs. controls. For the three polymorphisms within the NOS3 gene, no haplotype association was identified(p = 0.51). Similarly, a pseudo-haplotype analysis of all polymorphisms within the myosin genes did not reveal a significant association (p = 0.31). Pseudo-haplotype analysis of the five polymorphisms across all glutathione S-transferase (GST) genes revealed a significant association (p = 0.002). Across the 9 pseudo-haplotypes identified as being present in at least 5% of cases or controls, three haplotypes (G-G-A-A-T, G-A-A-G-C and A-A-A-A-C) were significantly more prevalent in cases compared to controls (Table 4; all p ≤ 0.012).
Table 4.
Pseudo-haplotype analysis of glutathione S-transferase genes (GSTZ1, GSTM2, GSTP1) showing pseudo-haplotypes with a frequency of at least 5% in either cases or controls
| Pseudo- Haplotype1 |
Frequency in cases |
Frequency in controls |
Odds ratio | 95% C.I. | Chi Square p-values |
|---|---|---|---|---|---|
| G-G-A-A-C | 0.1995 | 0.2443 | 0.657 | 0.426 ∼ 1.013 | 0.056 |
| G-G-A-A-T | 0.1706 | 0.0744 | 2.377 | 1.335 ∼ 4.231 | 0.003 |
| G-G-A-G-T | 0.0802 | 0.1268 | 0.528 | 0.295 ∼ 0.945 | 0.030 |
| G-A-A-G-C | 0.0753 | 0.0231 | 3.166 | 1.234 ∼ 8.119 | 0.012 |
| A-A-A-A-C | 0.0689 | 0.00005 | 1.369 | 0 ∼ 52823 | < 0.001 |
| G-G-A-G-C | 0.0683 | 0.0497 | 1.272 | 0.605 ∼ 2.677 | 0.525 |
| G-A-A-A-C | 0.0579 | 0.0977 | 0.503 | 0.2587 ∼ 0.977 | 0.040 |
| A-A-A-G-T | 0.0511 | 0.0384 | 1.224 | 0.526 ∼ 2.845 | 0.639 |
| G-G-B-A-C | 0.0304 | 0.0746 | 0.346 | 0.149 ∼ 0.801 | 0.010 |
Alleles are shown in order for the following polymorphisms: GSTZ1 p.Arg42Gly, GSTZ1 p.Lys32Glu, GSTM3 c.468+21_22insAGG (A, insertion; B, deletion), GSTP1 p.Ile105Val, GSTP1 p.Ala114Val. The overall analysis showed a significant association (p = 0.002).
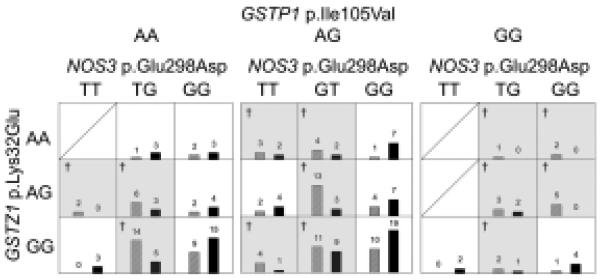
Finally, MDR analysis was performed across all 15 polymorphisms in the nine candidate genes. The best model for each number of variants is shown in Table 5. Similar to the single-gene analysis, the single-gene MDR model with highest prediction accuracy (57%; p = 0.011) consisted of the NOS3 p.Glu298Asp polymorphism. Across all of the MDR models (containing up to 5 loei), the most accurate predictor was the three-genotype model that included NOS3 p.Glu298Asp, GSTZ1 p.Lys32Glu, and GSTP1 p.Ile105Val, which predicted case/control status with 64% accuracy (p = 0.009). As shown in Fig. 1, specific allelic combinations of these three polymorphisms were identified as high and low risk (dark shading indicates higher risk). In particular, combinations including NOS3 p.Glu298Asp Glu-allele (G) homozygotes tended to have the lowest risk.
Table 5.
MDR models, showing prediction accuracy and cross-validation consistency (CVC) performed as measured by the number of times out of 10 subdivisions of the data the same loci appear in the model
| Model | Loci | Accuracy | CVC | ||||
|---|---|---|---|---|---|---|---|
| 1 locus | NOS3 p.Glu298Asp | 0.57 | 10 | ||||
| 2 locus | NOS3 p.Glu298Asp | GSTP1 p.Ile105Val | 0.55 | 5 | |||
| 3 locus | NOS3 p.Glu298Asp | GSTP1 p.Ile105Val | GSTZ1 p.Lys32Glu | 0.64 | 10 | ||
| 4 locus | NOS3 p.Glu298Asp | GSTP1 p.Ile105Val | GSTZ1 p.Lys32Glu | GSTZ1 p.Arg42Gly | 0.52 | 2 | |
| 5 locus | NOS3 p.Glu298Asp | GSTP1 p.Ile105Val | GSTZ1 p.Lys32Glu | GSTZ1 p.Arg42Gly | BDNF p.Val66Met | 0.63 | 8 |
Fig. 1.
A graphical representation of the three-gene MDR model with 64% prediction accuracy. Each cell shows the number of cases (left side of each cell; hatched bars) and controls (right side of each cell; solid bars) carrying that combination of the three genotypes from the GSTZ1 p.Lys32Glu, GSTP1 p.Ile105Val, and NOS3 p.Glu298Asp polymorphisms. Shaded cells (also denoted with the † symbol) are higher-risk, based on the proportion of cases vs. controls carrying that particular genotype combination.
Figure 2 shows a dendrogram of the interaction analysis of the 3-locus model, which shows a strong synergistic interaction between NOS3 p.Glu298Asp and GSTZ1 p.Lys32Glu, while GSTP1 p.Ile105Val appears to have an additive effect with the other two polymorphisms.
Fig. 2.
Interaction dendrogram showing strong redundancy between GSTZ1 p.Lys32Glu and NOS3 p.Glu298Asp, with a synergistic or additive interaction with GSTP1 p.Ile105Val, consistent with the best fitting 3-locus MDR model. The nature of the interaction is depicted in the legend.
4. Discussion
The present study is the first to report susceptibility genes for gentamicin-induced vestibular dysfunction. The NOS3 (ENOS) p.Glu298Asp polymorphism (Asp allele) was independently associated with risk for GM-induced vestibular dysfunction, while the three-gene combination of NOS3 (p.Glu298Asp), GSTZ1 (p.Lys32Glu), and GSTP1 (p.Ile105Val) had 64% accuracy in distinguishing cases from controls. While additional work will be needed to verify these results, the importance of these genes to oxidative stress, known to be a key factor in the mechanism of GM-induced hair cell damage, make them important contributors toward a potential screening tool useful for limiting the occurrence of GM-induced vestibular dysfunction [4].
In the present study, we have focused specifically on GM because of its particularly high vestibulotoxicity, common use, and the fact that GM is not metabolized, but simply excreted by the kidney [2], so any genetic infiuence on GM-related ototoxicity is hypothesized to occur at the level of the vestibular system specifically. As outlined above, NO and related ROS are important for the onset of hair cell degradation in response to GM-related compounds. Aminoglycosides are proposed to influence mitochondrial protein synthesis, resulting in NO and ROS generation and JNK activation, which then leads to apoptosis of hair cells and degradation of inner ear function [39]. Both scavenging of ROS and blockade of NO production have been shown to reduce ototoxic damage resulting from aminoglycosides [28-31,34,39]. In the present study, missense polymorphisms in three genes, NOS3, GSTZ1, and GSTP1 were associated with GM-induced vestibular dysfunction; all three genes are related to NO production and ROS in-activation.
The NOS3 p.Glu298Asp polymorphism has been shown to be related to NO production, with the 298Asp allele having lower levels of NO production in several studies [25,32,33]. Our data are not consistent with these previous findings in that we observed a higher proportion of 298Asp alleles in cases compared to controls; higher NO levels associated with ototoxicity would be expected of the 298Glu allele rather than the Asp allele. One possible explanation is that the p.Glu298Asp polymorphism is acting as a marker for other variants within the NOS3 gene region. Dendrogram analyses performed within the present study for 4-locus models were consistent with this, as the analyses showed a synergistic interaction between the NOS3 p.Glu298Asp and c.-813T>C (also known as T-786C) polymorphisms (data not shown). This can be better understood when these two NOS3 variants are combined as a new composite variable in an MDR analysis as discussed by Moore et al. [18]. When the data were analyzed this way for the present study, the constructed variable (p.Glu298Asp + c.-813T>C) was the best single variable predictor, with almost the same accuracy as the three-locus MDR model presented in Table 4 (analyses not shown). Previous studies have shown significant linkage disequilibrium between these two NOS3 polymorphisms [7,11], in addition to a third intron 4 tandem repeat polymorphism [11,14]. In fact, Hassan et al. [11] showed no influence of the p.Glu298Asp polymorphism alone on plasma NO levels, but haplotypes encompassing the c.-813T>C and intron 4 [c.582+250N27(4_5)] variants were related to NO levels. Thus, additional work is necessary to clarify the direct or indirect role of the p.Glu298Asp variant in NOS3 in GM-induced vestibular ototoxicity susceptibility in relation to the full haplotype structure of this gene region.
The glutathione S-transferase (GST) supergene family encodes a number of enzymes that catalyze the detoxification of various cytotoxic drugs and protect against DNA damage, possibly through direct ROS inactivation [12]. In fact, the activity of GST Pi, an endogenous inhibitor of JNK, has been shown to correlate with ototoxic sensitivity [37]. Ylikoski et al. [39] postulate that JNK activation is the critical step to hair cell degradation in response to ototoxic drugs, so the activity of GSTs, especially GST Pi, would be predicted to influence hair cell apoptosis. In the present study, both GSTZ1 p.Lys32Glu and GSTP1 p.Ile105Val were present in the final three-gene MDR predictive model, and both of these polymorphisms have been shown to be functional [5,35,42]. Specifically, the GSTZ1 p.Lys32Glu has been shown to affect enzyme activity in combination with the nearby Arg42Gly variant [5] and the GSTP1 105Val allele has been associated with lower enzyme activity [35,42]. Though the p.Arg42Gly polymorphism was not present in the final three-gene MDR model, it was present in the four- and five-locus MDR models and in the single gene allele association analyses. How these specific polymorphisms interact to increase susceptibility to GM-induced ototoxicity cannot be determined from the present study, though the MDR analysis suggests that interactions among the genes are important.
Several of the candidate genes selected for the present study did not demonstrate a significant association, either individually or in combination with other genes. For example, none of the myosin genes were found to be associated, despite their apparent importance to hair cell structure and function [35]. Similarly, although the mitochondrially encoded 12S RNA (MTRNR1) gene has been associated with aminoglycoside-induced hearing loss [9], that gene was not associated with GM-induced vestibular dysfunction in the current study or in a previous investigation [6]. Because several studies now indicate different susceptibilities for GM-induced hearing loss compared to GM-induced vestibulotoxicity, this result is not unexpected. Finally, BDNF limits ototoxic damage when provided simultaneously with GM [16] and reduced GM-related ototoxicity has been demonstrated with the combination of a NOS inhibitor with BDNF [28,30]; however, BDNF genotype was not associated with GM-induced vestibulotoxicity in the present study. That said, BDNF was present in the five-locus MDR model and had a tendency toward significance in the Armitage trend test (p = 0.09), so the possibility that BDNF acts as a modifying factor cannot be completely discounted.
The basis for the deviation from HWE in some of the polymorphisms is unclear. While we cannot completely eliminate the possibility of genotyping error, all quality control measures used in the present study demonstrated accurate and replicable genotype data. In addition, the fact that the direction of deviation from HWE differed in cases (inbreeding coefficient f = −0.11) and controls (inbreeding coefficient f = 0.28) for one of the significant single locus associations (NOS3 p.Glu298Asp) further supports the conclusion that the deviations were unlikely to be from genotyping error. As shown by Wittke-Thompson et al. [38] inbreeding coefficients in opposite directions are indicative of genetic association, and it is unlikely for the sign of the inbreeding coefficients to be opposite in cases and controls if genotyping analyses were done without respect to phenotype. In contrast, the GSTZ1 p.Lys32Glu analysis does not necessarily fit expected patterns of true association based on the pattern of deviations from HWE [38], suggesting caution in interpreting the findings for this variant. As emphasized previously, these results will require additional validation in an independent sample; however, the totality of the evidence provides considerable rationale for continued study of NOS3 as a susceptibility gene for GM-induced vestibulotoxicity, possibly in combination with GST genes.
The present study is not without limitations. Within the questionnaire data, dosages of GM were not known for the vast majority of subjects, so that information could not be included in the analysis. The population-based control sample was generally not exposed to gentamicin, which would have provided the most powerful contrast to the cases, as GM exposure without vestibulotoxicity would have eliminated susceptible individuals from the control group. Thus, a small number of individuals in the control population is expected to be susceptible; however, the inclusion of a small number of susceptible controls results in a reduction of power making the study design and results more conservative. Population structure, which was not tested in the present study, represents a possible limitation, though it is unlikely based on recent data from an extensive study performed in > 16,000 individuals of heterogeneous European descent [36]. The results showed that only a small fraction (n = 13) of > 465,000 polymorphisms demonstrated evidence of strong geographical variation, and the authors concluded that populations structure was minimal in the population [36]. Finally, we recognize that other candidate genes could be envisioned for this study. In particular, genotyping assays for polymorphisms in NOS1 (nNOS) and NOS2A (iNOS), both NO synthase genes important to NO production in the vestibular system [27], could not be optimized with the buccal cell samples in the present study. Nonetheless, we focused our initial candidate gene list on what we viewed as the most important targets of investigation and have identified an important combination of genes predicting 64% of responses to GM with respect to vestibular dysfunction from which more sophisticated future investigations can be initiated.
Acknowledgements
We are grateful to the participants and particularly to Ms. Lynn Brown, Director of Wobbler's Anonymous, for her commitment to this project. Winnie Kwok, Jade Clark, and Katie Voss contributed technical support. Funding was provided by NIH/NINDS grant NS046021.
List of abbreviations
- EM
expectation maximization
- GM
gentamicin
- GST
glutathione S-transferase
- HWE
Hardy-Weinberg equilibrium
- MDR
multi-dimensionality reduction
- MO
nitric oxide
- ROS
reactive oxygen species
- VOR
vestibulo-ocular reflex
References
- 1.Agerman K, Canlon B, Duan M, Ernfors P. Neurotrophins, NMDA receptors, and nitric oxide in development and protection of the auditory system. Ann NY Acad Sci. 1999;884:131–142. [PubMed] [Google Scholar]
- 2.Begg EJ, Barclay ML. Aminoglycosides – 50 years on. Br J Clin Pharmacol. 1995;39:597–603. [PMC free article] [PubMed] [Google Scholar]
- 3.Bitner-Glindzicz M, Rahman S. Ototoxicity caused by aminoglycosides. BMJ. 2007;335:784–785. doi: 10.1136/bmj.39301.680266.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black FO, Pesznecker S, Stallings V. Permanent gentamicin vestibulotoxicity. Otol Neurotol. 2004;25:559–569. doi: 10.1097/00129492-200407000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn AC, Tzeng HF, Anders MW, Board PG. Discovery of a functional polymorphism in human glutathione transferase zeta by expressed sequence tag database analysis. Pharmacogenetics. 2000;10:49–57. doi: 10.1097/00008571-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Braverman I, Jaber L, Levi H, Adelman C, Arons KS, Fischel-Ghodasian N, Shohat M, Elidan J. Audiovestibuar findings in patients with deafness caused by a mitochondrial susceptibility mutation and precipitated by an inherited nuclear mutation or aminoglycosides. Arch Otolaryngol Head Neck Surg. 1996;122:1001–1004. doi: 10.1001/archotol.1996.01890210073016. [DOI] [PubMed] [Google Scholar]
- 7.Colombo MG, Paradossi U, Andreassi MG, Botto N, Manfredi S, Masetti S, Biagini A, Clerico A. Endothelial nitric oxide synthase gene polymorphisms and risk of coronary artery disease. Clin Chem. 2003;49:389–395. doi: 10.1373/49.3.389. [DOI] [PubMed] [Google Scholar]
- 8.Dobie RA, Black FO, Pezsnecker SC, Stallings VL. Hearing loss in patients with vestibulotoxic reactions to gentamicin therapy. Arch Otolaryngol Head Neck Surg. 2006;132:253–257. doi: 10.1001/archotol.132.3.253. [DOI] [PubMed] [Google Scholar]
- 9.Fischel-Ghodsian N. Genetic factors in aminoglycoside toxicity. Ann NY Acad Sci. 1999;884:99–109. doi: 10.1111/j.1749-6632.1999.tb08639.x. [DOI] [PubMed] [Google Scholar]
- 10.Friedman TB, Hinnant JT, Fridell RA, Wilcox ER, Raphael Y, Camper SA. DFNB3 families and Shaker-2 mice: mutations in an unconventional myosin, myo 15. Adv Otorhinolaryngol. 2000;56:131–144. doi: 10.1159/000059094. [DOI] [PubMed] [Google Scholar]
- 11.Hassan A, Gormley K, O'Sullivan M, Knight J, Sham P, Vallance P, Bamford J, Markus H. Endothelial nitric oxide gene haplotypes and risk of cerebral small-vessel disease. Stroke. 2004;35:654–659. doi: 10.1161/01.STR.0000117238.75736.53. [DOI] [PubMed] [Google Scholar]
- 12.Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61:154–166. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 13.Jakulin A, Bratko I, Smrke D, Demsar J, Zupan B. Attribute interactions in medical data analysis. Lect Notes Artif Intell. 2003;2780:229–238. [Google Scholar]
- 14.Karasneh JA, Hajeer AH, Silman A, Worthington J, Ollier WE, Gul A. Polymorphisms in the endothelial nitric oxide synthase gene are associated with Behcet's disease. Rheumatology (Oxford) 2005;44:614–617. doi: 10.1093/rheumatology/keh561. [DOI] [PubMed] [Google Scholar]
- 15.Lerner SA, Schmitt BA, Seligsohn R, Matz GJ. Comparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicin. Am J Med. 1986;80:98–104. doi: 10.1016/0002-9343(86)90486-9. [DOI] [PubMed] [Google Scholar]
- 16.Lopez I, Honrubia V, Lee S-C, et al. The protective effect of brain-derived neurotrophic factor after gentamicin ototoxicity. Am J Otol. 1999;20:317–324. [PubMed] [Google Scholar]
- 17.Melchionda S, Ahituv N, Bisceglia L, et al. MYO6, the human homologue of the gene responsible for deafness in Snell's Waltzer mice, is mutated in autosomal dominant non-syndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore JH, Gilbert JC, Tsai CT, Chiang FT, Holden T, Barney N, White BC. A flexible computational frame-work for detecting, characterizing, and interpreting statistical patterns of epistasis in genetic studies of human disease susceptibility. J Theor Biol. 2006;241:252–261. doi: 10.1016/j.jtbi.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Richardson GP, Forge A, Kros CJ, Fleming J, Brown SDM, Steel KP. Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells. J Neurosci. 1997;17:9506–9519. doi: 10.1523/JNEUROSCI.17-24-09506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinne T, Bronstein AM, Rudge P, Gresty MA, Luxon LM. Bilateral loss of vestibular function: clinical findings in 53 patients. J Neurol. 1998;245:314–321. doi: 10.1007/s004150050225. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasieni PD. From genotypes to genes: doubling the sample size. Biometrics. 1997;53:1253–1261. [PubMed] [Google Scholar]
- 23.Schacht J. Antioxidant therapy attenuates aminoglycoside-induced hearing loss. Ann NY Acad Sci. 1999;884:125–130. [PubMed] [Google Scholar]
- 24.Smith WK, Sandooram D, Prinsley PR. Intratympanic gentamicin treatment in Meniere's disease: patients' experiences and outcomes. J Laryngol Otol. 2006;120:730–735. doi: 10.1017/S0022215106002283. [DOI] [PubMed] [Google Scholar]
- 25.Sofowora G, Dishy V, Xie HG, Imamura H, Nishimi Y, Morales CR, Morrow JD, Kim RB, Stein CM, Wood AJ. In-vivo effects of Glu298Asp endothelial nitric oxide synthase polymorphism. Pharmacogenetics. 2001;11:809–814. doi: 10.1097/00008571-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Song B-B, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hearing Res. 1996;94:87–93. doi: 10.1016/0378-5955(96)00003-2. [DOI] [PubMed] [Google Scholar]
- 27.Takumida M, Anniko M. Localization of nitric oxide synthase isoforms (NOS I, II and III) in the vestibular end organs of the guinea pig. ORL J Otorhinolaryngol Relat Spec. 1998;60:67–72. doi: 10.1159/000027567. [DOI] [PubMed] [Google Scholar]
- 28.Takumida M, Anniko M. Brain-derived neurotrophic factor and nitric oxide synthase inhibitor protect the vestibular organ against gentamicin ototoxicity. Acta Otolaryngol. 2002;122:10–15. doi: 10.1080/00016480252775661. [DOI] [PubMed] [Google Scholar]
- 29.Takumida M, Anniko M. Isosorbide delays gentamicin-induced vestibular sensory cell death. ORL J Otorhinolaryngol Relat Spec. 2005;67:276–281. doi: 10.1159/000089408. [DOI] [PubMed] [Google Scholar]
- 30.Takumida M, Anniko M, Shimizu A, Watanabe H. Neuroprotection of vestibular sensory cells from gentamicin ototoxicity obtained using nitric oxide synthase inhibitors, reactive oxygen species scavengers, brain-derived neurotrophic factors and calpain inhibitors. Acta Otolaryngol. 2003;123:8–13. doi: 10.1080/0036554021000028078. [DOI] [PubMed] [Google Scholar]
- 31.Takumida M, Popa R, Anniko M. Free radicals in the guinea pig inner ear following gentamicin exposure. ORL J Otorhinolaryngol Relat Spec. 1999;61:63–70. doi: 10.1159/000027643. [DOI] [PubMed] [Google Scholar]
- 32.Tanus-Santos JE, Desai M, Deak LR, Pezzullo JC, Abernethy DR, Flockhart DA, Freedman JE. Effects of endothelial nitric oxide synthase gene polymorphisms on platelet function, nitric oxide release, and interactions with estradiol. Pharmacogenetics. 2002;12:407–13. doi: 10.1097/00008571-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, de Leeuw PW, Smits P. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J Hypertens. 2002;20:2023–2027. doi: 10.1097/00004872-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K-I, Hess A, Michel O, Yagi T. Nitric oxide synthase inhibitor reduces the apoptotic change in the cisplatin-treated cochlea of guinea pigs. Anti-Canc Drugs. 2000;11:731–735. doi: 10.1097/00001813-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–280. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 36.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitlon DS, Wright LS, Nelson SA, Szakaly R, Siegel EL. Maturation of cochlear glutathione-S-transferases correlates with the end of the sensitive period for ototoxicity. Hearing Res. 1999;137:43–50. doi: 10.1016/s0378-5955(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 38.Wittke-Thompson JK, Pluzhnikov A, Cox NJ. Rationale inferences about departures from Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:967–986. doi: 10.1086/430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hearing Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- 40.Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum Hered. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
- 41.Zheng JL, Stewart RR, Gao WQ. Neurotrophin-4/5, brain derived neurotrophic factor, and neurotrophin-3 promote survival of cultured vestibular ganglion neurons and protect them against neurotoxcity of ototoxins. J Neurobiol. 1995;28:330–340. doi: 10.1002/neu.480280306. [DOI] [PubMed] [Google Scholar]
- 42.Zhong SL, Zhou SF, Chen X, Chan SY, Chan E, Ng KY, Duan W, Huang M. Relationship between genotype and enzyme activity of glutathione S-transferases M1 and P1 in Chinese. Eur J Pharm Sci. 2006;28:77–85. doi: 10.1016/j.ejps.2006.01.002. [DOI] [PubMed] [Google Scholar]