Abstract
Chemotherapy agents have been shown to induce the transcription factor NF-κB and subsequent chemoresistance in fibrosarcomas and other cancers. The mechanism of NF-κB-mediated chemoresistance remains unclear, with a previous report suggesting that doxorubicin induces this response independent of the inhibitory κB kinases (IKKs). Other studies have indicated that IKKβ, but not IKKα, is required. Mouse embryo fibroblasts (MEF) devoid of IKKα, IKKβ, or both subunits (DKO) were treated with doxorubicin. The absence of either IKKα or IKKβ or both kinases resulted in impaired induction of NF-κB DNA-binding activity in response to doxorubicin. To provide a valid clinical correlate, HT1080 human fibrosarcoma cells were transfected with small interference RNAs (siRNAs) specific for IKKα or IKKβ and then subsequently treated with doxorubicin. Knockdown of IKKα severely impaired the ability of doxorubicin to initiate NF-κB DNA-binding activity. However, a decrease in either IKKα or IKKβ resulted in decreased phosphorylation of p65 in response to doxorubicin. The inhibition of doxorubicin-induced NF-κB activation by the knockdown of either catalytic subunit resulted in increased cleaved caspase 3 and cleaved PARP, and increased apoptosis when compared to doxorubicin alone. The results of this study validate current approaches aimed at NF-κB inhibition to improve clinical therapies. Moreover, we demonstrate that IKKα plays a critical role in NF-κB-mediated chemoresistance in response to doxorubicin and may serve as a potential target in combinational strategies to improve chemotherapeutic response.
Keywords: IKKalpha, IKKbeta, NF-kappaB, Chemoresistance, Sarcoma
Introduction
Nuclear factor κB (NF-κB) is a transcription factor with critical roles in a number of inflammatory responses, as well as oncogenesis, metastasis and chemoresistance. There are five members of the NF-κB family: p50/p105 (NF-κB1), p52/p100 (NF-κB2), c-Rel, RelB, and p65 (RelA), characterized by the presence of the Rel homology domain which is involved in DNA binding, dimerization, and regulation by inhibitory proteins (1-3). NF-κB exists as hetero- and homodimers, with certain dimers (including the classic p50/p65 heterodimer) forming a complex with inhibitory κB proteins (IκB). Stimulation of cells with inflammatory cytokines leads to activation of the canonical pathway, which is mediated by phosphorylation of the IκB proteins by inhibitory κB kinases (IKKs) (2). The phosphorylated IκB proteins are targeted for ubiquitination and subsequent degradation by the 26S proteasome. Once freed from its binding proteins in the cytoplasm, NF-κB is able to accumulate in the nucleus and interact with regulatory elements and affect gene specific transcription (1, 4).
NF-κB activation is known to regulate an array of downstream anti-apoptotic genes, such as cIAP-2 and Bcl-xL (5, 6). It is this anti-apoptotic function of NF-κB that has implicated this family of transcription factors in the development, chemoresistance, and potential treatment of many cancers. In the field of oncology, NF-κB has been shown to be constitutively active in numerous neoplasms (7, 8). However, NF-κB can also be activated in response to treatment with specific chemotherapy agents or irradiation. Previous work in our laboratory demonstrated that NF-κB is vital to the inducible chemoresistance of colon cancers. More specifically, inhibiting NF-κB activation in response to chemotherapy significantly enhanced the cytotoxic effects of camptothecin (9, 10). This relationship has been reported in a variety of cancer cell types, including sarcomas (11, 12). Soft tissue sarcomas remain a relatively rare malignancy, affecting approximately 8000 new patients each year (13). Despite recent advances in understanding the biology of many of these soft tissue sarcomas, surgery remains the mainstay of treatment since most sarcomas are largely resistant to chemotherapy (14, 15). Surgery and radiotherapy can play important roles in the multimodality treatment of localized sarcomas, but the overall survival rate of less than 50% speaks to the chemoresistance of this malignancy in advanced stages (14, 15). The signaling mechanisms involved in NF-κB-mediated chemoresistance have not yet been completely elucidated. In fact, some studies have challenged the notion of chemoresistance, suggesting that NF-κB can play a pro-apoptotic role in response to specific chemotherapy agents, including doxorubicin (16-18).
Overall, these preclinical results have generated increased pharmacologic interest in agents aimed at inhibiting the activation of the NF-κB signaling pathway. Inhibition of the 26S proteasome, which is involved in regulating IκB protein degradation, remains the strategy that has been studied most extensively. The generalized proteasome inhibitors have been tested in several early phase human clinical trials in both hematologic malignancies and solid tumors. In fact, Velcade, a proteasome inhibitor approved by the Federal Drug Administration, has shown efficacy in multiple myelomas, both alone and in combination with doxorubicin (19, 20). Unfortunately, clinical trials in solid tumors using Velcade have been limited by toxicity and a lack of significant improvement in survival (21-23). Additionally, there is increasing evidence that proteasome inhibitors have broad effects, with varied responses both related and non-related to NF-κB activation. As a result, efforts have been redirected toward finding targets for inhibition that have a greater specificity for the NF-κB pathway.
One such potential target in the NF-κB pathway is the IKK complex, which consists of two catalytic subunits, IKKα and IKKβ, and a regulatory protein known as IKKγ/NEMO. This complex serves a critical role in the activation pathway of NF-κB by liberating it from its inhibitory molecules. Understanding the roles that these various subunits play in the activation of NF-κB in the setting of cancer and chemotherapy is critical to identifying more effective signaling targets. There are conflicting reports regarding the importance of the different IKK subunits in the NF-κB response to chemotherapy (24, 25). Similar to the classical response seen with Tumor Necrosis Factor α (TNFα), some cite an IKKβ dominance in mouse embryo fibroblasts (MEF) treated with doxorubicin (25). In a more recent report in a similar in vitro model, the same group suggests that an IKK-independent, PI3-kinase-dependent pathway is responsible for the late activation of NF-κB by doxorubicin (24). Delineating the role of the IKK subunits in NF-κB-mediated chemoresistance is crucial to the development of selective inhibitory strategies targeting these kinases. As such, examining this question using a clinically relevant chemotherapy agent in human cancer cells will be important in gaining a better understanding of the signaling steps leading to NF-κB-mediated chemoresistance.
In this study, we used a human fibrosarcoma model to examine the potential involvement of IKKα and IKKβ in controlling the activation of NF-κB in response to the standard chemotherapy agent, doxorubicin. Additionally, we will further elucidate whether NF-κB activation is pro- or anti-apoptotic in this experimental setting.
Materials and Methods
Cell Culture
The human fibrosarcoma cell line HT1080 was obtained form American Type Culture Collection (ATCC; Rockville, MD). The HT1080 cells are grown in Minimum Essential Medium (MEM) Alpha containing L-glutamine supplemented with 10% fetal bovine serum and 100μg/ml penicillin and 100μg/ml streptomycin. Cell cultures were maintained at 37°C with a mixture of 95% air and 5% CO2. Mouse embryo fibroblast (MEF) cells devoid of IKKα (IKKα−/−), IKKβ (IKKβ−/−), or both IKKα and IKKβ (DKO) (generously donated by Dr. I. Verma and Dr. M. Karin) were grown in Dulbecco's Minimal Essential Medium supplemented with 10% fetal bovine serum and antibiotics as above. Cells were treated with doxorubicin (1.5μg/ml) and harvested at the time points indicated (1, 3, 6, 18, 24 hours). The positive control used for these experiments was treatment with TNFα (50ng/ml) for 15 minutes.
Small Interference RNA (siRNA) Transfection
For transfection of siRNA in HT1080 cells 5×104 cells were plated in standard 6-well plates and incubated overnight. The transfections were then carried out according to the manufacturer's protocol. Briefly, the media was removed and replaced with transfection media containing siGENOME SMARTpool siRNA (sequences below) for siControl #1, siIKKα, or siIKKβ (final concentration 10-20nM) and DharmaFECT4 transfection reagent (Dharmacon, Inc., Lafayette, CO). The cells were maintained in transfection media for 24 hours. The transfection media was then removed and replaced with standard growth media. The cells were then grown for an additional 48 hours to achieve maximum protein knockdown. The cells were then treated with doxorubicin as described above. The knockdown of IKK proteins was confirmed with western blot.
Western Blot Analysis
The media was removed and the cells were washed with phosphate buffered saline (PBS) and then lifted in the same buffer. Cytoplasmic extracts were prepared using a hypotonic buffer [10mM Hepes (pH 7.6), 60 mM KCL, 1mM EDTA, 1mM DTT, 0.2% NP-40, 1mM PMSF with 1μl each of aprotinin, leupeptin, and pepstatin]. The extracts were isolated by sequential centrifugation at 2500rpm for 4 minutes to separate the nuclei and then at 13,000rpm for 5 minutes to remove any remaining cell fragments. Whole cell extracts were obtained using a lysis buffer [20mM Tris-HCL (pH 7.5), 150mM NaCl, 1mM Na2EDTA, 1mM EGTA, 1% Triton, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4] supplemented with protease and phosphatase inhibitors. The cells were incubated on ice in the whole cell lysis buffer for 5 minutes and the cellular fragments were then separated using centrifugation at 13,000rpm for 10 minutes.
Western blot analysis was then performed following the protocol provided by Invitrogen (Carlsbad, CA) by separating proteins (20-30μg) on NuPAGE 4-12% Bis-Tris gels. Antibodies for IκBα, β-Tubulin, GAPDH, and p65 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used at a dilution of 1:1000 in 5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in TBS-T. Antibodies for caspase 3, cleaved PARP, PARP (Cell Signaling Technology, Inc., Danver, MA) were also used at 1:1000 dilution, while antibodies for IKKα, IKKβ, phosphorylated p65 at serine 536, and cleaved caspase 3 (Cell Signaling Technology, Inc., Danver, MA) were used at a 1:500 dilution. The blots were incubated with the primary antibody overnight at 4°C. The membranes were then incubated with secondary anti-rabbit antibody diluted 1:10,000 in TBS-T for 1 hour and then developed using Amersham ECL Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, England).
TUNEL Assay
HT1080 cells transfected with siRNA as described above. The cells were then treated with doxorubicin (1.5μg/ml) for 24 hours. The cells were then trypsinized and isolated by centrifugation at 2500 rpm for 5 minutes. The cell pellet was then washed with cold PBS and fixed with 10% formalin. The cells were then embedded in paraffin, sectioned, and mounted on microscope slides. Apoptosis was then measured by labeling DNA strand breaks using the TUNEL method with the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) following the manufacturer's protocol. Results are reported as the percentage of 1000 cells that stained positive. Experiments were conducted in triplicate.
Electrophorectic Mobility Shift Assay (EMSA)
Cells were harvested after treatment as indicated above, and the EMSAs performed as previously reported (26). In brief, the cytoplasmic extracts were isolated as described above. The nuclei were then suspended in a high-salt buffer (20mM Tris pH 8.0, 420 mM NaCl, 1.5mM MgCl2, 0.2 mM EDTA, 1mM PMSF, 25% glycerol, and 1μl each of aprotinin, leupeptin, and pepstatin) for ten minutes. The samples were then placed in a centrifuge at 13,000 rpm for 10 minutes. The supernatant was then separated and protein concentration determined with Bradford assay. Approximately 5μg of nuclear extract was incubated with 1μg/μl polydIdC in binding buffer (50mM Tris pH 7.6, 5mM DTT, 2.5mM EDTA, 50% glycerol) with an oligonucleotide radiolabeled with [α32P]dCTP for 15 minutes at room temperature. The probe contains an NF-κB consensus binding site for the H-2κB promoter (5′-GGGGATTCCC-3′). The samples were then separated on a polyacrylamide gel and developed with autoradiography. For supershifts, the nuclear extract was incubated in binding buffer with 1μl of p65X, p50X (200μg/0.1ml; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), p52, RelB, or cRel (200μg/ml; Santa Cruz Biotechnology, Inc., Santa Crux, CA) for 15 minutes and then incubated for an additional 15 minutes with polydIdC and the radiolabeled probe.
Statistics
All experiments were conducted in triplicate to ensure the accuracy of the results. Statistical comparison was conducted for the TUNEL assay using normal approximation with the assistance of the University of North Carolina Biostatistics Core.
Results
Doxorubicin induces NF-κB DNA-binding in MEF cells through both IKKα and IKKβ
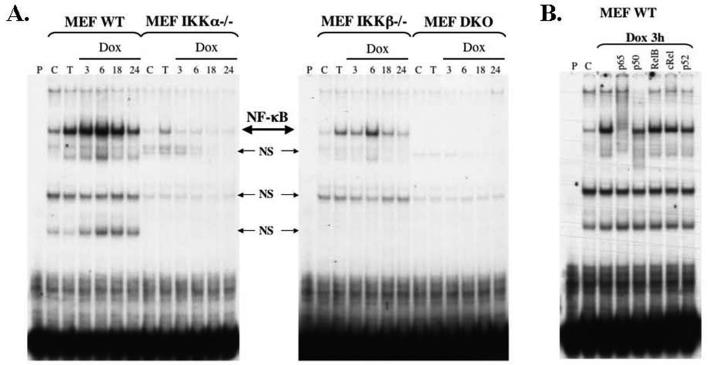
In order to examine the roles of the catalytic subunits of the IKK complex in doxorubicin-induced NF-κB activation, wild type (WT) MEF cells and MEF cells devoid of IKKα, IKKβ or both subunits were stimulated with doxorubicin (1.5μg/ml). Cells were harvested at 3h, 6h, 18h, and 24h time points and nuclear extracts were prepared and evaluated for NF-κB DNA-binding activity using EMSA (Figure 1A). Induction by TNFα served as a positive control and untreated cells served as a negative control. WT MEFs demonstrated basal NF-κB DNA-binding activity, which was significantly increased when exposed to TNFα or doxorubicin (Figure 1A). The time course of induction was similar to that seen in other in vitro human cancer models in response to clinically relevant chemotherapeutic agents (9, 12). Supershift analysis demonstrated that the activated form of NF-κB seen with doxorubicin consists of p65, p50 and p52 subunits as evidenced by the shifting of the complex in the p65 column and the decreased binding of the probe in the p50 and p52 columns (Figure 1B).
Figure 1.
(A) In mouse embryo fibroblasts (MEF), deletion of IKKα impairs nuclear binding of NF-κB (large arrow) in response to doxorubicin (Dox) to a greater extent then deletion of IKKβ. Wild type (WT), IKKα−/−, IKKβ−/− and double knockout (DKO) MEFs were stimulated with TNFα (T) or Dox at a final concentration of 1.5μg/ml for 3, 6, 18, or 24 hours. The results of these treatments were compared to untreated controls (C). The first lane in each gel (P) consists of only the radiolabeled probe, which is collected at the bottom of the gel. The IKKα−/− MEFs demonstrated impaired basal NF-κB DNA-binding activity as well as a significant decrease in induced NF-κB DNA-binding activity by both TNFα and dox. The results for IKKβ−/− MEFs were similar in regards to basal and TNFα-induced NF-κB DNA-binding, but the lack of IKKβ only mildly decreased the ability of doxorubicin to generate NF-κB activity. (B) Supershift analysis performed on the WT MEFs demonstrated that the activated NF-κB complex seen with doxorubicin in MEF cells consists mainly of p65 subunits and to a lesser extent p50 and p52 subunits. Moreover, the use of antibodies to NF-κB subunits clarifies that the additional lighter bands seen are non-specific (small arrows, NS).
In contrast to the WT MEFs, both the IKKα−/− MEFs and IKKβ−/− MEFs demonstrated impaired NF-κB DNA-binding activity at baseline and in response to both TNFα and doxorubicin (Figure 1A). More specifically, the lack of IKKα nearly completely abolished the doxorubicin-induced NF-κB activity, whereas the absence of IKKβ was less effective in deterring NF-κB DNA-binding activity in response to doxorubicin. These results support a significant role for IKKα in the NF-κB response to doxorubicin in MEF cells. It is important to note that the genetic depletion of either catalytic subunit in isolation was not sufficient to completely prevent the activation of NF-κB. Moreover, the fact that double knockout (DKO) MEFs are deficient in NF-κB DNA-binding activity basally and in response to both TNFα and doxorubicin (Figure 1A) indicates that the signaling from doxorubicin to NF-κB does in fact occur through both IKKα and IKKβ subunits.
Doxorubicin activates NF-κB and induces IκBα degradation in HT1080 cells
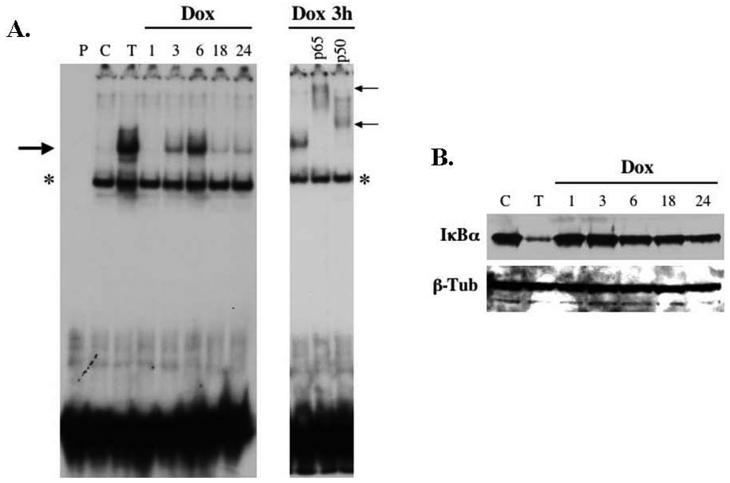
Although sarcomas are largely chemoresistant, doxorubicin remains a standard cytotoxic agent in the treatment of patients with systemic or locally advanced disease. Therefore we analyzed the pattern of NF-κB activation in HT1080 fibrosarcoma cells in response to doxorubicin (1.5μg/ml). Cytoplasmic and nuclear extracts were harvested following 1, 3, 6, 18, and 24 hours of treatment with doxorubicin. TNFα served as a positive control and untreated cells functioned as a negative control. Interestingly, stimulation with doxorubicin resulted in a biphasic pattern of NF-κB DNA-binding activity (Figure 2A). The peak activation was identified at 3-6 hours, similar to the MEF cells. However, rather than a steady decline of activity over time, the HT1080 cells demonstrated a small increase in activity seen again from 18 hours to 24 hours of treatment with doxorubicin. Supershift analysis confirmed that the activated NF-κB complex consisted of both p65 and p50 subunits (Figure 2A).
Figure 2.
(A) Doxorubicin (Dox) induces NF-κB activity in HT1080 fibrosarcoma cells in a bimodal pattern. HT1080 cells were stimulated with TNFα (50ng/ml) or doxorubicin (1.5μg/ml) and then harvested at 1, 3, 6, 18, and 24-hours. Nuclear extracts were evaluated by EMSA for NF-κB DNA-binding activity (large arrow). Again, the first lane (P) represents radiolabeled probe alone that and the results were compared to untreated cells (C). In the right panel, supershift analysis confirmed that the activated form of NF-κB consists of both p65 and p50 subunits (small arrows) and that the lower band represents a non-specific band (*). (B) Degradation of IκBα at later time points does not correlate with NF-κB nuclear activity. Cytoplasmic extracts of HT1080 cells were evaluated by western blot for IκBα protein levels and demonstrate a persistent decrease in IκBα over time despite the variable expression of nuclear NF-κB seen by EMSA.
Next, the effects of doxorubicin treatment on the protein levels of IκBα were examined. In contrast to the pattern seen with NF-κB DNA-binding seen on EMSA, the decrease in IκBα persisted throughout the treatment course (Figure 2B). The initial decrease in IκBα was identified at the 6-hour time point, which coincides with the first peak in NF-κB nuclear activity. However, IκBα protein levels continued to decrease at later time points despite the weaker NF-κB DNA-binding activity. We attribute the initial decrease in IκBα at the 6-hour time point to degradation by the 26S proteasome as it correlates with the initial increase in of NF-κB DNA-binding activity. However, the mechanism leading to the decrease in IκBα protein levels with prolonged exposure to doxorubicin remains unclear.
IKKα is critical to Doxorubicin-induced NF-κB DNA-binding activity in HT 1080 cells
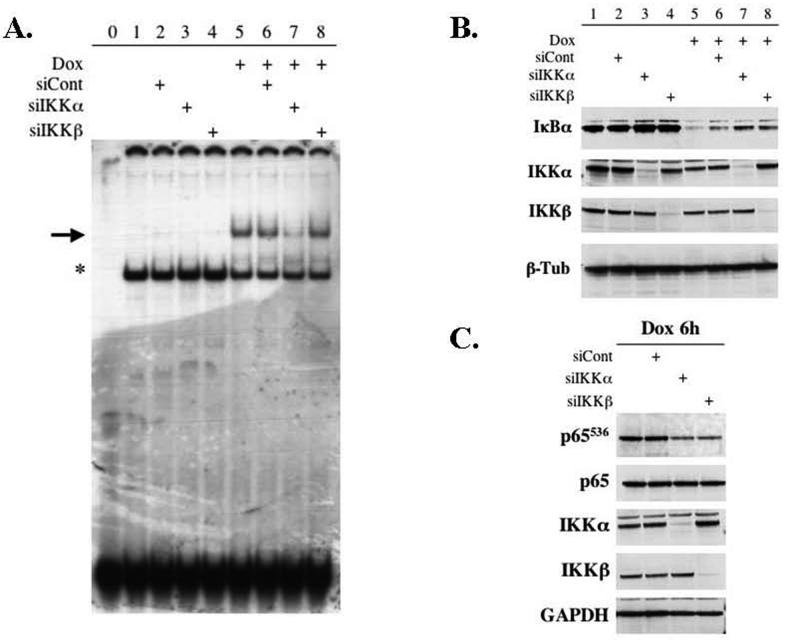
The roles for IKKα and IKKβ in the induction of NF-κB DNA-binding activity by doxorubicin in HT1080 cells were delineated using siRNA strategies to knock down the expression of IKK subunits. Small interference RNA for IKKα (10nM) or IKKβ (20nM) was transfected into HT1080 cells to achieve a selective decrease in each catalytic subunit. The cells were then stimulated with doxorubicin (1.5μg/ml) for 6 hours, which was previously identified as the point of maximal NF-κB DNA-binding activity. Nuclear extracts were evaluated by EMSA to assess NF-κB DNA-binding activity and cytoplasmic extracts were evaluated with western blot to determine the protein levels of the IKK subunits. The positive control in this experiment was HT1080 cells treated with doxorubicin alone. In order to control for effects of the transfection alone, a nonspecific control siRNA was employed. We demonstrated a decrease in doxorubicin-induced NF-κB DNA-binding only in the absence of IKKα (Figure 3A). Moreover, the knockdown of IKKβ did not significantly alter the activation of NF-κB at the nuclear level by doxorubicin (Figure 3A). Importantly, the siRNA treatments were successful in achieving a selective and substantial decrease in the IKKα and IKKβ subunits (Figure 3B). These results are similar to those seen in the MEF cells and support an important role for IKKα in the signaling of doxorubicin to NF-κB.
Figure 3.
(A) Depletion of IKKα causes a significant decrease in NF-κB DNA-binding (large arrow) in HT1080 cells treated with doxorubicin (Dox) for 6 hours. HT1080 fibrosarcoma cells were transfected with siRNA as described. The cells were then stimulated with Dox (1.5μg/ml) for 6 hours. Nuclear extracts were evaluated using EMSA and compared to radiolabeled probe alone (Lane 0) and untreated cells (Lane 1). (B) Cytoplasmic extracts were evaluated by Western blot confirming successful selective depletion of IKKα and IKKβ using siRNA strategies. Moreover, the lack of either catalytic subunit resulted in a mild increase in IκBα at baseline and resulted in a mild decrease in the ability of Dox to induce the degradation of IκBα. (C) Whole cell extracts were prepared from HT1080 cells following siRNA transfection and exposure to Dox for 6 hours. Evaluation again confirmed specific knockdown of the intended IKK subunit. Moreover, examination of the state of phosphorylated p65 at the serine residue at position 536 (p65536) demonstrates that the lack of either IKKα or IKKβ results in decreased phosphorylation of p65 in response to Dox. Together these results suggest that both IKKα and IKKβ play a significant independent role in the activation of NF-κB following treatment with chemotherapy.
Both IKKα and IKKβ are important to IκBα degradation and p65 phosphorylation
In order to further evaluate the activation of NF-κB by doxorubicin, the effects of knocking down IKKα and IKKβ on IκBα degradation and p65 phosphorylation were studied. For IκBα degradation, selective depletion of either IKKα or IKKβ was achieved using siRNA and the HT1080 cells were stimulated with doxorubicin for 6 hours. Cellular extracts were then prepared and evaluated for IκBα protein levels. Treatment with doxorubicin alone caused a substantial degradation of IκBα. Interestingly, the knockdown of either catalytic subunit resulted in an increase in IκBα at baseline (Figure 3B, lanes 3 and 4) as well as a decrease in the doxorubicin-induced degradation of IκBα (Figure 3B, lanes 7 and 8). As such, the functions of IKKα and IKKβ appeared to mirror each other and have comparable roles in the signaling of doxorubicin to NF-κB in response to doxorubicin.
Additionally, we analyzed the effects of IKKα and IKKβ on the phosphorylation state of the p65 subunit as a result of exposure to doxorubicin. The phosphorylation site analyzed in these experiments was the serine residue at position 536. This particular phosphorylation site has been shown to be important in NF-κB signaling in response to various cytokines (27, 28). Moreover, both IKKα and IKKβ have been shown to be capable of phosphorylating this site (28, 29). For these experiments, whole cell extracts were prepared following siRNA transfection and doxorubicin treatment for 6 hours. The exposure to doxorubicin generated a high level of phosphorylated p65 that was not affected by the transfection of the control siRNA (Figure 3C). However, the depletion of either IKKα or IKKβ significantly decreased the amount of phosphorylation of p65. The results clearly show that in HT1080 cells both IKKα and IKKβ are required for phosphorylating the p65 subunit in response to doxorubicin. These results again support a critical role for both IKKα and IKKβ in the signaling pathway that enables fibrosarcoma cells to activate NF-κB when exposed to the DNA damage agent, doxorubicin.
Deletion of either IKKα or IKKβ increases the cytotoxicity of doxorubicin in HT1080 cells
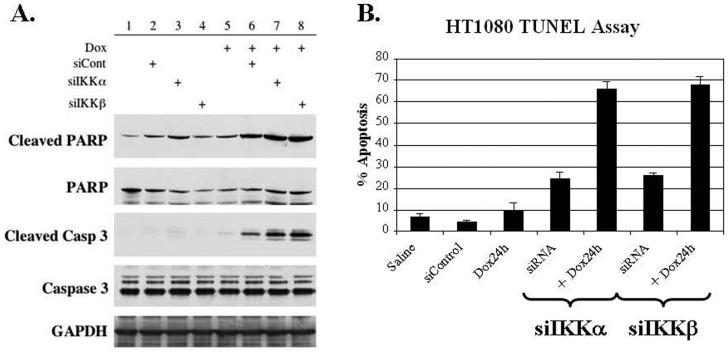
Having identified that IKKα has a dominant role in NF-κB DNA-binding activity, and that both IKKα and IKKβ subunits are important kinases in the phosphorylation of the p65 subunit in the face of doxorubicin, the question remains whether the deletion of IKKα or IKKβ would affect the overall survival of HT1080 cells treated with doxorubicin. In order to answer this question, siIKKα or siIKKβ was transfected into HT1080 cells that were subsequently stimulated with doxorubicin. The outcomes of these combination treatments on the rate of apoptosis in HT1080 cells were evaluated in two ways. First, cells treated with doxorubicin for 6 hours were harvested and the cellular extracts were evaluated for processing of caspase 3 and PARP, both markers of apoptosis. We demonstrate that transfection alone with either siControl, siIKKα, or siIKKβ was able to induce some cleavage of PARP at baseline. Notably, the knockdown of IKKα generated the largest increase in cleaved PARP and the only detectable levels of cleaved caspase 3 in unstimulated cells (Figure 4A, lanes 1-4). When evaluating the effects of the deletion of IKKα and IKKβ on the cytotoxicity of doxorubicin, the results demonstrate that the absence of either catalytic subunit results in a significant increase in both cleaved caspase 3 and cleaved PARP when compared to doxorubicin alone (Figure 4A, lanes 5-8). It is important to note that transfection of the control siRNA also increased the levels of these apoptotic markers when combined with doxorubicin suggesting that transfection alone can be mildly toxic when combined with chemotherapy. Nevertheless, the depletion of IKKα or IKKβ resulted in even greater toxicity than the control siRNA in the doxorubicin treatment groups confirming that both of these subunits are important to the NF-κB mediated chemoresistance of HT1080 cells to doxorubicin.
Figure 4.
(A) Following siRNA transfection and exposure to doxorubicin (Dox) for 6 hours, cellular extracts were prepared and evaluated for markers of apoptosis. The ability of Dox to initiate apoptosis is significantly enhanced by preventing the activation of NF-κB through the knockdown of IKKα or IKKβ resulting in increased cleaved caspase 3 and increased cleaved PARP. (B) Apoptosis following 24 hours of exposure to Dox was quantitated using a TUNEL assay. The knockdown of IKKα combined with doxorubicin (Dox) treatment significantly increased the percentage of apoptosis by 57% (54-59%, 95% CI) when compared to Dox alone and by 42% (39%-44%, 95% CI) when compared to siRNA treatment alone. Similarly, the combination of siIKKβ and Dox resulted in a significant 59% (56-62%, 95% CI) increase in apoptosis when compared with Dox alone and a 43% (40%-45%, 95% CI) when compared with siRNA treatment alone. The experiments were performed in triplicate.
Next, in order to confirm the results seen with cellular markers of apoptosis and to more quantitatively measure the rates of apoptosis, a TUNEL assay was conducted. HT1080 cells were transfected with siIKKα, siIKKβ, or siControl and subsequently treated with doxorubicin for 24 hours. The cells were stained as previously described and the number of positive, or apoptotic, cells per 1000 total cells were counted. The experiment was conducted in triplicate and the results are reported as the overall percentage of apoptotic cells. The combination of IKKα deletion and doxorubicin resulted in a 57% (54-59%, 95% CI) increase in the incidence of apoptosis compared to doxorubicin alone and a 42% (39-44%, 95% CI) increase when compared to siIKKα alone (Figure 4B). When the cells were treated with siIKKβ, the results were similar with a 59% (56-62%, 95% CI) increase in apoptosis compared to chemotherapy alone and a 43% (40-45%, 95% CI) increase compared to siIKKβ alone. Moreover, the percentage of apoptosis in combination treatment groups was also greater than the sum of the individual treatments.
Together, these results confirm that the activation of NF-κB in fibrosarcoma cells functions in an anti-apoptotic manner and is directly linked to the resistance of these cells to doxorubicin. Furthermore, both IKKα and IKKβ subunits serve a critical role in the signaling pathways involved in the NF-κB-mediated chemoresistance of fibrosarcomas.
Discussion
The transcription factor NF-κB has been implicated as an important mediator in a number of cellular processes from inflammation to cancer. In neoplasms, NF-κB has received specific attention for its role in oncogenesis (2, 4). In addition, inducible chemoresistance is attributed to the activation of NF-κB, which in turn stimulates the transcription of anti-apoptotic genes enabling cells to overcome chemotherapy-induced apoptosis, or programmed cell death (5, 6). Previous studies from our laboratory and others have supported this concept by demonstrating that inhibition of NF-κB activation sensitizes neoplastic cells to the genotoxic effects of their relevant anticancer therapies (9, 10, 12). Coincidentally, there has been increasing pharmacologic interest in inhibition strategies aimed at NF-κB in an effort to improve response rates in chemoresistant cancers. Currently, the only NF-κB inhibitors used clinically are generalized proteasome inhibitors, which clearly have broad effects. As a result, these strategies in early phase clinical trials have been limited in solid tumors by lack of efficacy and increased toxicity (21-23). More recent efforts have focused on identifying more selective inhibitory targets in the NF-κB pathway.
As the role of NF-κB in the chemotherapy response is being elucidated, several recent studies have challenged the notion that the induction of NF-κB leads to an anti-apoptotic response; the authors suggest that depending on the cellular context, NF-κB activation can be required for chemotherapy to cause cell death (16, 17, 30, 31). Therefore, delineating the role of NF-κB activation in specific tumors in response to clinically relevant chemotherapies is critical. Then, further analysis of the mechanisms behind the induction of NF-κB could identify more effective targets for NF-κB inhibition strategies with the potential to improve cytotoxic response rates.
In this regard, physiological stimuli, such as TNFα or LPS, have been reported to activate the “canonical pathway” leading to IKKβ-dependent phosphorylation and degradation of IκBα and translocation of NF-κB to the nucleus (32). On the other hand, IKKα has been implicated in the noncanonical or alternative pathway, leading to the processing of p100 and the generation of free p52/RelB dimers (33, 34). In chemotherapy models, initial studies examining NF-κB induction in response to chemotherapy agents, like doxorubicin, published similar results labeling IKKβ as the critical catalytic subunit (25). Recently, it has also been shown that doxorubicin can initiate IκBα degradation and NF-κB activation independent of either IKKα or IKKβ using the same in vitro models (24). Moreover, some studies indicate that sumoylation of NEMO is important in NF-κB activation in response to DNA damage agents reportedly through a complex interaction involving the p53-inducible death-domain-containing protein (PIDD) (35, 36). As these results unfold, the role of the IKK subunits in response to DNA damaging agents remains unclear; whether IKKα and IKKβ serve independent, overlapping or complementary roles is yet to be determined. Further clarification of these mechanisms will be important in isolating potential therapeutic targets within the IKK complex relative to NF-κB-mediated chemoresistance.
In this study, we sought to further clarify the roles of IKKα and IKKβ in the activation of NF-κB in response to doxorubicin, a DNA-damage agent known to induce NF-κB activity in a variety of malignancies (30, 31, 37). By first examining the effects of doxorubicin in MEF cells devoid of either IKKα, IKKβ, or both subunits, we demonstrated that early NF-κB activation is clearly not IKK-independent as suggested in other published reports (24). In addition, IKKα was found to have a more significant role compared to IKKβ in the doxorubicin-induced NF-κB DNA-binding activity as measured by EMSA. However, deleting IKKα did not result in complete abolition of NF-κB activity, suggesting that the induction of NF-κB by doxorubicin is not entirely independent of IKKβ.
In regards to the role these IKKs play in an oncologic setting, we felt it imperative to examine these relationships using a human cancer cell line in which doxorubicin has clinical relevance. Doxorubicin, in combination with ifosfamide, is well recognized to be a treatment of locally advanced and metastatic sarcomas, although the reality remains that clinical response rates are less than 30% and overall survival rates remain dismal for advanced stage disease (14). As such, we elected to use HT1080 human fibrosarcoma cells manipulated with siRNA strategies to deplete either IKKα or IKKβ in order to further study the significance of both catalytic subunits in the doxorubicin-induced activation of NF-κB.
Interestingly, doxorubicin-induced NF-κB DNA-binding activity was bimodal with its strongest peak at 3-6 hours post-treatment and a reproducible second weaker increase in DNA-binding noted at 18-24 hours. Others have found that NF-κB activity can oscillate over time in response to the TNFα as a function of the levels of inhibitory κB proteins in the cell, but this work has not been previously reported when cells have been exposed to chemotherapy (38). Our analysis demonstrated that IκBα protein levels did decrease throughout the duration of doxorubicin treatment and did not correlate with the level of nuclear activity of NF-κB as measured by EMSA. As such, it is possible that this delayed decrease in inhibitory κB proteins in the face of doxorubicin is the source for the second increase in NF-κB nuclear activity. Further studies will be needed in order to determine significance of these oscillations in nuclear activity and their affects on apoptosis and gene expression.
In order to further clarify the roles of IKKα and IKKβ in the NF-κB response to doxorubicin, we focused on the early maximal activation of NF-κB seen following 6 hours of exposure to doxorubicin. In our in vitro model, the depletion of the IKKα subunit resulted in significant inhibition of doxorubicin-induced NF-κB DNA-binding activity, while the knockdown of IKKβ had no effect. In order to better understand the significance of these two subunits in the signaling pathways that generate NF-κB activation, we examined IκBα degradation and the phosphorylation of p65. Rather than identifying a dominant subunit, as in the case of NF-κB DNA-binding activity, we demonstrated a shared responsibility in both of these processes. More specifically, the knockdown of IKKα and IKKβ blunted the ability of doxorubicin to initiate the degradation of IκBα as well as decreased the phosphorlyation of p65 at the serine536 residue following doxorubicin treatment. This further supports the concept that both IKKα and IKKβ possess the ability to phosphorylate the p65 subunit, and stresses that the absence of either catalytic subunit results in a less profound activation of NF-κB by doxorubicin.
Having identified similar, important roles for both the IKKα and IKKβ subunits in the NF-κB response to doxorubicin, it was critical to evaluate what effect losing one of these subunits would have on the cytotoxicity of the drug. The evaluation of cellular markers of apoptosis (cleaved PARP and cleaved caspase 3) and quantification of the overall percentage of apoptosis using TUNEL staining revealed that knocking down either IKKα or IKKβ results in a significant increase in the cytotoxic effects of doxorubicin. Although there was some toxicity associated with the transfection process itself, the combination therapies involving deletion of IKKα or IKKβ together with doxorubicin resulted in greater toxicity than the sum of the individual treatments.
Given the complexity of the signaling pathway, it will be important to continue exploring the role of these catalytic subunits as well as the importance of the regulatory subunit NEMO in the NF-κB response to DNA damage. Additionally, recent reports have suggested that the NF-κB activity is regulated through p53. Since HT1080 cells maintain wild type p53, it will be important to examine our model of chemoresistance in p53 null sarcoma cells (39).
In conclusion, the results of our study support a novel and important role for IKKα in NF-κB-mediated chemoresistance in fibrosarcomas. This further validates the potential for developing biological means of inhibiting the catalytic IKK subunits. These preclinical studies using human cancer cell lines and clinically relevant cytotoxic agents are important to identify mechanisms of chemoresistance and identify potential targets for future therapeutic strategies. In these aggressive malignancies, a large proportion of the patients present with advanced incurable stage of disease, with treatments limited to systemic chemotherapy. Ultimately, pharmacologic agents aimed at selective inhibition of the NF-κB response to current chemotherapy agents will lead to combinational approaches to improve outcomes in these chemoresistant malignancies.
| Target | Sense | Antisense |
|---|---|---|
| IKKα | 1. CAAAGAAGCUGACAAUACUUU 2. CCAGAUACUUUCUUUACUAUU 3. GAAGUUCGGUUUAGUAGCCUU 4. AAAUAUGGCAUCUCCUUAAUU |
1. 5′-PAGUAUUGUCAGCUUCUUUGUU 2. 5′-PUAGUAAAGAAAGUAUCUGGUU 3. 5′-PGGCUACUAAACCGAACUUCUU 4. 5′-PUUAAGGAGAUGCCAUAUUUUU |
| IKKβ | 1. GGAAGUACCUGAACCAGUUUU 2. CCAAUAAUCUUAACAGUGUUU 3. GGAUUCAGCUUCUCCUAAAUU 4. GUGGUGAGCUUAAUGAAUGUU |
1. 5′-PAACUGGUUCAGGUACUUCCUU 2. 5′-PACACUGUUAAGAUUAUUGGUU 3. 5′-PUUUAGGAGAAGCUGAAUCCUU 4. 5′-PCAUUCAUUAAGCUCACCACUU |
Acknowledgments
Funding for this research was supported by the following grants: R01-CA73756, R01-CA75080, K08-CA098240, GI SPORE P50-CA10699-01, T32-CA09688
Abbreviations
- IKK
Inhibitory κB kinase
- siRNA
small interference RNA
- DKO
double knockout
- MEF
mouse embryo fibroblast
- NF-κB
nuclear factor κB
- IκB
inhibitor κB
- NEMO
NF-κB essential modulator
- TNFα
tumor necrosis factor alpha
- WT
wild type
- EMSA
electrophorectic mobility shift assay
- TUNEL
terminal transferase dUTP nick end labeling
- PIDD
p53-inducible-death-domain-containing-protein
- LPS
lipopolysaccharide
REFERENCES
- 1.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. The Journal of clinical investigation. 2001 Feb;107(3):241–6. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006 Oct 30;25(51):6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 3.Verma IM. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Annals of the rheumatic diseases. 2004 Nov;63(Suppl 2):ii57–ii61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell death and differentiation. 2006 May;13(5):738–47. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 5.Hinz M, Loser P, Mathas S, Krappmann D, Dorken B, Scheidereit C. Constitutive NF-kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells. Blood. 2001 May 1;97(9):2798–807. doi: 10.1182/blood.v97.9.2798. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Wang X, Evers BM. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. The Journal of biological chemistry. 2003 Dec 19;278(51):51091–9. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- 7.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr., Sledge GW., Jr. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Molecular and cellular biology. 1997 Jul;17(7):3629–39. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999 Jan;5(1):119–27. [PubMed] [Google Scholar]
- 9.Cusack JC, Liu R, Baldwin AS. NF- kappa B and chemoresistance: potentiation of cancer drugs via inhibition of NF- kappa B. Drug Resist Updat. 1999 Aug;2(4):271–3. doi: 10.1054/drup.1999.0094. [DOI] [PubMed] [Google Scholar]
- 10.Cusack JC, Jr., Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer research. 2001 May 1;61(9):3535–40. [PubMed] [Google Scholar]
- 11.Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000 Feb 24;19(9):1123–31. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 12.Wang CY, Mayo MW, Baldwin AS., Jr. Science. 5288. Vol. 274. New York, NY: Nov 1, 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB; pp. 784–7. [DOI] [PubMed] [Google Scholar]
- 13.Society AC. Cancer Facts and Figures 2006. American Cancer Society; Atlanta: 2006. [Google Scholar]
- 14.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Annals of surgery. 1998 Sep;228(3):355–65. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linehan DC, Lewis JJ, Leung D, Brennan MF. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000 Apr;18(8):1637–43. doi: 10.1200/JCO.2000.18.8.1637. [DOI] [PubMed] [Google Scholar]
- 16.Ashikawa K, Shishodia S, Fokt I, Priebe W, Aggarwal BB. Evidence that activation of nuclear factor-kappaB is essential for the cytotoxic effects of doxorubicin and its analogues. Biochemical pharmacology. 2004 Jan 15;67(2):353–64. doi: 10.1016/j.bcp.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Molecular cell. 2004 Mar 26;13(6):853–65. doi: 10.1016/s1097-2765(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 18.Campbell KJ, Witty JM, Rocha S, Perkins ND. Cisplatin mimics ARF tumor suppressor regulation of RelA (p65) nuclear factor-kappaB transactivation. Cancer research. 2006 Jan 15;66(2):929–35. doi: 10.1158/0008-5472.CAN-05-2234. [DOI] [PubMed] [Google Scholar]
- 19.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002 Nov 15;20(22):4420–7. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. The New England journal of medicine. 2003 Jun 26;348(26):2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 21.Alberts SR, Foster NR, Morton RF, et al. PS-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group (NCCTG) randomized phase II study. Ann Oncol. 2005 Oct;16(10):1654–61. doi: 10.1093/annonc/mdi324. [DOI] [PubMed] [Google Scholar]
- 22.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006 Nov 1;24(31):5025–33. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 23.Ryan DP, Appleman LJ, Lynch T, et al. Phase I clinical trial of bortezomib in combination with gemcitabine in patients with advanced solid tumors. Cancer. 2006 Nov 15;107(10):2482–9. doi: 10.1002/cncr.22264. [DOI] [PubMed] [Google Scholar]
- 24.Tergaonkar V, Bottero V, Ikawa M, Li Q, Verma IM. IkappaB kinase-independent IkappaBalpha degradation pathway: functional NF-kappaB activity and implications for cancer therapy. Molecular and cellular biology. 2003 Nov;23(22):8070–83. doi: 10.1128/MCB.23.22.8070-8083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer cell. 2002 Jun;1(5):493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 26.Mayo MW, Wang CY, Cogswell PC, et al. Science. 5344. Vol. 278. New York, NY: Dec 5, 1997. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras; pp. 1812–5. [DOI] [PubMed] [Google Scholar]
- 27.Mattioli I, Sebald A, Bucher C, et al. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol. 2004 May 15;172(10):6336–44. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- 28.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003 Jun 1;170(11):5630–5. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T. The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. The Journal of biological chemistry. 2003 Jan 10;278(2):919–26. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- 30.Campbell KJ, O'Shea JM, Perkins ND. Differential regulation of NF-kappaB activation and function by topoisomerase II inhibitors. BMC cancer. 2006;6:101. doi: 10.1186/1471-2407-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho WC, Dickson KM, Barker PA. Nuclear factor-kappaB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-kappaB-dependent transcription in cancer cells. Cancer research. 2005 May 15;65(10):4273–81. doi: 10.1158/0008-5472.CAN-04-3494. [DOI] [PubMed] [Google Scholar]
- 32.Li ZW, Chu W, Hu Y, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. The Journal of experimental medicine. 1999 Jun 7;189(11):1839–45. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends in immunology. 2004 Jun;25(6):280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes & development. 2004 Sep 15;18(18):2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 35.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell. 2005 Dec 16;123(6):1079–92. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 36.Mabb AM, Wuerzberger-Davis SM, Miyamoto S. PIASy mediates NEMO sumoylation and NF-kappaB activation in response to genotoxic stress. Nature cell biology. 2006 Sep;8(9):986–93. doi: 10.1038/ncb1458. [DOI] [PubMed] [Google Scholar]
- 37.Romano MF, Avellino R, Petrella A, Bisogni R, Romano S, Venuta S. Rapamycin inhibits doxorubicin-induced NF-kappaB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer. 2004 Dec;40(18):2829–36. doi: 10.1016/j.ejca.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann A, Levchenko A, Scott ML, Baltimore D. Science. 5596. Vol. 298. New York, NY: Nov 8, 2002. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation; pp. 1241–5. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong MB, Bian X, Liu Y, et al. Neoplasia. 11. Vol. 8. New York, NY: Nov, 2006. Signaling from p53 to NF-kappa B determines the chemotherapy responsiveness of neuroblastoma; pp. 964–74. [PMC free article] [PubMed] [Google Scholar]