Abstract
Bromelain (Br), a proteolytic enzyme extracted from the stem of the pineapple, is known to possess anti-inflammatory activity and has been shown to reduce blood viscosity, prevent the aggregation of blood platelets, and improve ischemia-reperfusion (I/R) injury in a skeletal muscle model. We investigated the capacity of Br to limit myocardial injury in a global I/R model. Adult male Sprague-Dawley rats were divided into two groups: control (PBS) and Br at 10 mg/kg in PBS administered via intraperitoneal injection (twice/day) for 15 consecutive days. On day 16, the hearts were excised and subjected to 30 min of global ischemia followed by 2 h of reperfusion. Br treatment showed higher left ventricular functional recovery throughout reperfusion compared with the controls [maximum rate of rise in intraventricular pressure (dP/dtmax), 2,225 vs. 1,578 mmHg/s at 2 h reperfusion]. Aortic flow was also found to be increased in Br treatment when compared with that in untreated rats (11 vs. 1 ml). Furthermore, Br treatment reduced both the infarct size (34% vs. 43%) and the degree of apoptosis (28% vs. 37%) compared with the control animals. Western blot analysis showed an increased phosphorylation of both Akt and FOXO3A in the treatment group compared with the control. These results demonstrated for the first time that Br triggers an Akt-dependent survival pathway in the heart, revealing a novel mechanism of cardioprotective action and a potential therapeutic target against I/R injury.
Keywords: apoptosis, myocardial infarction, Ananas comosus, forkhead transcription factor
Myocardial ischemia-reperfusion (I/R) injury occurs in a wide spectrum of disorders ranging from cardiac arrest to acute myocardial infarction and represents a major public health concern. Ischemia induces several pathological changes due to lack of oxygen supply to the myocardium (16), and postischemic reperfusion worsens the injury. Modulation of the adaptive response to ischemic heart disease has become a major research interest. Pharmacological preconditioning plays a prominent role in reducing such tissue damage in response to I/R injury. In this respect, bromelain (Br), which is a descriptor for a family of sulfhydryl proteolytic enzymes extracted from the stem of Ananas comosus, the common pineapple plant (13, 23), has shown promise. Br is composed of several distinct cysteine proteolytic fractions ranging in size from 15 to 27 kDa and is commonly delivered as a powder in a gelatin or enteric-coated capsule. Reports suggest that oral administration of Br inhibits time-dependent thrombus formation in a laser thrombosis model and reduces human platelet aggregation both in vitro and in vivo (10). Br, when combined with rutin and trypsin, was also shown to have a protective effect on the skeletal muscle during I/R injury in a rabbit hindlimb model, as demonstrated by a prevention of no flow and a preservation of the muscle tissue (11). Previous studies have shown that Br has the capacity to reduce angina (10), exert antihypertensive action (17), and significantly reduce the incidence of coronary infarct when administered with potassium and magnesium orotate (12). Although earlier reports suggested the protective role of Br against I/R injury, its mechanism of action is not known. Therefore, the objective of the present study was to investigate the effect of Br pretreatment on the degree of I/R injury in an ex vivo isolated rat heart model. Moreover, the upregulation of survival kinases is known to attenuate the process of apoptosis. In particular, the serine or threonine kinase Akt is well established to play an important role in endothelial and cardiomyocyte cell biology that activates an antiapoptotic or prosurvival signaling cascade (22). In addition, reports suggest that the targets of phospho (p)-Akt action are localized in the nucleus (18). Akt regulates the activity of a variety of other targets that includes the proapoptotic protein Bad, caspase-9, and the members of the forkhead box transcription factor/protein (FOXO) family such as FOXO1, FOXO3A, and FOXO4 (4, 5). FOXOs inhibit Fas ligands and Bcl-2 like the protein Bim. In addition, Akt-dependent phosphorylation leads to an inhibition of forkhead transcription factor activity and, thereby, prevents proapoptotic signaling (22). Hence, in the present study we investigated the effect of Br on myocardial functions, infarct size, apoptosis, and the status of p-Akt and p-FOXO following I/R injury. As expected, Br treatment was found to exert a cardioprotective effect as demonstrated by the reduction in both infarct size and the degree of apoptosis in association with an improvement in functional changes in the myocardium such as heart rate (HR), left ventricular developed pressure (LVDP), maximum rate of rise in intraventricular pressure (dP/dtmax), aortic flow (AF), and coronary flow (CF). In addition, we have observed an increased phosphorylation of Akt and FOXO on Br treatment. The cardioprotective effect demonstrated by Br in our present study may occur via the phosphorylation of cytosolic and/or nuclear FOXO by Akt. Clearly, this study documents for the first time the involvement of the Akt-FOXO pathway in Br-mediated cardioprotection in ischemic-reperfused myocardium.
MATERIALS AND METHODS
Animal maintenance and treatment
All animals used in this study received humane care in compliance with the principles of the laboratory animal care formulated by the National Society for Medical Research and with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (Publication No. 85-23, Revised 1996). The experimental protocol was approved by the Institutional Animal Care Committee of the University of Connecticut Health Center (Farmington, CT). Male Sprague-Dawley rats weighing between 250 and 275 g were used for the study.
Br treatment
Br (EC 3.4.22.32, Lot No. 1965; Vital Nutrients, Middletown, CT) was used at a dose of 10 mg/kg dissolved in PBS prepared fresh every day. Each animal received two intraperitoneal injections (0.5 ml) per day, 6–8 h apart, beginning 15 days before the isolation and ex vivo ischemic induction of the heart. The dose used was based on in vivo dose-response studies performed in our laboratory with 3, 10, and 30 mg/kg body wt. We found that 3 mg has no effect on cardiac function, whereas 10 and 30 mg/kg of Br demonstrated similar cardioprotection. Therefore, we selected 10 mg/kg for our present study. Br was independently tested for authenticity, potency (2,400–2,660 gelatin digestive units/gm), microbial contamination, residual solvents, heavy metals, and aflatoxins (Vital Nutrients; ChromaDex, Clearwater, FL; and Pharmline, Florida, NY). Ananas comosus (common pineapple) was identified by the raw material provider (Indonesia) using organoleptic techniques, and Br identity was confirmed with a Fourier transform infrared spectrometer (Pharmline).
Isolated working heart preparation
Rats were anesthetized with pentobarbital sodium (80 mg/kg ip) (Abbott, Baxter Health Care, Deerfield, IL) and heparinized (500 IU/kg iv; Elkins-Sinn, Cherry Hill, NJ). After a sufficient depth of anesthesia thoractomy was performed, the hearts were perfused in the retrograde Langendorff mode at 37°C at a constant perfusion pressure of 100 cmH2O (10 kPa) for a 5-min washout period. The perfusion buffer used in this study consisted of a modified Krebs-Henseleit bicarbonate buffer (KHB) containing (in mM) 118 NaCl, 4.7 KCl, 1.7 CaCl2, 25 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, and 10 glucose. The Langendorff preparation was switched to the working mode following the washout period as previously described (14, 15, 20). The working mode was introduced by switching the flow to the left atrium from the aortic root with a constant preload of 17 cmH2O and an afterload of 100 cmH2O (14, 15, 20).
At the end of 10 min, after the attainment of steady-state cardiac function, baseline functional parameters were recorded. The circuit was then switched back to the retrograde mode, and the hearts were perfused for 15 min with KHB buffer and were then subjected to global ischemia for 30 min and then to reperfusion for 2 h. The first 10 min of reperfusion were in the retrograde mode to allow for postischemic stabilization and thereafter in the antegrade working mode to allow for the assessment of functional parameters, which were recorded at 30, 60, 90, and 120 min of reperfusion.
Cardiac function
Aortic pressure was measured using a pressure transducer (Micro-Med) connected to a sidearm of the aortic cannula, and the signal was amplified using a Heart Performance Analyzer (model 400; Micro-Med). HR, LVDP, and the first derivative of developed pressure (dP/dt) were all derived or calculated from the continuously obtained pressure signal. AF was measured using a calibrated flow meter (Gilmont Instrument, Barrington, IL), and CF was measured by a timed collection of the coronary effluent dripping from the heart.
Estimation of infarct size
At the end of reperfusion, a 1% (wt/vol) solution of triphenyltetrazolium chloride in phosphate buffer was infused into the aortic cannula at 37°C. The hearts were excised and stored at −70°C. Sections of frozen heart were fixed in 10% formalin, placed between two coverslips, and digitally imaged using a Epson scanner. To quantitate the areas of interest in pixels, Scion Image (a public-domain software package) was used. The infarct size was quantified and expressed in pixels (14, 15, 20).
Determination of cardiomyocyte cell apoptosis
Terminal dUTP nick-end labeling (TUNEL) assay was performed in deparafinized sections of 4 uM thick with an Apop tag kit (Oncor) (14, 15, 20). Specific DNA fragmentation at nucleosomal units is one of the most characteristic biochemical features of apoptosis. Apoptotic DNA fragmentation is detectable using molecular biological assays, such as DNA gel electrophoresis or in situ labeling of DNA nicks [terminal deoxynucleotidyl transferase (TdT)-mediated dUTP in situ nick-end labeling (TUNEL)]. We adapted the TUNEL procedure to demonstrate the extent of genomic cell death.
In brief, the formaldehyde-fixed left ventricle was embedded in paraffin, cut into transverse sections (4 μm thick), and deparaffinized with a graded series of xylene and ethanol solutions. Immunohistochemical detection of apoptotic cells was carried out using TUNEL, in which residues of digoxigenin-labeled dUTP are catalytically incorporated into the DNA by TdT, an enzyme that catalyzes a template-independent addition of nucleotide triphosphate to the 3′-OH ends of double- or single-stranded DNA. The incorporated nucleotide was incubated with a sheep polyclonal anti-digoxigenin antibody followed by a FITC-conjugated rabbit anti-sheep IgG as a secondary antibody as described by the manufacturer (Apop Tag Plus; Oncor, Gaithersburg, MD). The sections (n = 5 animals) were washed in PBS three times, blocked with normal rabbit serum, and incubated with mouse monoclonal antibody-recognizing sarcomeric actin (Sigma) followed by staining with tetramethylrhodamine isothiocyanate (TRITC)-conjugated rabbit anti-mouse IgG (200:1 dilution).
Isolation of nuclear and cytosolic protein fractions
The protein was isolated according to the kit protocol of CelLytic NuCLEAR Extraction kit from Sigma (St. Louis, MO). In brief, 100 mg of tissue were homogenized in 1 ml of buffer containing 100 mM HEPES (pH 7.9) with 15 mM MgCl2, 100 mM KCl, and 0.1 M DTT solution and centrifuged at 10,000 g for 20 min. The supernatant was transferred to a fresh tube and was the cytosolic fraction. The pellet was resuspended in 150 μl of extraction buffer containing 1.5 μl of 0.1 M DTT and 1.5 μl protease inhibitor cocktail. The solution was allowed to stand on ice for 30 min by shaking at brief intervals followed by centrifugation at 20,000 g for 5 min. The supernatant was transferred to a clean chilled tube and contained the nuclear protein fraction. The cytosolic and nuclear total protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce, Rockville, IL).
Western blot analysis
To quantify the phosphorylation of Akt and FOXO3A (Cell Signaling), we performed Western blot analysis using various specific primary antibodies. The cytosolic and nuclear proteins were run on SDS-PAGE typically using 10% (acrylamide-to-bis ratios). The separated proteins were electrophoretically transferred to Immobilon-P membranes (Millipore, Bedford, MA) using a semidry transfer system (Bio-Rad, Hercules, CA). Protein standards (Bio-Rad) were run in each gel. The blots were blocked in Tris-buffered saline-Tween 20 (TBS-T) containing 20 mM Tris base (pH 7.6), 137 mM NaCl, and 0.1% Tween 20 supplemented with 5% (wt/vol) nonfat dry milk for 1 h; blots were incubated overnight at 4°C with the p- and non-p-Akt and FOXO3A primary antibodies, respectively. Membranes were washed three times in TBS-T before incubation for 1 h with horseradish peroxidase-conjugated secondary antibody diluted 1:2,000 in TBS-T and 5% (wt/vol) nonfat dry milk. After incubation, membranes were washed three times with TBS-T for 10 min each, blots were treated with enhanced chemiluminescence (Amersham) reagents, and the required proteins were detected by autoradiography for variable lengths of time with Kodak X-Omat film (14, 15, 20). GAPDH and histone H3 were used as the loading controls for the cytosolic and nuclear fractions, respectively (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
The values for myocardial functional parameters, total and infarct volumes, and infarct sizes are all expressed as means ± SE. The ANOVA test was first carried out followed by Bonferroni correction to test for any differences between the mean values of all groups. The results were considered significant if P < 0.05.
RESULTS
Effect of Br on myocardial function
A significant difference in the cardiac functions (dP/dtmax, LVDP, AF, and CF) was observed even at the baseline level (Fig. 1) in the Br group compared with control. However, no significant difference was observed in HR (Fig. 1B), both at the baseline level (before ischemia) as well as during reperfusion in the Br group compared with the control group. The Br group demonstrated a significant increase in LVDP both at the baseline level and after 30 min of ischemia (Fig. 1A) throughout the reperfusion time compared with the control (81 vs. 76 at 120 min of reperfusion). dP/dtmax is an index for the contractile ability of the heart to generate force for the ejection of blood from the ventricle. The Br group demonstrated a significant increase in dP/dtmax at the baseline level. After 120 min of reperfusion, the postischemic values of dP/dtmax (Fig. 1C) were significantly increased in the Br-treated group (2,225 vs. 1,578 mmHg/s) compared with control. A similar pattern was observed in AF (11 vs. 1; Fig. 1D) as well as in CF (Fig. 1E) in the Br group compared with the control. Thus the cardioprotective effects of Br-treated animals were demonstrated by a significant recovery of postischemic myocardial function.
Fig. 1.
Effect of bromelain (Br) on left ventricular developed pressure (LVDP; A), heart rate (B), maximum rate of rise in intraventricular pressure (dP/dtmax) (C), aortic flow (D), and coronary flow (E). The isolated hearts from control (Con) and Br-10-treated rats were subjected to 30 min of global ischemia followed by 2 h of reperfusion. Results are expressed as means ± SE. *P < 0.05, significant difference between Br and Con rats; n = 6 animals in each group.
Effects of Br on myocardial infarct size
Infarct size (Fig. 2A) was significantly higher in the control hearts subjected to 30 min ischemia and 2 h reperfusion compared with the hearts that were not subjected to the I/R protocol (almost at the baseline level; data not shown). The values were noticeably reduced in the Br-treated group compared with the control nontreated group (34% vs. 43%).
Fig. 2.
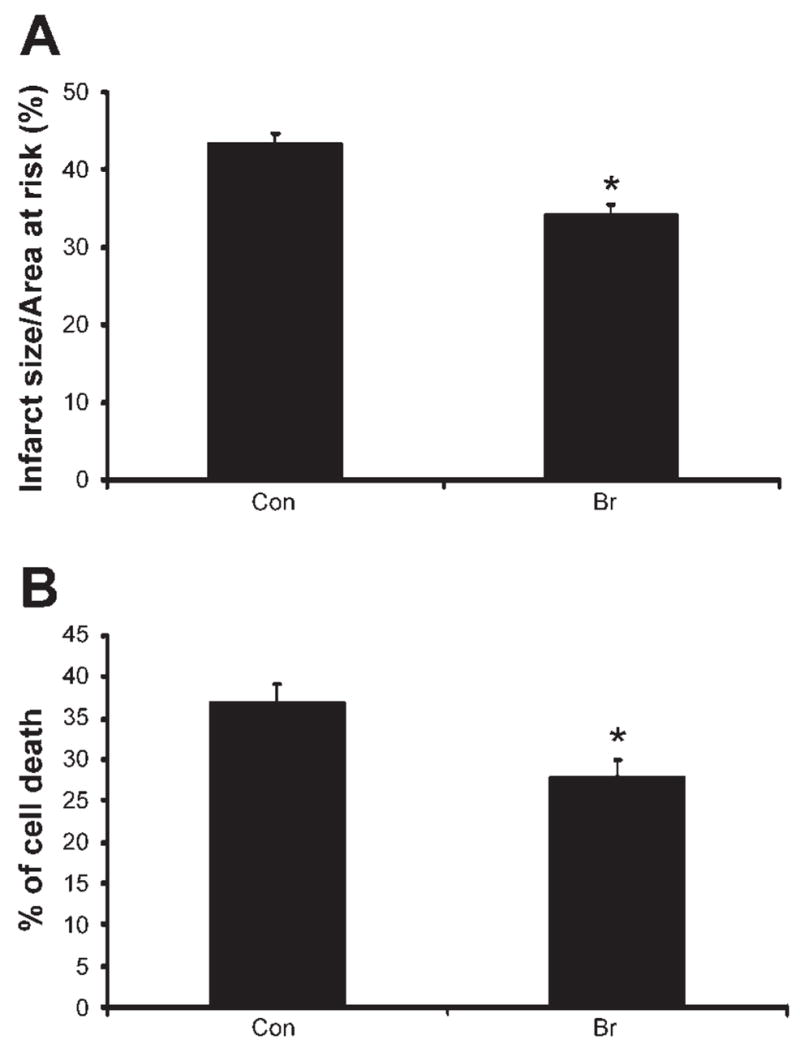
Effect of Br on infarct size (A) and cardiomyocyte apoptosis (B). The isolated hearts from Con and Br rats were subjected to 30 min of global ischemia followed by 2 h of reperfusion. Results are expressed as means ± SE. *P < 0.05, significant difference between Br and Con rats; n = 6 animals in each group.
Effects of Br on myocardial apoptosis
Double antibody staining with α-sarcomeric actin (Fig. 2B) by TUNEL assay was used to measure the cardiomyocyte apoptosis. Treatment with Br showed a significant decrease in the cardiomyocyte apoptosis (28% vs. 37%) compared with the control group. The decreased cardiomyocyte apoptosis in the Br-treated group might be due to an increased expression of p-Akt and FOXO3A levels.
Effects of Br on phosphorylation of Akt and FOXO3A
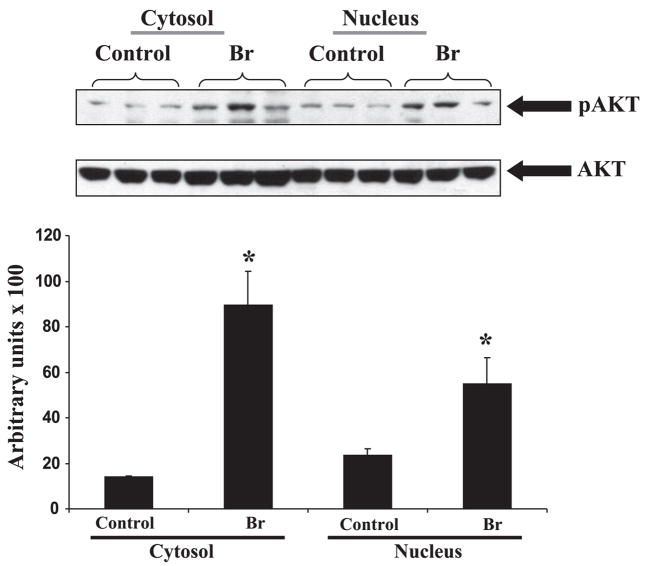
The phosphorylation of Akt was found to be increased both in the cytosolic and the nuclear fraction following I/R (Fig. 3) in the Br group compared with the controls. Similarly, Br treatment significantly enhanced the phosphorylation of FOXO3A levels (Fig. 4A) following I/R compared with control. We speculate that Br mediated phosphorylated Akt translocates to the nucleus where it phosphorylates FOXO3A, which is an important event to trigger a survival signal in the ischemic myocardium. Akt-mediated phosphorylation of FOXOs occurs in the nucleus, where it prevents DNA binding as well as creates docking sites for 14-3-3 proteins. FOXO3A is phosphorylated by Akt (8), which is expelled from the nucleus and sequestered in an inactive complex with 14-3-3.
Fig. 3.
Top: representative Western blot from left ventricle tissue isolated from each group showing the effect of Br on the expression of phosphorylated (p)-Akt in Br rats. p-Akt was expressed as 60 kDa, respectively. Akt was used as loading control. Bottom: the quantitative estimation of p-Akt between treated and nontreated groups in cytosolic and nuclear fractions. *P < 0.05, increase in the expression of p-Akt in Br-treated group compared with the nontreated controls.
Fig. 4.
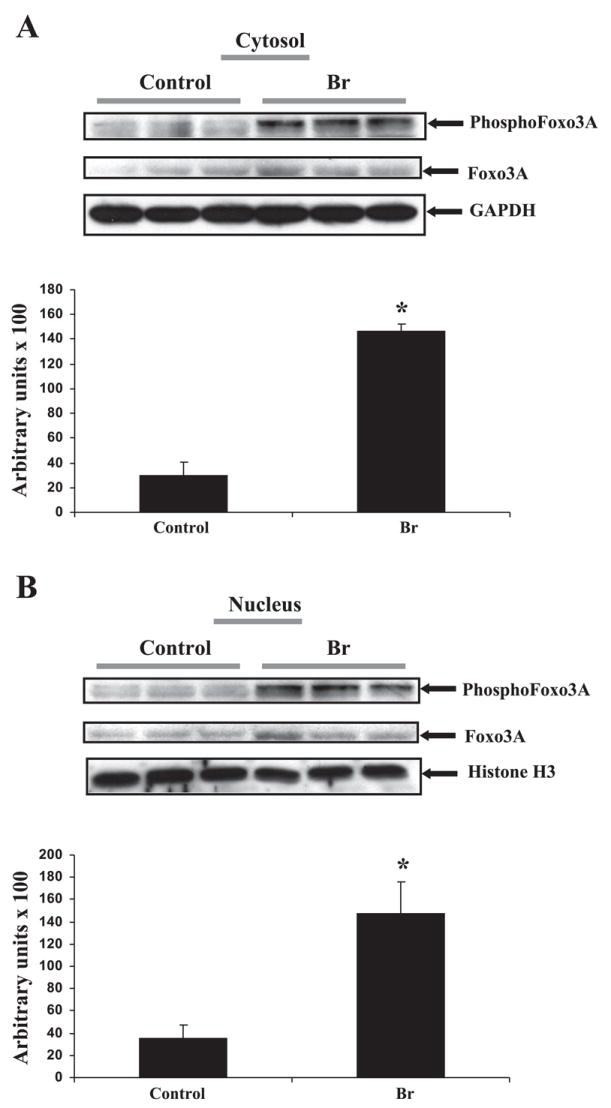
Representative Western blot from left ventricle tissue isolated from each group showing the effect of Br on the expression of p-FOXO3A in Br rats. p-FOXO3A was expressed as 60 kDa, respectively. FOXO3A was used as loading control. A: cytosolic fraction. B: nuclear fraction. Histone and GAPDH were used as the loading controls for nuclear and cytosolic protein, respectively. A and B, bottom: quantitative estimation of p-FOXO between treated and nontreated groups in cytosolic and nuclear fractions. *P < 0.05, increase in the expression of p-FOXO3A in Br-treated group compared with the nontreated controls.
DISCUSSION
Our study demonstrates for the first time that the intraperitoneal administration of Br provides cardioprotection by limiting myocardial injury in a global I/R model. This was determined by an improved postischemic ventricular recovery and a reduction in the myocardial infarct size as well as a reduction in the degree of apoptosis following I/R injury when compared with those values of the nontreated control rats. The present study was designed to pharmacologically precondition the heart with Br before the induction of ischemia, to slow down the rate of development of ischemic injury, and to avoid postischemic reperfusion injury. There are several important findings of the present study that indicate that Br may function as a pharmacological preconditioning agent. Br was found to precondition the heart, as evidenced by its ability to lower the infarct size by reducing apoptosis and improved postischemic functional recovery. It was also found to be a potent coronary vasodilator, as documented by increased CF.
From our observations, we suggest that the Br-mediated cardioprotection might be mediated by the activation of the Akt-FOXO signaling cascade. In our present study, we have observed an increased phosphorylation of Akt on Br treatment, which might have resulted in decreased cardiomyocyte apoptosis and myocardial infarct size following I/R injury. Apoptosis occurs in a wide variety of cardiovascular disorders and is now known as one of the prime causes that attributes to the compromised cardiac function (9). Reperfusion of the ischemic myocardium is associated with apoptotic cell death in concert with DNA fragmentation. It was recently documented that apoptosis is the dominant form of myocardial cell death in the infarct area immediately after infarction as well as during the later stages in the remote viable myocardium (3). Serine or threonine phosphorylation of Akt plays a crucial role in the cell survival cascade (22) since Akt activation phosphorylates multiple protein substrates, which regulate the gene transcription of which one is the set of FOXO proteins. An increased expression of Akt has also been associated with better myocardial contractility (6). In addition, the phosphorylation of Akt was shown to play an important role in facilitating growth factor-mediated cell survival and in blocking apoptotic cell death (7).
Recently, the Akt-FOXO signaling cascade and the regulation and translocation of FOXO have been suggested and frequently cited as important signaling mechanisms for the prosurvival pathway. The FOXO subfamily of forkhead transcription factors is a downstream target of Akt. This subfamily consists of three members: FOXO1 (FKHR), FOXO3A (FKHRL-1), and FOXO4 (AFX), which are all inactivated by Akt (2, 5). FOXO transcription factors have been implicated in regulating diverse cellular functions, including differentiation, metabolism, proliferation, and survival (1, 19). Active FOXO transcription factors have been associated with the activation of several proapoptotic genes, thereby activating apoptosis. However, the phosphorylation of FOXO by Akt has been shown to bring about the exclusion of FOXO from the nucleus and the inhibition of the forkhead transcriptional program.
Br treatment has demonstrated an increased phosphorylation of FOXO3A both in the nucleus and the cytoplasm, thereby inhibiting the proapoptotic signaling pathway. We assume after nuclear phosphorylation of FOXO3A by p-Akt that it is expelled from the nucleus and gets inactivated. It has been proposed that the phosphorylation of the forkhead domains in FOXO by Akt leads to the disruption of its DNA binding capacity (21). Furthermore, this phosphorylated state of FOXO seems to expose a high-affinity binding site for 14-3-3 proteins (21). This FOXO is then shuttled to the cytosol where it becomes a target for the ubiquitin-proteasome system (21). The improvement in cardiac functions on Br treatment might be attributed to the decreased cardiomyocyte apoptosis by the activation of the prosurvial Akt and, thereby, the inhibition of the proapoptotic FOXO3A.
For the first time, the results of our study demonstrate that the beneficial effects of Br are due to its ability to phosphorylate Akt, which in turn leads to the phosphorylation of FOXO3A. This phosphorylation sequence can be linked to the reduction of apoptotic cell death. We demonstrate that under our experimental circumstances, Br significantly reduced the extent of myocardial infarction and improved postischemic cardiac function, providing evidence that Br could mediate cardioprotection at least in part against I/R-induced injury.
In conclusion, Br demonstrated a significant cardioprotective effect by inhibiting the degree of apoptosis and reducing infarct size, leading to improved cardiac function, which may be due to the phosphorylation of Akt and FOXO3A. Therefore, adjunctive therapies by natural products such as Br can target specific molecular pathways involved in cell survival, which may prove to be efficient in the treatment of human heart disease and ischemic pathology.
Acknowledgments
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-56803, HL-69910, and HL-85804 and National Institutes of Health Grant NCCAM5F32-AT001569.
References
- 1.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–199. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93:8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 4.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Cittadini A, Monti MG, Iaccarino G, Di Rella F, Tsichlis PN, Di Gianni A, Stromer H, Sorriento D, Peschle C, Trimarco B, Sacca L, Condorelli G. Adenoviral gene transfer of Akt enhances myocardial contractility and intracellular calcium handling. Gene Ther. 2006;13:8–19. doi: 10.1038/sj.gt.3302589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta SR. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb E, Haffner R, von Ruden T, Wagner EF, Oren M. Down-regulation of wild-type p53 activity interferes with apoptosis of IL-3-dependent hematopoietic cells following IL-3 withdrawal. EMBO J. 1994;13:1368–1374. doi: 10.1002/j.1460-2075.1994.tb06390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maulik N, Das DK. Apoptosis, heart failure, ischemic heart disease. Heart Fail Rev. 1999;4:1–9. [Google Scholar]
- 10.Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001;58:1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumayer C, Fugl A, Nanobashvili J, Blumer R, Punz A, Gruber H, Polterauer P, Huk I. Combined enzymatic and antioxidative treatment reduces ischemia-reperfusion injury in rabbit skeletal muscle. J Surg Res. 2006;133:150–158. doi: 10.1016/j.jss.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Nieper H. Decrease of the incidence of coronary heart infarct by Mg and K oraotate and bromelain. Acta Med Empirica. 1977;12:614–618. [Google Scholar]
- 13.Ota S, Horie K, Hagino F, Hashimoto C, Date H. Fractionation and some properties of the proteolytically active components of bromelains in the stem and the fruit of the pineapple plant. J Biochem (Tokyo) 1972;71:817–830. doi: 10.1093/oxfordjournals.jbchem.a129831. [DOI] [PubMed] [Google Scholar]
- 14.Penumathsa SV, Koneru S, Thirunavukkarasu M, Zhan L, Prasad K, Maulik N. Secoisolariciresinol diglucoside: relevance to angiogenesis and cardioprotection against ischemia-reperfusion injury. J Pharmacol Exp Ther. 2007;320:951–959. doi: 10.1124/jpet.106.114165. [DOI] [PubMed] [Google Scholar]
- 15.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, Menon VP, Otani H, Maulik N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole-Wilson P. Who are the enemies? Lack of oxygen. Eur Heart J. 2002;4(Suppl G):G15–G19. [Google Scholar]
- 17.Sebekova K, Dammrich J, Fierlbeck W, Krivosikova Z, Paczek L, Heidland A. Effect of chronic therapy with proteolytic enzymes on hypertension-induced renal injury in the rat model of Goldblatt hypertension. Am J Nephrol. 1998;18:570–576. doi: 10.1159/000013411. [DOI] [PubMed] [Google Scholar]
- 18.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 19.Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, Walsh K. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 20.Thirunavukkarasu M, Penumathsa SV, Juhasz B, Zhan L, Cordis G, Altaf E, Bagchi M, Bagchi D, Maulik N. Niacin-bound chromium enhances myocardial protection from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H820–H826. doi: 10.1152/ajpheart.00134.2006. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster KA. Aktion in the nucleus. Circ Res. 2004;94:856–859. doi: 10.1161/01.RES.0000126699.49835.5D. [DOI] [PubMed] [Google Scholar]
- 23.Yamada F, Takahashi N, Murachi T. Purification and characterization of a proteinase from pineapple fruit, fruit bromelain FA2. J Biochem (Tokyo) 1976;79:1223–1234. doi: 10.1093/oxfordjournals.jbchem.a131176. [DOI] [PubMed] [Google Scholar]