Abstract
Retinal rods and cones share a phototransduction pathway involving cyclic GMP1. Cones are typically 100 times less photosensitive than rods and their response kinetics are several times faster2, but the underlying mechanisms remain largely unknown. Almost all proteins involved in phototransduction have distinct rod and cone variants. Differences in properties between rod and cone pigments have been described, such as a 10-fold shorter lifetime of the meta-II state (active conformation) of cone pigment3, 4, 5, 6 and its higher rate of spontaneous isomerization7, 8, but their contributions to the functional differences between rods and cones remain speculative. We have addressed this question by expressing human or salamander red cone pigment in Xenopus rods, and human rod pigment in Xenopus cones. Here we show that rod and cone pigments when present in the same cell produce light responses with identical amplification and kinetics, thereby ruling out any difference in their signalling properties. However, red cone pigment isomerizes spontaneously 10,000 times more frequently than rod pigment. This high spontaneous activity adapts the native cones even in darkness, making them less sensitive and kinetically faster than rods. Nevertheless, additional factors are probably involved in these differences.
Human or salamander red cone pigment, together with green fluorescent protein (GFP) for facilitating screening, was introduced as a transgene into Xenopus under the control of the cytomegalovirus (CMV) promoter. In a wild-type or GFP control Xenopus retina, an antibody to red cone pigment labelled only the outer segments of sporadic red cones. In a frog expressing transgenic red cone pigment, however, immunolabelling included the abundant rod outer segments (Fig. 1a). The concentrated labelling of rod outer segments suggested that the localization signal that targets cone pigment to the outer segment is also recognized by rods. The immunostaining was usually non-uniform across the whole retina, suggesting variable expression of the transgenic pigment among the rods (see below).
Figure 1.
Xenopus rods expressing transgenic human and salamander red cone pigments. (a) Frozen sections of Xenopus retinas immunostained for red cone pigment. All transgenic animals also expressed GFP for screening purposes. GFP Control, transgenic animal expressing GFP marker only; Human Red, transgenic animal expressing human red cone pigment; Salam Red, transgenic animal expressing salamander red cone pigment. RPE, retinal pigment epithelium; ROS, rod outer segment; RIS, rod inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 10 μm. (b) Flash intensity-response families from rods of the corresponding genotypes. Suction-pipette recording. 20-msec flash at time 0, delivering 0.12, 0.41, 1.55, 5.19, 25.6, 85.9, 328 or 1100 photons (520 nm) μm-2. For the wild-type and GFP rods, the lowest flash intensity shown is 0.12 photons μm-2; for transgenic rods expressing human salamander red cone pigment, the lowest flash intensity shown is 0.41 photons μm-2. (c) Dark current noise in control and transgenic rods. Note the increased noise in cone-pigment-expressing rods (“dark” traces of the two right panels). The “light” trace was recorded in saturating light, delivering 1.52 × 107 photons (520 nm) μm-2 sec-1.
Outer-segment membrane current was recorded from single principal (‘red’) rods with a suction pipette. Rods expressing transgenic cone pigment showed no marked changes in their flash responses (Fig. 1b), but their sensitivity was, on average, half that of wild-type or GFP control rods (Fig. 2a and Table 1). In darkness, these rods also showed considerably higher current noise, which was suppressible by light (Fig. 1c). The photosensitivity of the noise, together with its kinetic characteristics (see below), suggested that it originated in the phototransduction pathway. In other control experiments with transgenic human rod pigment expressed in Xenopus rods, no increase in dark noise was observed (data not shown).
Figure 2.
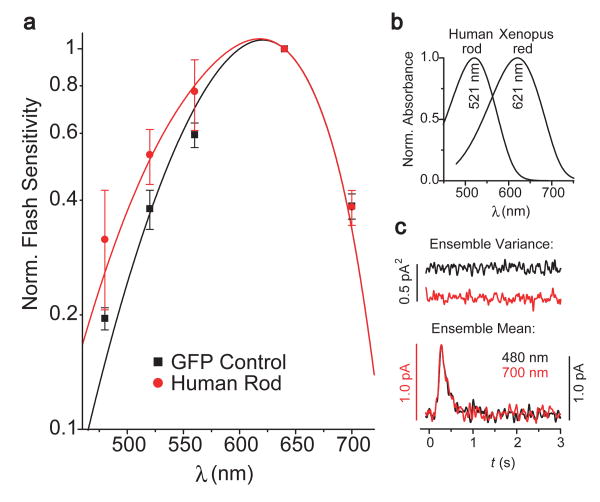
Spectral shift and dim-flash responses in rods expressing transgenic cone pigment. (a) Red-shift in action spectrum induced by human red cone pigment. Averaged flash sensitivity, Sf, for rods expressing GFP alone (n=15) was 2 times those expressing GFP together with human red cone pigment (n=17). Sf was derived from a series of identical dim flashes at 520, 560, 640, and 700 nm, respectively. Error bars give SEM. The data are fit by the A2 spectral template for Xenopus “red” rod pigment (black) and for a combination of 99.970% Xenopus rod pigment and 0.030% human red cone pigment A2 spectral templates (red). The dashed black trace is identical to solid black trace, but shifted vertically (in order to compensate for the desensitization of rods expressing cone pigment) for comparison with red trace. Note the increased sensitivity at long wavelengths for rods expressing cone pigment. Inset: A2 spectral templates for Xenopus “red” rod pigment (λmax = 521 nm) and human red cone pigment (λmax = 617 nm) (see Methods for details). (b) Comparison of dim-flash responses at 520 nm and 700 nm for a rod expressing human red cone pigment. Repeated dim flashes delivered 0.41 photon μm-2 at 520 nm or 1.34 × 103 photons μm-2 at 700 nm. The effective collecting area of this cell was unusually small (4.2 μm2). According to the spectral templates, the two wavelengths should discriminate for one or the other pigment by 2.68-fold and 1,133-fold, respectively. With the percentage of transgenic cone pigment in this cell estimated to be 0.039%, 31% of the flash responses at 700 nm should have been triggered by cone pigment. The same mathematical waveform (red traces) fits the mean responses at both wavelengths, indicating identical kinetics. The ratio between response ensemble variance (σ2, upper black traces) and mean (m, lower black traces) at response peak was 0.61 pA at 520 nm and 0.56 pA at 700 nm. In computing σ2, the beginning part (i.e., prior to flash) of each response trace was artificially superposed; because of the high dark noise, this caused the progressive drift (increase) in the variance trace. (c) Left, magnified view of boxed part of action spectrum in (a) to show the resolution of the pigment ratio. Dashed red lines are drawn with 0.030±0.010% cone pigment. Right, similar averaged data for salamander red cone pigment. Solid red line is 0.033%, and dashed red lines are 0.033±0.010%, cone pigment. (d) Effect of mutating the putative phosphorylation sites of transgenic human red cone pigment on rod response kinetics. The intensity of flashes delivered at time 0 are given in the Methods.
Table 1. Comparison of parameters for wild-type Xenopus rods and Xenopus rods expressing transgenic cone pigment.
| WT control (N = 9) | GFP control (N = 15) | Human Rod Pigment (N = 9) | Human Red Cone Pigment (N = 17) | Salamander Red Cone Pigment (N = 12) | |
|---|---|---|---|---|---|
| Dark Current (pA) | 10.5±0.5 | 16.8±1.2 | 15.0±0.5 | 13.2±1.2 | 11.9±0.9 |
| Single-Photon Response (pA) | 0.55±0.03 (520 nm) | 0.61±0.05 (520 nm) | 0.55±0.03 (520 nm) | 0.48±0.02 (520 nm)
0.48±0.03 (700 nm) |
0.35±0.04 (520 nm)
0.30±0.06 (700 nm) |
| Time-to-Peak (sec) | 1.24±0.04 (520 nm) | 1.32±0.04 (520 nm) | 1.22±0.05 (520 nm) | 1.03±0.04 (520 nm)
1.03±0.04 (700 nm) |
1.02±0.06 (520 nm)
0.90±0.07 (700 nm) |
| Flash Sensitivity at 520 nm (photons-1 μm2)a | 0.46±0.02 | 0.55±0.04 | 0.54±0.05 | 0.36±0.02 | 0.30±0.04 |
| Thermal Isomerization Rate (R* s-1)b | 0.17±0.03 | 0.17±0.02 | 0.06±0.01 | 0.73±0.16 | 0.77±0.27 |
Values given as mean±SEM
Flash sensitivity values have been normalized with respect to the dark current.
These are raw rates derived from power spectral fits. For control rods, the continuous component of the dark noise has not been subtracted. For rods expressing transgenic pigment, the noise observed in control rods has not been subtracted (see text).
The increase in dark noise in rods expressing transgenic red cone pigment suggested that cone pigment was more prone to spontaneous isomerization than rod pigment, and that it was coupled functionally to the rod phototransduction pathway. Such coupling was confirmed by a small red shift in the spectral sensitivity of rods expressing cone pigment (Fig. 2a, c), caused by a λmax of ∼ 620 nm for red cone pigment as compared with ∼ 520 nm for rod pigment9,10,11, with 11-cis-dehydroretinal (vitamin A2) as the chromophore (which Xenopus retina has almost exclusively; Fig. 2a, inset, and Methods).
On the basis of the average expression of cone pigment described below, 520-nm light should stimulate essentially only rod pigment, whereas 700-nm light should stimulate cone pigment with ‒25% probability (31% for the cell in Fig. 2b). In a series of identical dim flashes, the averaged response waveform were unexpectedly identical at both wavelengths (Fig. 2b, d, left), indicating that the responses triggered by rod and cone pigments had the same kinetics.
The response amplitude triggered by a single pigment molecule, derived from the response ensemble variance-to-mean ratio in the dim-flash experiment, was also very similar at 520 and 700 nm (∼ 0.61 pA versus 0.56 pA; Fig. 2b and Table 1). The variance-to-mean ratio at 700 nm does not have a simple meaning because the response ensemble consists of mixed responses from rod and cone pigments. However, computer simulations of two mixed-response populations, having various amplitude ratios and occurring according to the Poisson distribution with realistic parameters to mimic our experiments (Supplementary Information), suggested that the responses produced by single rod and cone pigment molecules were unlikely to differ by more than twofold in amplitude. A more definitive analysis based on the dark-current noise indicated that the two amplitudes are essentially identical (see below).
The percentage of transgenic cone pigment in host rods was actually very small. Given the identical responses produced by rod and cone pigments, this percentage can be estimated by fitting a composite absorption spectrum to the measured action spectrum, especially at long wavelengths. This gave 0.030 ± 0.005% (n = 17) for human and 0.033 ± 0.017% (n = 12) for salamander red cone pigment (Fig. 2a, c), which is broadly comparable to the expression of transgenic rod pigment in Xenopus rods12. These estimates are insensitive to uncertainties of ± 3 nm in the λmax values for the spectral templates of the two pigments.
We also generated Xenopus expressing a mutant human red cone pigment lacking all putative phosphorylation sites in the carboxy terminus to remove any inactivation by pigment kinase, and found that the host rods showed longer-lasting responses at 700 nm than at 520 nm (Fig. 2d, right). This experiment verifies that transgenic red cone pigment is truly functional and that, despite the rapid decay of the meta-II state of red cone pigment, its shutoff is still dominated by phosphorylation and arrestin binding preceding the decay, as it is for rod pigment (see also ref 13). Because the responses produced by endogenous rod pigment and transgenic wild-type red cone pigment have identical waveforms, the pigment kinase and arrestin in rods must act identically on rod and cone pigments (Supplementary Information).
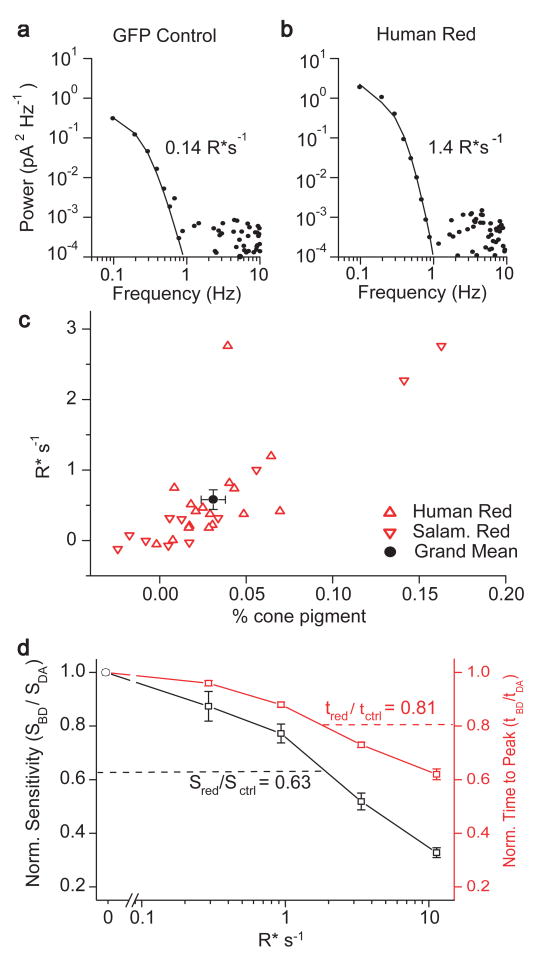
We analysed the dark-current noise in rods by computing its power density spectrum. For GFP control and wild-type rods, this power spectrum (after subtracting the power spectrum of residual noise in saturating light) agreed fairly well with that derived from the measured single-photon response of endogenous rod pigment (Fig. 3a), as expected14. The fit gave an average spontaneous isomerization (R*) rate for the rod pigment of 0.17 ± 0.02 R* s-1 (n = 15) in GFP control rods (0.14 R* s-1 for the cell is Fig. 3a), and 0.17 ± 0.03 R* s-1 (n = 9) in wild-type rods. A correction has to be made to remove a ‘continuous’ component14 of the dark noise originating from intrinsic phosphodiesterase activity15. Assuming this continuous noise to be comparable in variance to the discrete noise14, the power density for the ‘discrete’ noise, reflecting pigment isomerization only, should be roughly halved, corresponding to ∼ 0.1 R* s-1 (at 21–23 °C).
Figure 3.
Analysis of dark current noise. (a) Power density spectrum of the dark noise from a GFP-control rod (filled circles) fitted by the power spectrum computed from the single-photon response from endogenous rod pigment (continuous trace). The derived rate of thermal isomerization for this cell was 0.14 R* sec-1. (b) The same analysis for a rod expressing human red cone pigment. The derived rate of thermal isomerization was 1.4 R* sec-1. (c) Collected plot of the net rate of thermal isomerization of transgenic cone pigment (R* sec-1) against the estimated percentage of cone pigment in individual host rods (open red symbols). Average dark noise of control rod (0.17 R* sec-1) already subtracted. Cone pigment expression was calculated as in Fig 2. The negative percentages for several cells with essentially undetectable expression were due to measurement scatter, which made the measured flash sensitivity at 700 nm fall below the absorption spectrum for the endogenous rod pigment. Black circle gives grand mean ± SEM for the transgenic rods expressing human or salamander red cone pigments. (d) The decrease in flash sensitivity and reduction in time-to-peak of the dim-flash response due to transgenic red cone pigment could be reproduced in GFP-control rods by a background light producing 1-2 R* sec-1. Abscissa, intensity of steady background light on GFP-control rods. Left ordinate, ratio of dim-flash sensitivities in the presence (SBD) and absence (SDA) of background light for GFP-control rods. Right ordinate, ratio of times-to-peak of dim-flash responses in the presence (tBD) and absence (tDA) of background light for GFP-control rods. The ratios Sred/Sctrl and tred/tctrl are from grand-averaged dim-flash sensitivities and response times-to-peak of rods with and without transgenic cone pigment, respectively, calculated as follows. From Table 1, the grand-averaged dim-flash sensitivity (at 520 nm), normalized with respect to the dark current, was 0.33 photon-1 μm2 for rods expressing human or salamander red cone pigment, versus 0.52 photon-1 μm2 for wild type, GFP-control, and human-rhodopsin-expressing Xenopus rods combined, giving a ratio of 0.63. Correspondingly, the ratio for the times-to-peak was 0.81. Note that both ratios intersect with the background-light data at almost the same background light intensity, as expected. This value of ca. 1.8 R* sec-1 is broadly similar to the spontaneous isomerization rate estimated from power spectral analysis of the dark noise in rods expressing transgenic cone pigment.
For rods expressing transgenic red cone pigment, the power spectrum of the much higher dark noise was also fit well by that derived from the single-photon response of rod pigment triggered with dim 520-nm flashes (Fig. 3b). This fit confirmed that the response kinetics were indeed identical whether generated by rod or cone pigment. Given that the responses produced by single rod and cone pigment molecules had essentially identical amplitudes (see below), the fit gave an average spontaneous isomerization rate of 0.73 ± 0.16 R* s-1 (n = 17) for cells expressing human red cone pigment (1.4 R* s-1 for the cell in Fig. 3b) and 0.77 ± 0.27 R* s-1 (n = 12) for cells expressing salamander red cone pigment. Subtracting all endogenous dark noise in the control rods described above gave rates for human and salamander cone pigments of 0.56 R* s-1 and 0.60 R* s-1, respectively. The net rate in individual cells ranged rom 0 to 2.8 R* s-1, a variation attributed to the variable expression of cone pigment, as suggested by immunostaining (see above). Indeed, the calculated net rate in each cell was roughly proportional to the estimated percentage of cone pigment (Fig. 3c).
The low expression of the transgenic cone pigment actually has an advantage. In three rods expressing cone pigment, the increase in dark noise, although substantial, showed occasional breaks to reveal the quiet dark-current baseline (for example, Fig. 1c, third column, top trace). In these cases, an objective estimate of the unitary amplitude (that is, the response amplitude triggered by a single isomerized cone pigment molecule) underlying the noise can be calculated from σ2/m × [∫ f(t)dt/∫ f2(t)dt] (ref. 16), where σ2 is the steady-current variance, m is the mean current relative to this baseline, and f(t) is a function normalized to unity at peak and with a power spectrum that fits the dark-noise spectrum.
Before calculation, the σ2 and m components reflecting spontaneous isomerization of endogenous rod pigment (σr2 and mr) were evaluated and subtracted from the overall σ2 and m values, using σr2 = νrαr2 ∫f2 (t)dt and mr= νrαr∫f(t)dt, (ref. 16), where νr is the average isomerization frequency (0.1 R* s-1, as measured above) and αr is the response peak amplitude triggered by a single rod pigment molecule measured with dim 520-nm flashes (Supplementary Information).
The response triggered by a single cone pigment molecule derived in this way, 0.55 ± 0.01 pA (n = 3), agreed well with the rod pigment single photon response of 0.49 ± 0.02 pA measured in the same cells with dim 520-nm flashes. This agreement indicates that rod and cone pigments indeed generate responses of essentially identical size. With the unitary amplitude known, the rate of cone pigment events could be calculated from either of the above equations and was determined to be 1.13 ± 0.16 R* s-1, which becomes 1.23 ± 0.16 R* s-1 after adding νr. This value is close to the value of 1.09 ± 0.14 R* s-1 derived by power spectral analysis in the same three cells.
The high thermal isomerization rate of the transgenic cone pigment was equivalent to a steady background light and should adapt the host rod even in darkness. This adaptation could explain why rods expressing cone pigment were less sensitive than normal. Indeed, the average sensitivity ratio between transgenic and control rods in darkness (0.63; Fig. 3d) could be reproduced in control rods by exposing them to a steady light of 1.8 R* s-1, which is broadly similar to the equivalent light derived from the noise analysis described above. The same conclusion can be drawn by using the response ‘time to peak’ as the parameter for comparing transgenic and control rods (Fig. 3d).
Separately, by using the cones of Xenopus expressing transgenic human rod pigment, we could study the converse situation, that is, rod pigment functioning in a cone environment. We chose the abundant ‘red’ cones for recording. The functional coupling of transgenic rod pigment to the host cone transduction pathway was detected by a blue shift in the action spectrum (Fig. 4a). The fit of the measured action spectrum by a linear combination of the A2 absorption templates for human rod pigment and Xenopus cone pigment (Fig. 4b and Methods) indicated that, on average, ± 12% of the action spectrum was contributed by transgenic rod pigment. Why a transgenic pigment is expressed at a considerably higher percentage in cones than in rods is not clear at present. Again, dim-flash responses elicited at 480 and 700 nm showed identical kinetics (Fig. 4c, bottom).
Figure 4.
Xenopus red cones expressing transgenic human rod pigment. (a) Blue shift in action spectrum. Flash sensitivity from cones expressing GFP alone or together with human rod pigment was averaged (n=10 each) and normalized at 640 nm. Error bars give SEM. The data are fit by the A2 spectral template for Xenopus red cone pigment (black) and for a combination of 88% contribution from the A2 template of Xenopus red cone pigment and 12% contribution from the A2 template of transgenic human rod pigment (red). Note the increased sensitivity at short wavelengths for cones expressing rod pigment. In principle, the flash sensitivity at 560 nm ought to be the same in the absence and presence of transgenic rod pigment because this is near the isosbetic point (see b). The fact that this was not the case most probably reflected a slight uncertainty in the measurements at these wavelengths, because the two sets of data matched well at long wavelengths (consistent with the dominance of the contribution from the endogenous cone pigment in this spectral region). Constraining a sensitivity invariance at 560 nm would give 2.7% (instead of 12%) contribution from the A2 template of the human rod pigment. (b) A2 spectral templates for Xenopus red cone pigment (λmax = 621 nm) and human rod pigment (λmax = 521 nm) (see Methods). At 480 nm, the rod pigment would be preferentially stimulated by 5.4-fold, while at 700 nm the cone pigment would be preferentially stimulated by 1,322-fold. (c) Identical kinetics of dim-flash responses at 480 nm and 700 nm for a Xenopus cone expressing transgenic human rod pigment. The scales for the two responses have been adjusted so that the mean response at 480 nm (actual amplitude was 1.11 pA) matches that at 700nm (0.93 pA). The responses represent averages from 30 flash trials each. At each wavelength, the ensemble variance of the response was negligible, indicating that the unitary response was extremely small. In this cell, the percentage of transgenic rod pigment was estimated to be 6.6%. Because the probability of absorption at 480 nm is 5.4-fold higher for human rod pigment than Xenopus red cone pigment, it can be calculated that ca. 28% of the response at 480 nm should have been triggered by rod pigment. Flash intensity at 700 nm delivered the equivalent of 1.37 × 103 photon μm-2 at 621 nm (λmax for endogenous red cone pigment). With an average effective collecting area of 0.08 μm2 for the Xenopus red cone outer segment (4.3 μm length ×1.6 μm mean diameter, unpolarized illumination incident transversely at the outer segment, and assuming a transverse optical density of 0.012 μm-1 and a quantum efficiency of isomerization of 0.67), this intensity should produce ca. 110 cone-pigment isomerizations. Thus, the single-photon response triggered by the endogenous red cone pigment is about 0.93 pA/110 = 0.008 pA in this experiment, consistent with the extremely small variance of the flash responses. This value is ca. 70-fold less than that for the single-photon response of a Xenopus red rod.
From the above ∼ 12% and the absorption templates, the transgenic rod pigment should contribute, on average, ∼ 42% of the light response at 480 nm (28% for the cell in Fig. 4c) but only 0.01% at 700 nm. Thus, the kinetic invariance at the two wavelengths strongly suggested that transgenic rod pigment and endogenous cone pigment produced identical response waveforms. At the same time, there was no detectable increase in ensemble variance during the dim-flash response at 480 or 700 nm (Fig. 4c, top), consistent with extremely small unitary amplitudes in both cases and therefore ruling out the possibility that a much larger response was produced by transgenic rod pigment than by endogenous cone pigment. These conclusions mirrored those derived above from rods expressing transgenic cone pigment.
In summary, rod and cone pigments are completely equivalent with respect to signalling downstream in phototransduction: first, the active lifetimes of both are dictated by shutoff regulated by phosphorylation and arrestin binding rather than by meta-II decay; second, the pigment kinase and arrestin in a rod or cone act identically on rod and cone pigments; and third, rod and cone pigments have identical efficacies when coupled to a given (rod or cone) transducin. Nonetheless, the two do differ in that cone pigment (or at least red cone pigment) is intrinsically much more prone to spontaneous isomerization.
How large is this difference? The spontaneous isomerization rate of rod pigment in control Xenopus rods is ∼ 0.1 R* s-1 (see above), corresponding to a molecular rate constant of ∼ 3.7 × 10-11 s-1 (Methods). Similar calculations give a molecular rate constant of ∼ 6.7 × 10-7 s-1 for salamander red cone pigment (1.8 × 104-fold higher). Applying this rate constant to the native salamander red cone (Methods) gives ∼ 175 R* s-1. This rate is 3.5 - 7.0 times lower than that previously estimated from native salamander red cones8,17. For human red cone pigment, the disparity is even larger between our estimated rate and that measured from native monkey red cones (Supplementary Information). These disparities suggest that a significant portion of the dark noise in a native cone does not originate from the pigment.
It is possible that, in native cones, spontaneous transducin activity contributes to the noise. In addition, although some spontaneous transduction activity in cones is dependent on GTP8 and therefore triggered by either pigment or transducin, not all dark transduction activity has to originate exclusively from these two sources, but instead can come from spontaneous phosphodiesterase activity (Supplementary Information). Finally, some dark noise in native cones previously ascribed to the transduction process8, 17 might not originate from the outer segment at all. Unlike the rod cGMP-gated channel, which behaves as a constant-current generator by showing a flat current–voltage relation at physiological membrane potentials1, the cone cGMP-gated channel shows substantial slope conductance at these potentials1 and therefore can be influenced by voltage noise at the inner segment or cell body. This noise is also light sensitive, because the cGMP-gated channels are closed in the light.
How much adaptation can be brought about by the spontaneous isomerization activity of cone pigment? For salamander red cones, the equivalent light of 175 R* s-1 will desensitize these cells six- to sevenfold, which accounts for the bulk of the overall sensitivity difference between salamander rods and red cones (14- to 15-fold8, 18, note that folds multiply; Supplementary Information). In this regard, it is interesting to note that salamander green rods and blue cones share the same pigment and also have remarkably similar single-photon response amplitudes and kinetics19. Although this sharing of the same pigment by classes of rods and cones is rather unusual (for example, it is not found in primates), the situation nonetheless suggests that the phototransduction components downstream from pigment may not have much of a role in determining the difference in absolute sensitivity between rods and cones.
In primates2, rods and red cones differ in sensitivity by ∼ 100-fold, perhaps half of which (∼ 10-fold) is possibly accounted for by the spontaneous isomerization of red cone pigment (Supplementary Information). The same conclusion can be drawn from published data for fish20, assuming a comparable rate constant for fish and salamander red cone pigment. Thus, in general, the high rate of spontaneous isomerization of the red cone pigment seems to be an important factor underlying the difference in absolute sensitivity between rods and red cones in darkness. At the same time, additional mechanisms are probably involved. These other factors could be quantitative differences in the functional coupling between transduction proteins acting downstream from the pigment, which might produce different amplifications at the respective stages21 and also quantitatively different Ca2+ feedbacks18, 22. Biochemical experiments in fish have indicated that such differences do exist21, but so far these experiments have been done at very low concentrations of free Ca2+ (calculated as ∼ 30 nM or lower) and therefore might reflect merely a highly adapted state, which may well differ between rods and cones. The issue on downstream components remains to be elucidated. Finally, this study deals only with the red pigment, and it will be important to examine the green and blue pigments as well.
Methods
Generation of transgenic frogs
All constructs were prepared in pCS2+-based vectors, designed for optimal protein expression in Xenopus. The expression of pigment and GFP genes was controlled by a CMV promoter, which can drive transgene expression in both rods and cones of Xenopus. In generating the (presumably) phosphorylation-defective mutant of human red cone pigment, all ten putative phosphorylation sites (S345, S348, S349, S351, T353, S356, S357, S359, S360 and S362) in the C terminus were changed to alanine. All cloning and mutagenesis were done with standard methods. We produced transgenic Xenopus embryos by using restriction-enzyme-mediated integration as described23. Hatched frogs were kept under a 12 h/12 h light/dark cycle before use.
Immunocytochemistry
Fresh eyecups from a pithed frog were fixed overnight at 4 °C in PBS containing 4% paraformaldehyde. After further overnight incubation in PBS plus 30% sucrose at 4 °C, the eyecups were embedded in OCT (Tissue-Tek) and sectioned at 10–20 μm. The retinal sections were processed for immunocytochemistry with polyclonal antibody JN492 (ref. 24) specific for red cone pigment, followed by Cy3-conjugated goat anti-rabbit IgG. Despite the use of the promiscuous CMV promoter, the immunolabelling was predominantly located in the rod outer segments, which could indicate particularly strong transgene expression in rods combined with specific targeting, or perhaps instability of the transgenic pigment protein in other retinal cell types. By contrast, GFP expressed under the CMV promoter was much more widespread in the retina (data not shown).
Suction pipette recordings
Small frogs (beyond stage 66, the peak of metamorphosis; 2–5 cm long) dark-adapted overnight were double-pithed and the eyes were removed and hemisected. The retina was peeled off and chopped, and the pieces were placed in a recording chamber and perfused with Ringer solution containing 110 mM NaCl, 2.5 mM KCl, 1.6 mM MgCl2, 1.0 mM CaCl2, 10 mM dextrose, 10 mM HEPES and 0.1 μg ml-1 bovine serum albumin (pH 7.8). Under infrared illumination, the outer segment of a rod or cone photoreceptor projecting from a retinal fragment was drawn into a tight-fitting glass pipette for recording and stimulated with calibrated 20-ms flashes or bright steady light. The signals were low-pass filtered at 20 Hz (8-pole Bessel) and digitized at 100 Hz for further analysis. For power spectral analysis, the signals were low-pass filtered at 20 Hz (8-pole Butterworth) and digitized at 100 Hz.
To determine the effect of mutating the phosphorylation sites in human red cone pigment (Fig. 2d), Flashes delivered 0.41, 1.55, 5.19, 25.6, 85.9 and 328 photons μm-2 at 520 nm, and 1.38 × 103, 4.38 × 103, 2.33 × 104, 7.41 × 104, 2.87 × 105 and 9.13 × 105 photons μm-2 at 700 nm for rods expressing the wild-type cone pigment; and 0.35, 1.35, 4.54, 22.4, 75.2 and 287 photon μm-2 at 520 nm, 1.52 × 103, 4.82 × 103, 2.56 × 104, 8.15 × 104, 3.15 × 105 and 1.0 × 106 photons μm-2 at 700 nm for rods expressing the mutated cone pigment.
Single-photon response and power spectral analysis
The amplitude of the single-photon response was calculated as the ensemble amplitude variance-to-mean ratio of the transient peak of the responses to a series of 30–100 identical dim flashes. In experiments involving power density spectra, the average response to a dim-flash series was scaled to the single-photon response amplitude and then fit by a function consisting of the convolution of four single exponentials with optimally adjusted time constants. This function was used for computing the power spectrum of the single-photon response. Power spectra of the noise in both darkness and bright light were calculated using Clampfit8 (Axon Instruments) in 10.24-s segments with 50% overlap, from ten sweeps of 40.96 s each. The dark-minus-light difference spectrum was obtained by subtraction. The rate of thermal isomerization of the visual pigment was estimated by scaling the power spectrum of the single-photon response to fit the difference spectrum and dividing this scaling factor by the acquisition time (10.24 s).
For the response kinetics of Xenopus cones expressing human rod pigment (Fig. 4c), the single-photon response was calculated as follows. The flash intensity at 700 nm delivered the equivalent of 1.37 × 103 photon μm-2 at 621 nm (λmax for endogenous red cone pigment). With an average effective collecting area of 0.08 μm2 for the Xenopus red cone outer segment (length 4.3 μm by mean diameter 1.6 μm, unpolarized illumination incident transversely at the outer segment, assuming a transverse optical density of 0.012 μm-1 and a quantum efficiency of isomerization of 0.67), this intensity should produce about 110 cone pigment isomerizations. The single-photon response triggered by the endogenous red cone pigment was therefore about 0.008 pA (mean response at 700 nm (0.93 pA) divided by 110).
Pigment absorption spectrum templates
We adopted published visual pigment templates11 for describing the absorption spectra of the various pigments. The best fit of the A2 template to our spectral sensitivity measurements from GFP control rods (Fig. 2a) gave λmax = 521 nm for the Xenopus red rod pigment, consistent with previous reports9,10,11. For salamander red cone pigment with A2 chromophore, we used λmax = 620 nm (ref. 25). For the polymorphic form of the human red cone pigment used here, λmax = 557 nm with A1 chromophore26 was adopted. We used an empirical expression27 to derive its corresponding λmax with A2 chromophore and obtained λmax = 617 nm. For the pigment of the principal Xenopus ‘red’ cones, the fit of our data to the A2 template11 gave λmax = 621 nm, which is slightly higher than reported9. For human rod pigment, λmax = 498 nm (ref. 28) with A1 chromophore would give λmax = 521 nm with A2 chromophore29.
The small amount (a few per cent) of A1 pigment presumably present in the Xenopus retina10 was ignored in this work. Its inclusion would produce a negligible difference in the estimated expression of the transgenic pigment.
Pigment content of cells
In our experiments, the Xenopus rod outer segment had a mean diameter of 6.4 μm. On average, 40 μm of the outer segment length was recorded by the suction pipette. Thus the recorded outer segment volume was 1.3 × 103 μm3. Adopting a standard pigment concentration of 3.5 mM30 in an outer segment, this volume corresponds to 2.7 × 109 pigment molecules. The corresponding number of cone pigment molecules in a transgenic Xenopus rod can be calculated by simply multiplying the rod pigment number with the corresponding percentage of total pigment content derived from the red-shift experiments (0.030% for human red cone pigment and 0.033% for salamander red cone pigment). For salamander red cones, its outer segment is on average 4 μm in diameter × 10 μm in length19. At 3.5 mM30, the total cone pigment content is therefore 2.6 × 108 molecules. These numbers are used for calculating pigment thermal rate constants or the overall rate of thermal isomerization in a cell.
Calculation of flash sensitivity and time-to-peak ratios
In Fig. 3d, the grand-mean dim-flash sensitivity ratio (Sred/Sctrl) and response ‘time to peak’ ratio (tred/tctrl) of rods with or without transgenic cone pigment, respectively, were calculated as follows. From Table 1, the grand-mean dim-flash sensitivity (at 520 nm), normalized with respect to the dark current, was 0.33 photon-1 for rods expressing human or salamander red cone pigment, as compared with 0.52 photon-1 μm2 for wild type, GFP control and human-rhodopsin-expressing Xenopus rods combined, giving a ratio of 0.63. Correspondingly, the ratio for the time to peak was 0.81.
Supplementary Material
Acknowledgments
We thank J. Lai, Y. Liang and R. Bruno for help with generating transgenic frogs; J. Nathans for the human rhodopsin and red cone opsin cDNAs and the antibody to red opsin; V. Bhandawat for suggesting the program for computer simulations of random events; and D. A. Baylor, M. E. Burns, S. Kawamura, E. N. Pugh Jr, F. Rieke, J. L. Schnapf, Y. Shichida and members of the Yau laboratory for discussions and comments on the manuscript. This work was supported by the US National Eye Institute.
References
- 1.Yau KW. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- 2.Baylor DA. Photoreceptor signals and vision. The Proctor Lecture. Invest Ophthalmol Vis Sci. 1987;28:34–49. [PubMed] [Google Scholar]
- 3.Okada T, et al. Circular dichroism of metaiodopsin II and its binding to transducin: a comparative study between meta II intermediates of iodopsin and rhodopsin. Biochemistry. 1994;33:4940–4946. doi: 10.1021/bi00182a024. [DOI] [PubMed] [Google Scholar]
- 4.Imai H, Imamoto Y, Yoshizawa T, Shichida Y. Difference in molecular properties between chicken green and rhodopsin as related to the functional difference between cone and rod photoreceptor cells. Biochemistry. 1995;34:10525–10531. doi: 10.1021/bi00033a026. [DOI] [PubMed] [Google Scholar]
- 5.Imai H, Terakita A, Tachibanaki S, Imamoto Y, Yoshizawa T, Shichida Y. Photochemical and biochemical properties of chicken blue-sensitive cone visual pigment. Biochemistry. 1997;36:12773–12779. doi: 10.1021/bi970809x. [DOI] [PubMed] [Google Scholar]
- 6.Starace DM, Knox BE. Activation of transducin by a Xenopus short wavelength visual pigment. J Biol Chem. 1997;272:1095–1100. doi: 10.1074/jbc.272.2.1095. [DOI] [PubMed] [Google Scholar]
- 7.Barlow HB. Visual Problems of Colour. Vol. 2. HM Stationery Office; London: 1958. pp. 617–630. [Google Scholar]
- 8.Rieke F, Baylor DA. Origin and functional impact of dark noise in retinal cones. Neuron. 2000;26:181–186. doi: 10.1016/s0896-6273(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 9.Witkovsky P, Levine JS, Engbretson GA, Hassin G, MacNichol EF., Jr A microspectrophotometric study of normal and artificial visual pigments in the photoreceptors of Xenopus laevis. Vision Res. 1981;21:867–873. doi: 10.1016/0042-6989(81)90187-5. [DOI] [PubMed] [Google Scholar]
- 10.Palacios AG, Srivastava R, Goldsmith TH. Spectral and polarization sensitivity of photocurrents of amphibian rods in the visible and ultraviolet. Vis Neurosci. 1998;15:319–331. doi: 10.1017/s0952523898152136. [DOI] [PubMed] [Google Scholar]
- 11.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- 12.Moritz OL, Tam BM, Papermaster DS, Nakayama T. A functional rhodopsin–green fluorescent protein fusion protein localizes correctly in transgenic Xenopus laevis retinal rods and is expressed in a time-dependent pattern. J Biol Chem. 2001;276:28242–28251. doi: 10.1074/jbc.M101476200. [DOI] [PubMed] [Google Scholar]
- 13.Lyubarsky AL, Chen CK, Simon MI, Pugh EN., Jr Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J Neurosci. 2000;20:2209–2217. doi: 10.1523/JNEUROSCI.20-06-02209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol (Lond) 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996;71:2553–2572. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz B, Miledi R. The statistical nature of the acetylcholine potential and its molecular components. J Physiol (Lond) 1972;224:665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampath AP, Baylor DA. Molecular mechanism of spontaneous pigment activation in retinal cones. Biophys J. 2002;83:184–193. doi: 10.1016/S0006-3495(02)75160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakatani K, Yau KW. Sodium-dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J Physiol (Lond) 1989;409:525–548. doi: 10.1113/jphysiol.1989.sp017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma JX, et al. A visual pigment expressed in both rod and cone photoreceptors. Neuron. 2001;32:451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 20.Miller JL, Korenbrot JI. Phototransduction and adaptation in rods, single cones, and twin cones of the striped bass retina: a comparative study. Vis Neurosci. 1993;10:653–667. doi: 10.1017/s0952523800005356. [DOI] [PubMed] [Google Scholar]
- 21.Tachibanaki S, Tsushima S, Kawamura S. Low amplification and fast visual pigment phosphorylation as mechanisms characterizing cone photoresponses. Proc Natl Acad Sci USA. 2001;98:14044–14049. doi: 10.1073/pnas.241396898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JL, Picones A, Korenbrot JI. Differences in transduction between rod and cone photoreceptors: an exploration of the role of calcium homeostasis. Curr Opin Neurobiol. 1994;4:488–495. doi: 10.1016/0959-4388(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 23.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signalling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- 25.Makino CL, Groesbeek M, Lugtenburg J, Baylor DA. Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores. Biophys J. 1999;77:1024–1035. doi: 10.1016/S0006-3495(99)76953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merbs SL, Nathans J. Absorption spectra of human cone pigments. Nature. 1992;356:433–435. doi: 10.1038/356433a0. [DOI] [PubMed] [Google Scholar]
- 27.Whitmore AV, Bowmaker JK. Seasonal variation in cone sensitivity and short-wave absorbing visual pigments in the rudd Scardinius erythrophthalmus. J Comp Physiol A. 1989;166:103–115. [Google Scholar]
- 28.Bowmaker JK, Dartnall HJ. Visual pigments of rods and cones in a human retina. J Physiol (Lond) 1980;298:501–511. doi: 10.1113/jphysiol.1980.sp013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harosi FI. An analysis of two spectral properties of vertebrate visual pigments. Vision Res. 1994;34:1359–1367. doi: 10.1016/0042-6989(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 30.Hárosi FI. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975;66:357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.