Abstract
Females alter their mate choices as they transition through different reproductive stages; however, the proximal mechanisms for such behavioral fluctuation are unclear. In many taxa, as females transition through different reproductive stages, there is an associated change in hormone levels; therefore, we examined whether fluctuation in hormone levels serves as a proximal mechanism for within-individual variation in mate choice in female túngara frogs (Physalaemus pustulosus). We manipulated hormone levels of females by administering 0, 10, 100, 500 or 1000 IU of human chorionic gonadotropin (HCG), which is a ligand for luteinizing hormone (LH) receptors and will therefore cause increased gonadal hormone production. Phonotaxis assays were conducted to measure three aspects of mate choice behavior before and after HCG administration; receptivity (response to a conspecific mate signal), permissiveness (response to a signal that is less attractive than conspecific signals) and discrimination (ability to discern signal differences). The probability of response to a conspecific and an artificial hybrid signal significantly increased at the highest HCG doses. The difference in mean response time between pre- and post-HCG tests was significantly different for both the receptivity and permissiveness tests among the five doses. Increased permissiveness, however, was not due to decreased discrimination because females could discriminate between calls even at the highest HCG doses. These hormonal manipulations caused the same behavioral pattern we reported in females as they transitioned through different reproductive stages (Lynch, K.S., Rand, A.S., Ryan, M.J., Wilczynski, W., 2005. Plasticity in female mate choice associated with changing reproductive states. Anim. Behav. 69, 689–699), suggesting that changes in hormone levels can influence the female’s mate choice behavior.
Keywords: Male choice, Receptivity, Reproductive hormones, Anuran
Introduction
Variation in female mate choice potentially influences the strength of directional selection on male advertisement signals and possibly even the strength of selection on the choice itself; however, our current understanding of the causes for variation in female mate choice is limited (see Jennions and Petrie, 1997 for review). Within-individual variation in female mate choice arises when a female shows variation over time in the types of males she will accept as mates. Such alteration in female mate choice arises due to developmental, environmental and/or social factors (Crowley et al., 1991; Milinski and Bakker, 1993; Pruett-Jones, 1992 for review of mate copying; Endler and Houde, 1995; Rand et al., 1997); however, a less studied mechanism that contributes to alteration in mate choice behavior is short-term changes in female condition (Thornhill, 1984; Proctor, 1991; Poulin, 1994; Simmons, 1994; Penton-Voak et al., 1999). For instance, female pied flycatchers (Ficedula hypoleuca) in good body condition will travel further to assess available mates than females in poor condition (Slagsvold et al., 1988). Female scorpionflies (Hylobittacus apicalis) will accept males that bring small nuptial gifts only when they are hungry, however, when they are satiated, they accept only males with large nuptial gifts (Thornhill, 1984). Parasitized female bullies (Gobiomorphus breviceps) accept significantly smaller males as mates relative to unparasitized female bullies (Poulin, 1994). There are also examples of short-term changes in female condition influencing mate choice in humans. For instance, women show stronger preferences toward masculine male faces during the fertile phase of the menstrual cycle (i.e., ovulatory stage) as opposed to the luteal stage in which they are infertile (Penton-Voak et al., 1999). Such changes in the condition of the female clearly contribute to alterations in mate decisions.
Cyclic changes in the female’s hormonal state may induce a short-term change in female condition that contributes to variation in mate choice. Elevation in hormone levels often coincides with the approach of the time when the female must release eggs or oviposit, and we have previously shown that as this time approaches, female túngara frogs (Physalaemus pustulosus) modify their mate choice behaviors. Theoreticians predict that such alteration of mate choice behavior occurs as a consequence of time constraints (Real, 1990; Crowley et al., 1991), which can be imposed upon the mate-searching female as she approaches the time at which she must release her eggs, and empirical studies have supported these predictions (Lea et al., 2000; Lynch et al., 2005). Furthermore, age-related declines in reproductive capability or the approach of the breeding season’s end have also been shown to act as constraints upon mate-searching females (Forsgren, 1997; Qvarnstrom et al., 2000; Kodric-Brown and Nicoletto, 2001; Moore and Moore, 2001; Veen et al., 2001). Few studies, however, have examined these constraints from a proximal perspective. For instance, it is also possible that such changes in mate choice behavior are induced by elevated hormone levels which cause females to become receptive to courting males, and as female receptivity reaches some threshold, the female lowers the threshold for mate acceptance and therefore broadens the range of mate signals she is willing to accept. In fact, it has long been known that the induction of female receptive behaviors is hormone-dependent in a variety of taxa including birds (Noble, 1973; Delville and Balthazart, 1987), amphibians (Diakow and Nemiroff, 1981; Schmidt, 1984, 1985; Boyd, 1994), mammals (Tetel et al., 1994; Cushing and Carter, 1999) and reptiles (Alderete et al., 1980; Rhen and Crews, 2000; Rhen et al., 1999, 2000). It has not been well studied, however, whether these changes in receptive behavior are accompanied by changes in the range of mate signals that female will accept. In one recent study, testosterone-treated female dark-eyed juncos (Junco hyemalis) were less discriminating in their mate choices than control females (McGlothlin et al., 2004). Although there are few hormone manipulation studies that provide empirical support for these predictions, it has been shown that variation in mate choice decisions can be associated with changes in reproductive stage (Lea et al., 2000; Bosch and Boyero, 2004; Lynch et al., 2005), and that, in a few anuran species, changes in reproductive stage are associated with changes in hormonal state (Licht et al., 1983; Pierantoni et al., 1984; Iela et al., 1986; Itoh and Ishii, 1990; Harvey et al., 1997; Medina et al., 2004; Lynch and Wilczynski, 2005). Such studies suggest that fluctuation in hormonal state may serve as a proximal mechanism for altered mate choice.
Anurans are often used as a model in studies of female mate choice because the females base their mate choices almost entirely on the male’s acoustic advertisement signal (Wells, 1977). Their reproductive behavior is relatively stereotyped, therefore making it easy to observe female mate choice both in the field and in the laboratory (Ryan, 1985). We assayed individual variation in mate choice by repeatedly testing mate preferences of female túngara frogs, a neotropical species in which differences in male signals within a population influence female preference (Ryan, 1980, 1985, 1997; Rand and Ryan, 1981; Rand et al., 1992; Ryan and Rand, 1990, 2001; Wilczynski et al., 1995, 1999). We used phonotaxis tests to assay three aspects of acoustic-based mate preferences in the túngara frog; receptivity, permissiveness and discrimination. Receptivity is operationally defined as a positive phonotactic response to any conspecific male signal, and permissiveness is defined as the female’s selectivity and is assessed by a response to an artificial hybrid call that is known to be less attractive than the conspecific call. Testing permissiveness asks whether the female is so motivated to mate that she will accept an unattractive signal; therefore, permissiveness can be thought of as a measure of receptivity that asks whether the female is so receptive to mate that she will broaden the range of signals that she will accept. Discrimination is the female’s ability to discern the difference between a conspecific call and the artificial hybrid call when given the choice to do so (Lynch et al., 2005). We examined these aspects of mate choice behavior both before and after hormone manipulation to determine whether hormonal state acts as a proximal mechanism for altered mate choice behavior.
Our objective in this study is to determine whether manipulations of gonadotropic hormones, which ultimately increase gonadal hormones such as estrogen and androgens, will influence females to express the same pattern in mate choice behavior as seen in wild-caught females as they transitioned through different reproductive stages. That is, as wild-caught females neared the time to oviposit, they increased motivation to mate and became less selective in their mate choices (Lynch et al., 2005). By using a gonadotropin, we manipulate the female’s entire reproductive state so that the female will be induced to release eggs. Such an initial broad approach to this question will allow us to determine whether a heightened reproductive state corresponds with the point at which the female displays increased receptivity and less selectivity in her mate choices, however, these experiments will not provide an exact physiological mechanism by which such behavioral modification can occur.
Methods
The túngara frog is a neotropical species in which reproduction occurs during the rainy season (approximately May to December). Females will lay multiple clutches of eggs during a single breeding season with gravid periods separated by approximately 4 to 6 weeks (Ryan, 1985). It has been noted, however, that female túngara frogs will oviposit during the dry season months if an adequate rainfall occurs (S. Rand, personal communication).
All female túngara frogs used in this study were from a colony maintained at the University of Texas at Austin. Frogs in the colony were maintained in 5- or 10-gal aquariums with damp moss in groups of five and fed 1-week old crickets three times per week. The frogs were housed with simulated, but clock-shifted, equatorial light/dark cycles so that dusk began at 14:00 and dawn began at 02:00.
All females had previously mated with a male (or released eggs) at least once before used for phonotaxis tests in this study, which allowed us to be sure that all females were reproductively able. Phonotaxis tests were conducted in July 2003. The mean weight of females was 2.45 g, and the mean snout–vent length was 29.36 mm.
During the phonotaxis test, the subject was placed into an acoustic chamber measuring 1.8 × 2.7 m with acoustic foam on the walls to reduce reverberation. Two speakers were placed 2.7 m apart at equal distances from the center of the chamber. The peak intensity of the acoustic stimulus was set at 80 dB SPL (re 20μP) in the center of the chamber where the female was initially released. All acoustic stimuli were synthesized on a Dell computer with unpublished software produced by J. Schwartz. Phonotaxis tests were conducted from approximately 13:00 to 19:00.
To initiate the phonotaxis test, the female was placed under a funnel in the center of the chamber for three minutes while acoustic stimuli are antiphonally broadcast from opposing speakers with a 0.5-s interval between the stimuli. The side on which each stimulus is presented was alternated to control for side bias. After the funnel was lifted, the female was allowed 15 min to approach either speaker. If the female came within 10 cm of a speaker, it was considered a response to the stimulus being broadcast from that speaker. If the female remained stationary for at least 2 consecutive min, failed to move from the release site within 5 min or did not approach a speaker within 15 min, she was considered unresponsive to the acoustic stimuli. In total, each female completed four phonotaxis tests during each trial: the first receptivity test, a permissiveness test, a discrimination test and a final receptivity test.
Most of these tests were repeatedly used with wild-caught females to determine how each of these behaviors changed as they progressed through different reproductive stages. Lynch et al. (2005) reported that receptivity and permissiveness fluctuated in unison so that the frequency of these behaviors was low in the unamplexed stage (i.e., unmated stage) but increased during the amplexed stage (i.e., mated stage that is near the time of ovipositing). Both receptivity and permissiveness simultaneously declined in the post-mated stage (i.e., after egg release). The increase in permissive mate choices during the amplexed stage was not due to a decrease in discrimination because females maintained discriminatory responses during this stage. Discrimination, however, did decline in the post-mated stage. Here, we use similar phonotaxis tests in order to determine whether hormone fluctuations may play a role in such behavioral flexibility.
Receptivity phonotaxis tests
The female’s receptive state was examined in the first phonotaxis test. In this test, the female heard two conspecific mate signals, a whine alone and a whine–chuck. A response to either of these signals is sufficient to consider the female as receptive to mate signals. That is, this test did not measure which conspecific signal the female responded to, only whether she responded to one or the other. The same stimuli were repeated in the last phonotaxis test, and the female needed to respond to a conspecific call in both the first and last test in order to be considered fully receptive. Females that approached a speaker in only one of the two tests were not considered receptive, as we could not be sure that an apparent response in only one test indicated receptivity or was simply a random movement toward one of the speakers.
Permissiveness phonotaxis test
Immediately following the first receptivity test, the female was re-tested to determine if she would approach an artificial hybrid call. The artificial hybrid whine was synthesized by varying spectral and temporal components of the P. pustulosus whine so that the call would be intermediate between the calls of P. pustulosus and P. enesefae, a closely related species. Details describing the synthesis of this call are discussed in Ryan et al. (2003). This artificial hybrid whine has previously been shown to elicit a response from only 25% of females (Ryan et al., 2003), indicating this call is less attractive to P. pustulosus females than are conspecific mate calls. The hybrid whine was paired against white noise with equal amplitude and duration. Females that respond to white noise were excluded from statistical analyses because it is not possible to interpret the meaning of this response. This single-choice design was used because a female túngara exhibits strong discrimination in favor of a conspecific signal when present, yet displays some degree of recognition toward heterospecific calls when the conspecific call is not available (Ryan et al., 2003). This type of design allowed us to examine how hormonal state influences the strength of female preferences.
Discrimination phonotaxis test
Immediately following the permissiveness test, we examined the female’s ability to discern the difference between the conspecific whine and the hybrid whine. A response to a conspecific whine over the hybrid whine indicates that the female is able to discern the difference between the two whines; that is, she is maintaining her discrimination. Conversely, a response to a hybrid whine indicates that the female has not maintained normal discriminatory responses.
Effects of HCG administration on mate choice behaviors
Females completed phonotaxis assays (pre-HCG tests) and were randomly placed into one of five dose groups in which they received human chorionic gonadotropin (HCG; Sigma). HCG is a ligand for luteinizing hormone (LH) receptors, which causes gonadal activation and therefore, the production of gonadal hormones. Doses of HCG include: 0 (control; N = 8), 10 (N = 8), 100 (N = 8), 500 (N = 16) or 1000 IU (N = 20). HCG was dissolved in 0.9% saline solution and given in a subcutaneous injection in a volume of 50 μl. The same phonotaxis tests described above were repeated approximately 20–24 h after HCG administration (post-HCG tests). The observer scoring the behavior was unaware of the treatment each subject received.
Egg laying and mate choice response
HCG induces egg laying in approximately 24 h in most females that are treated with high doses (i.e., 500 or 1000 IU). In the 500 IU group, 75% of females released eggs after injection with HCG and in the 1000 IU, 68.75% of females released eggs after injection with HCG. Only 8% of females in the 0, 10 and 100 IU groups laid eggs. We used Chi-squared goodness of fit to compare mate choice behavior between egg layers (N = 25) and non-egg layers (N = 8) to determine whether mate choice flexibility depended on the presence of mature oocytes.
Hormone assays
Upon completion of phonotaxis tests, blood samples were collected via the orbital sinus from five subjects in each dose group. These procedures were approved by the University of Texas IACUC. The blood was centrifuged, and the plasma layer was stored at −20°C until assayed. Plasma volumes ranged from 5 μl to 20 μl. Twenty μl of tritiated estrogen or testosterone (approximately 1000 cpm) was added to each plasma sample for recovery determination. Plasma samples were extracted using 3 ml of diethyl ether. The mean recovery after extraction was 71 ± 0.09% and 95 ± 0.16% for estrogen and androgens respectively. Hormone assays were conducted with enzyme immunoassay (EIA) kits purchased from Caymen Chemical. These kits were validated prior to use in this study by extracting hormone from a pooled sample of frog plasma. We then diluted the pooled plasma to be three or four different concentrations that were within the most sensitive portion of the standard curve. The assay kit estimated the hormone concentration at each of the dilutions to be within 20% of each other. Each sample was assayed in duplicate, and each sample was measured at a minimum of two dilutions. Inter-assay variation was 12% for estrogen whereas androgens were measured on a single plate. Intra-assay variation was 9.9% and 9.5% for estrogen and androgens respectively. Cross-reactivity in the estrogen kit was 0.1% for testosterone and 5 α-DHT, 0.07% for 17 α-estradiol and 0.03% for progesterone and the detection limit is 8 pg/ml. The detection limit for testosterone EIA kits is 6 pg/ml. Testosterone EIA kits have a 27.4% and 18.9% cross-reactivity with 5α-dihydrotestosterone and 5β-dihydrotestosterone respectively. Therefore, we refer to testosterone measurements simply as androgens.
Statistical analyses
We used two analyses to assess variation in receptive and permissive behaviors. First, we tested whether the probability of a response to a mate signal changed between the pre- and post-HCG tests using Chi-squared goodness of fit. We used the frequency of responses in the post-HCG condition as expected and the frequency of response in the pre-HCG condition as observed. These tests were not used in the lower dose groups (0, 10 and 100 IU) because low response frequencies yielded low expected values. Second, we tested whether individual females changed their responses between the pre- and post-HCG phonotaxis tests by using a Kruskal–Wallis test to analyze the difference in the female’s response time before and after HCG treatment. Response time was measured as the time between when the funnel was lifted, allowing the female freedom to move and the time at which she reached the 10 cm point around a speaker. These scores were recorded in seconds, and non-responsive females received the maximum number of seconds (900 s). These analyses were done for both the receptivity and permissiveness phonotaxis tests.
We tested responses of females after treatment with HCG to determine whether hormonal manipulation influenced discrimination. We used a binomial test to examine whether the probability of a discriminatory response was significantly different from the probability of a random response.
We examined whether HCG administration significantly altered the plasma concentration of estrogen and androgens using one-way ANOVA with Tukey’s post hoc comparisons. Alpha values were set at 0.05 for all statistical tests, and all reported values are mean ± SE.
Results
Effects of HCG administration on mate choice behaviors
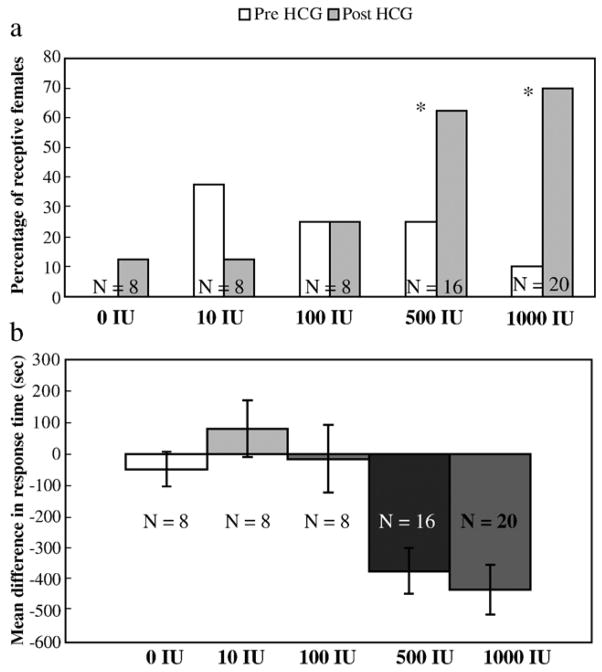
At the highest doses of HCG (500 and 1000 IU), receptivity and permissiveness were increased, but there was no change in discrimination. Female receptivity among the five doses of HCG was examined by determining if females increased the number of responses after treatment and whether females responded in less time after treatment (Figs. 1a and b respectively). At 500 IU of HCG, there is a significant increase in the probability of a response to the conspecific call during the receptivity tests after HCG treatment (N = 16; χ2 = 9.6; df = 1; P < 0.002). There is also a significant increase in the probability of a receptive response at the 1000 IU dose of HCG after treatment (N = 20; χ2 = 34.28; df = 1; P < 0.001). These data show that the probability of a receptive response (i.e., positive phonotactic response to a conspecific call) increases at the 500 and 1000 IU levels after treatment with HCG.
Fig. 1.
(a) Percent of receptive females tested that responded to conspecific calls in the receptivity tests before and after 4 doses of HCG (in International Units, IU) and a control vehicle injection. The percent of females responding to the conspecific call before HCG administration was compared to the percent responding after HCG administration using Chi-squared goodness of fit; asterisk indicates significant pre- vs. post-injection responses. (b) Mean (±SE) difference in response time before and after HCG treatment during the receptivity tests as a function of HCG dose (in International Units, IU). Negative mean response times indicate faster responding after receiving treatment. Kruskall–Wallis test indicates a significant difference among doses (P < 0.001).
The Kruskal–Wallis test showed a significant difference in the amount of time to respond to a conspecific mate call between the pre- and post-HCG tests (χ2 = 21.1; df = 4; P < 0.001) among the five doses. The mean differences in response time were −47.5 s ± 55.1 s, 81.75 s ± 90.1 s, −15.93 s ± 107.4 s, −372.22 s ± 73.3 s and −431.2 s ± 79.2 s for the 0 IU, 10 IU, 100 IU, 500 IU and 1000 IU dose groups respectively. These data show that female receptivity is significantly higher at 500 and 1000 IU doses, and that the 500 IU dose is the threshold for which receptive behaviors appear.
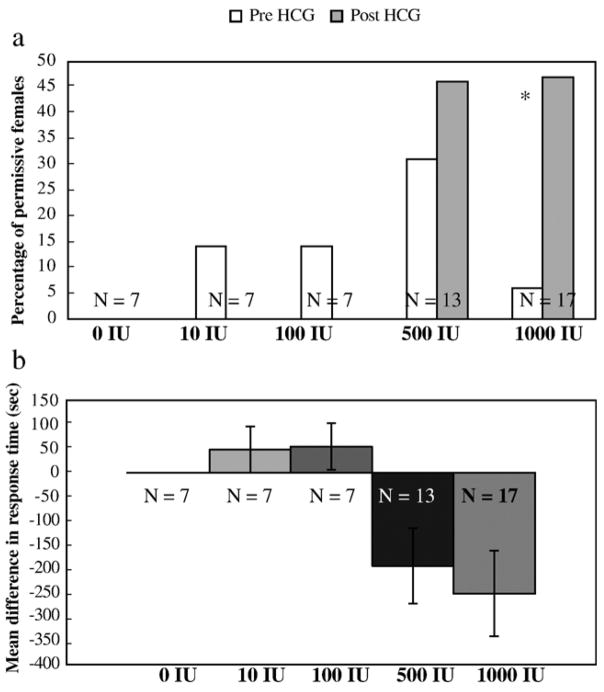
Female permissiveness among the five doses of HCG was examined by determining if females were more likely to respond to the less attractive hybrid call after treatment and whether treatment influenced time to respond to the less attractive call (Figs. 2a and b respectively). Among all the dose groups, nine females responded to white noise and were therefore excluded from this analysis. At 1000 IU of HCG, a significant increase appeared in the probability of a response to the less attractive hybrid call during the permissiveness phonotaxis test after HCG treatment (N = 17; χ2 = 11.57; df = 1; P < 0.001).
Fig. 2.
(a) Percent of females responding permissively (i.e., to an unattractive artificial hybrid call) before and after 4 doses of HCG (in International Units, IU) and a control vehicle injection. The percent of females responding to the hybrid call before HCG administration was compared to the percent responding after HCG administration using Chi-squared goodness of fit; asterisk indicates significant pre- vs. post-injection responses. (b) Mean (±SE) difference in response time before and after HCG treatment during the permissiveness tests as a function of HCG dose (in International Units, IU). Negative mean response times indicate faster responding after receiving treatment. Kruskall–Wallis test indicates a significant difference among doses (P = 0.046).
A Kruskal–Wallis test showed a significant difference in the amount of change in female response time to the hybrid call between the pre- and post-HCG tests (χ2 = 9.7, df = 4, P = 0.046) among the five doses. The mean differences in response time were 0 s ± 0 s, 48.43 s ± 48.43 s, 54.71 s ± 54.71 s, −194.23 s ± 87.42 s and −251.53 s ± 96.88 s for the 0 IU, 10 IU, 100 IU, 500 IU and 1000 IU dose groups respectively. These results show that female permissiveness is significantly higher at 500 and 1000 IU doses, and that the 500 IU dose is the threshold for which permissive behaviors appear.
We examined whether hormone manipulation influenced discrimination between the conspecific and hybrid whine (Fig. 3). At the three lower doses of HCG (0, 10, 100 IU), few females responded during the discrimination test, making it difficult to detect the probability of a non-random response. At the two highest doses (500 IU, N = 10; 1000 IU, N = 11), females responded to the conspecific whine over the hybrid whine significantly more often than chance (two-tailed binomial test P = 0.012 and P = 0.001 respectively).
Fig. 3.
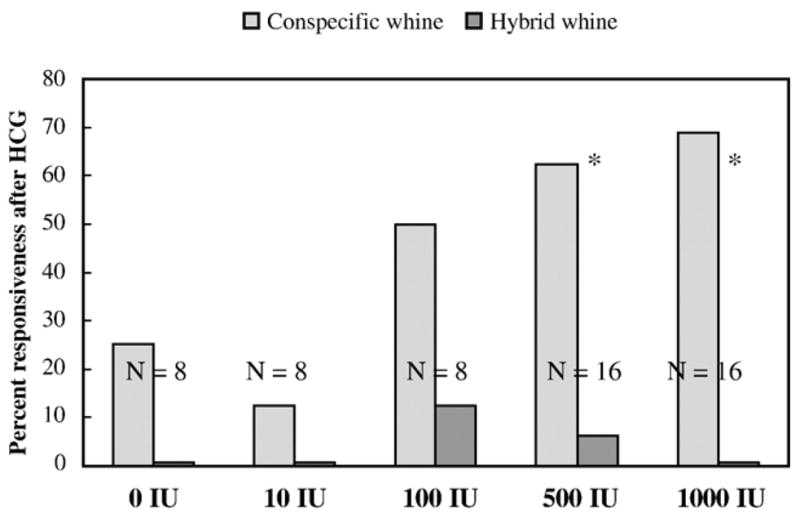
Percent of females responding to a conspecific call vs. an artificial hybrid call in a two choice discrimination test after 4 doses of HCG (in International Units, IU) and a control vehicle injection. N equals the number of subjects tested in each treatment. Asterisks indicate significant difference in percent responding to the different calls. A higher response to the conspecific whine over the hybrid whine indicates that the female was able to discern the difference between these two stimuli.
Egg laying and mate choice response
Eight females injected with a high dose of HCG did not lay eggs and of those eight, one responded receptively before injection. After injection, there were only two females that responded receptively. No females that did not lay eggs yet received a high dose of HCG chose permissively before injection, whereas one female responded permissively after injection (N = 8). These comparisons were not analyzed statistically due to the small values and the obvious lack of difference between pre- and post-HCG response. On the other hand, there were 25 females that laid eggs after HCG injection, and these comparisons did show significant differences in pre-and post-HCG response. All females that responded to the white noise were excluded from this analysis. Five females that laid eggs responded receptively before HCG injection, whereas 20 females responded receptively after injection (N = 25; χ2 = 52.25; P < 0.001). Five females responded permissively before injection, which increased to ten after injection (N = 20; χ2 = 5.0; P = 0.025).
Hormone assays
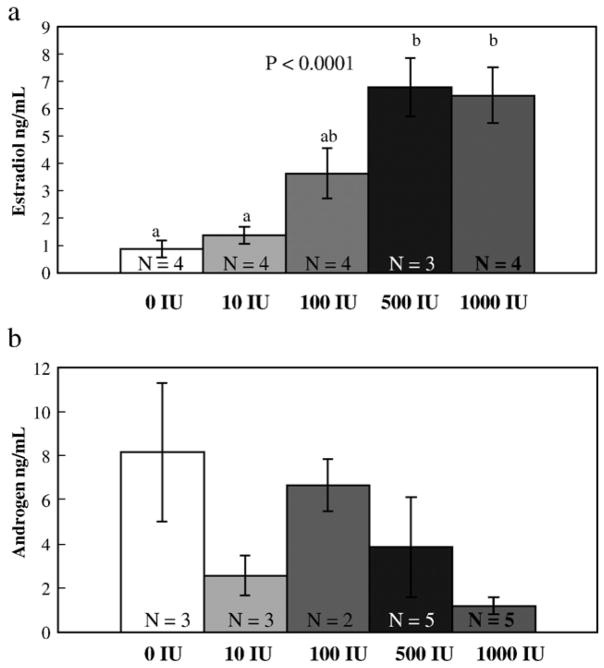
Estrogen concentration significantly increased in a dose-dependent manner after HCG treatment (Fig. 4a; N = 19; df = 4, 14; F = 12.55; P < 0.0001). Tukey’s post hoc tests revealed that both 500 and 1000 IU dose groups had significantly elevated estrogen concentrations in relation to the control group (P = 0.001 for both 500 and 1000 IU). These high doses of HCG also had significantly elevated estrogen levels in relation to the 10 IU group (P = 0.003 and P = 0.002 for 500 and 1000 IU). The estrogen concentration in the 100 IU group was marginally different from the 500 IU group (P = 0.097). There was no difference in estrogen concentration between 500 and 1000 IU dose groups (P = 0.99). In addition, there was no significant difference in the mean concentration of androgen among the different dose groups (Fig. 4b; N = 18; df = 4, 13; F = 2.108; P = 0.14).
Fig. 4.
(a) Mean (±SE) plasma estrogen concentration and (b) androgen concentration as a function of HCG dose (in International Units, IU). N equals the number of subjects tested in each treatment. The different letters represent groups that are significantly different from one another based on one-way ANOVA with a Tukey’s post hoc analysis.
Discussion
Manipulating the females’ reproductive state using high doses of HCG influenced females to show receptive behaviors. Such increases in receptivity were accompanied by changes in the female’s mate selectivity. That is, the female increased the range of mate signals she was willing to accept as receptivity increased. This is the same pattern in mate choice behavior we observed in wild-caught females as they approached the time at which they must release their eggs in the field. Furthermore, as we observed in wild-caught females, the HCG induced increase in the type of signals the female was willing to respond to (i.e., permissiveness) was not due to a decrease in discrimination. It is more likely, however, that HCG decreased the female’s choosiness rather than her discrimination.
Estrogen concentration also increased in a dose-dependent manner but plateaus at 500 IU of HCG. The mean estrogen concentration with high doses of HCG is 6.5 to 6.8 ng/ml, which is within the physiological range of wild-caught females that are actively reproducing (i.e., amplexing; Lynch and Wilczynski, 2005). In addition, wild-caught females in amplexus also show maximal receptive and permissive behaviors (Lynch et al., 2005). Consequently, evidence from both hormone manipulation studies and natural hormone fluctuation studies show a peak in estrogen when receptive and permissive behaviors are maximal, suggesting that estrogen contributes to the expression of these behaviors. Alternatively, it is also possible that the HCG injection activated LH receptors in the brain, which may play a role in evoking receptive behaviors. However, this conclusion is not consistent with the results presented by Kelley (1982) in which she showed that luteinizing hormone releasing hormone (LHRH), but not HCG, was effective at maximizing receptive behavior in steroid-primed, ovariectomized female Xenopus laevis. It is also possible that HCG exerts behavioral effects by elevating other hormones. For instance, Schmidt (1984) showed that the action of HCG could be inhibited if given with indomethacin, an inhibitor of prostaglandin synthesis, suggesting that HCG may exert behavioral effects by elevating prostaglandin levels. Also, in female X. laevis neither estrogen or progesterone alone was effective at reinstating receptive behaviors in ovariectomized females, however, when administered together females showed an increase in receptive behavior. The frequency of these behaviors, however, did not increase significantly until there was an additional treatment with gonadotropin (Kelley, 1982). Also, progesterone alone or in combination with arginine vasotocin (AVT) primes females before the administration of other drugs used to induce receptivity in a female anuran (Schmidt, 1984; 1985). In addition, other studies have found peptide hormones such as AVT and LHRH, as well as other hormones such as prostaglandins, to be effective at inducing receptivity in female amphibians (Diakow and Nemiroff, 1981; Kelley, 1982; Boyd, 1994; Schmidt, 1985). In short, although our results suggest that estrogen is involved in inducing receptive and also permissive mate choices, it may not be necessary and sufficient for the alteration in female mate choice behavior. Our data at present are not sufficient to identify the mechanism for these effects.
High doses of HCG also induced females to become gravid and release their eggs. Therefore, it is also possible that there are endogenous peripheral influences that contribute to female receptivity. For instance, female leopard frogs (Rana pipiens) must ovulate and pass eggs through the oviduct in order to display receptive behavior (Diakow et al., 1988). This change in receptive behavior can be induced in ovariectomized leopard frogs by artificially distending the female with fluid (Diakow et al., 1978). These studies indicate that there are peripheral neural responses, especially in the oviduct, that contribute to the induction of receptivity. A closer examination of our data shows that females that received high doses of HCG (500 or 1000 IU) yet never laid eggs after HCG treatment did not significantly increase receptive and permissive behaviors after hormone treatment. On the other hand, females that received high doses of HCG and were retaining mature eggs that could be released after treatment did significantly increase receptive and permissive behaviors after hormone treatment. In other words, the significant increase in receptive and permissive responses observed in females treated with high doses of HCG occurs only in females that are retaining at least some mature eggs that can be released once HCG is administered. The mechanism by which these peripheral factors influence behavior is unclear. It is possible that eggs retained within the oviduct can initiate hormone release, such as prostaglandin, which is needed for receptive behaviors. It is also possible that retained eggs cause distention of the oviduct, and the somatosensory stimulation facilitates receptivity. In sum, hormonal and physiological factors may work together to influence female mate choice behavior.
There is no significant pattern in androgen concentration among the HCG doses, however, the trend suggests that androgen levels decline as dose increases. Although our assays are unable to discern whether the androgen pattern is due to a change in testosterone, dihydrotestosterone or both, previous studies have reported that reproductively active female anurans exhibit significantly higher circulating levels of testosterone than dihydrotestosterone (Harvey et al., 1997; Wilczynski et al., 2003; Medina et al., 2004). In addition, levels of testosterone in some female anurans are comparable to male levels and are generally higher than the concentration of estrogen in the plasma (d’Istria et al., 1974; Licht et al., 1983; Harvey et al., 1997; Wilczynski et al., 2003; Medina et al., 2004). Although the behavioral consequences of elevated testosterone in female anurans are unclear, our results show that when females express peak receptive and permissive behaviors, androgen levels decline. This is consistent with our previous finding in which androgen levels significantly decline in wild-caught females when they go from an unamplexed to an amplexed state (Lynch and Wilczynski, 2005). Such a result can be explained if testosterone is primarily responsible for our estimation of circulating androgens because a decline in testosterone levels may reflect aromatization of testosterone into estrogen.
Theoretical models of mate choice behavior suggest that constraints can influence the outcome of a female’s decision on which male to accept (Real, 1990; Crowley et al., 1991; Sullivan, 1994). The data presented here suggest that time constraints imposed upon the female due to HCG induced egg release may serve as an ultimate mechanism that can impact her mate choice decisions. Such a result is consistent with our observation of wild-caught females that are naturally approaching the time at which they must oviposit because those females also showed an increase in permissive mate choices. Furthermore, the present study demonstrates that hormonal changes caused by HCG or possibly HCG itself can induce females to become receptive to male courtship cues, and such changes in receptivity are accompanied by an increase in the females permissive mate choices.
Acknowledgments
This research was supported by grant IBN 0078150 from the National Science Foundation to D.C. Cannatella, M.J. Ryan and W. Wilczynski and NIMH grant RO1 2 MH 057066 to W. Wilczynski. K. Lynch was supported by grant T32 MH 18831. We thank Jennifer Gench with her help in conducting phonotaxis assays and Cristina Gridi-Papp for the animal care.
References
- Alderete MR, Tokarz RR, Crews D. Luteinizing hormone-releasing hormone (LHRH) and thyrotropin releasing hormone (TRH) induction of female sexual receptivity in the lizard, Anolis carolinensis. Neuroendocrinology. 1980;30:200–205. doi: 10.1159/000123001. [DOI] [PubMed] [Google Scholar]
- Bosch J, Boyero L. Reproductive stage and phonotactic preferences of female midwife toads (Alytes cisternasii) Behav Ecol Sociobiol. 2004;55:251–256. [Google Scholar]
- Boyd SK. Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Horm Behav. 1994;28:232–240. doi: 10.1006/hbeh.1994.1020. [DOI] [PubMed] [Google Scholar]
- Crowley PH, Travers SE, Linton MC, Cohn SL, Sih AS, Sargent CR. Mate density, predation risk and the seasonal sequence of mate choices: a dynamic game. Am Nat. 1991;137:567–596. [Google Scholar]
- Cushing BS, Carter CS. Prior exposure to oxytocin mimics the effects of social contact and facilitates sexual behavior in females. J Neuroendocrinol. 1999;11:765–769. doi: 10.1046/j.1365-2826.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- Delville Y, Balthazart J. Hormonal control of female sexual behavior in the Japanese Quail. Horm Behav. 1987;21:288–309. doi: 10.1016/0018-506x(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Diakow C, Nemiroff A. Vasotocin, prostaglandin and female reproductive behavior in the frog, Rana pipiens. Horm Behav. 1981;15:86–93. doi: 10.1016/0018-506x(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Diakow C, Wilcox JN, Woltmann R. Female frog reproductive behavior elicited in the absence of ovaries. Horm Behav. 1978;11:183–189. doi: 10.1016/0018-506x(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Diakow C, Scharff C, Arnow L. Egg-oviduct interaction initiates reproductive behavior. Horm Behav. 1988;22:131–138. doi: 10.1016/0018-506x(88)90036-0. [DOI] [PubMed] [Google Scholar]
- d’Istria M, Delrio G, Botte V, Chieffi G. Radioimmunoassay of testosterone, 17 β-oestradiol and oestrone in the male and female plasma of Rana esculenta during sexual cycle. Steroids Lipids Res. 1974;5:42–48. [PubMed] [Google Scholar]
- Endler JA, Houde AE. Geographic variation in female preferences for male traits in Poecillia reticulate. Evolution. 1995;49:456–468. doi: 10.1111/j.1558-5646.1995.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Forsgren E. Mate sampling in a population of sand gobies. Anim Behav. 1997;53:267–276. [Google Scholar]
- Harvey LA, Propper CR, Woodley SK, Moore MC. Reproductive endocrinology of the explosively breeding desert spadefoot toad, Scaphiopus couchii. Gen Comp Endocinol. 1997;105:102–113. doi: 10.1006/gcen.1996.6805. [DOI] [PubMed] [Google Scholar]
- Iela L, Rastogi RK, Delrio G, Bagnara JT. Reproduction in the Mexican leaf frog, Pachymedusa dacnicol or: III. The female Gen Comp Endorinol. 1986;63:381–392. doi: 10.1016/0016-6480(86)90137-1. [DOI] [PubMed] [Google Scholar]
- Itoh M, Ishii S. Changes in plasma levels of gonadotropins and sex steroids in the toad, Bufo japonicus, in association with behavior during breeding season. Gen Comp Endocrinol. 1990;80:451–464. doi: 10.1016/0016-6480(90)90194-q. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Female sex behaviors in the south African clawed frog, Xenopus laevis: gonadotropin-releasing, gonadotropic and steroid hormones. Horm Behav. 1982;16:158–174. doi: 10.1016/0018-506x(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Kodric-Brown A, Nicoletto PF. Age and experience affect female choice in the guppy (Poecilia reticulate) Am Nat. 2001;157:316–323. doi: 10.1086/319191. [DOI] [PubMed] [Google Scholar]
- Lea J, Halliday T, Dyson M. Reproductive stage and history affect the phonotactic preferences of female midwife toads, Alytes muletensis. Anim Behav. 2000;60:423–427. doi: 10.1006/anbe.2000.1482. [DOI] [PubMed] [Google Scholar]
- Licht P, McCreery BR, Barnes R, Pang R. Seasonal and stress related changes in plasma gonadotropins, sex steroids and corticosterone in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol. 1983;50:124–145. doi: 10.1016/0016-6480(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Gonadal steroids vary with reproductive stage in a tropically breeding female Anuran. Gen Comp Endocrinol. 2005;143:51–66. doi: 10.1016/j.ygcen.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Rand AS, Ryan MJ, Wilczynski W. Plasticity in female mate choice associated with changing reproductive states. Anim Behav. 2005;69:689–699. [Google Scholar]
- McGlothlin JW, Neudorg DLH, Casto JM, Nolan V, Ketterson ED. Elevated testosterone reduces choosiness in female dark-eyed juncos (Junco hyemalis): evidence for a hormonal constraint on sexual selection? Proc R Soc London, Ser B. 2004;271:1377–1384. doi: 10.1098/rspb.2004.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina MF, Ramos I, Crespo CA, González-Calvar S, Fernández SN. Changes in serum sex steroid levels throughout the reproductive cycle of Bufo arenarum females. Gen Comp Endocrinol. 2004;136:143–151. doi: 10.1016/j.ygcen.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Milinski M, Bakker TCM. Costs influence sequential mate choice in stickleback, Gasterosteus aculeatus. Proc R Soc London, Ser B. 1993;250:229–233. [Google Scholar]
- Moore PJ, Moore AJ. Reproductive aging and mating: the ticking of the biological clock in female cockroaches. Proc Natl Acad Sci U S A. 2001;98:9171–9176. doi: 10.1073/pnas.161154598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. Hormonal control of receptivity in female quail (Coturnix coturnix japonica) Horm Behav. 1973;4:61–72. [Google Scholar]
- Penton-Voak IS, Perrett DI, Castles D, Burt M, Koyabashi T, Murray LK. Female preferences for male faces changes cyclically. Nature. 1999;399:741–742. doi: 10.1038/21557. [DOI] [PubMed] [Google Scholar]
- Pierantoni R, Iela L, Delrio G, Rastogi RK. Seasonal plasma sex steroid levels in the female Rana esculenta. Gen Comp Endocrinol. 1984;53:126–134. doi: 10.1016/0016-6480(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Poulin R. Mate choice decisions by parasitized upland bullies, Gobiomorphus breviceps. Proc R Soc London, Ser B. 1994;256:183–187. [Google Scholar]
- Proctor HC. Courtship in the water mite Neumania papillator: males capitalize on female adaptations for predation. Anim Behav. 1991;42:589–598. [Google Scholar]
- Pruett-Jones SG. Independent versus non-independent mate choice: do females copy each other? Am Nat. 1992;140:1000–1009. doi: 10.1086/285452. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom A, Pärt T, Sheldon BC. Adaptive plasticity in mate preferences linked to differences in reproductive effort. Nature. 2000;405:344–347. doi: 10.1038/35012605. [DOI] [PubMed] [Google Scholar]
- Rand AS, Ryan MJ. The adaptive significance of a complex vocal repertoire in a neotropical frog. Zeitschrift für Tierpsychology. 1981;57:209–214. [Google Scholar]
- Rand AS, Ryan MJ, Wilczynski W. Signal redundancy and receiver permissiveness in acoustic mate recognition by the túngara frog, Physalaemus pustulosus. Am Zool. 1992;32:81–90. [Google Scholar]
- Rand AS, Bridarolli ME, Dries L, Ryan MJ. Light levels influence female choice in túngara frogs: predation risk assessment? Copeia. 1997;2:447–450. [Google Scholar]
- Real LA. Search theory and mate choice: I. Models of single-sex discrimination. Am Nat. 1990;136:376–405. [Google Scholar]
- Rhen T, Crews D. Organization and activation of sexual and agonistic behavior in the leopard gecko, Eublepharis macularius. Neuroendocrinology. 2000;71:252–261. doi: 10.1159/000054543. [DOI] [PubMed] [Google Scholar]
- Rhen T, Ross J, Crews D. Effects of testosterone on sexual behavior and morphology in adult female leopard geckos, Eublepharis macularius. Horm Behav. 1999;36:119–128. doi: 10.1006/hbeh.1999.1530. [DOI] [PubMed] [Google Scholar]
- Rhen T, Sakata JT, Zeller M, Crews D. Sex steroid levels across the reproductive cycle of female leopard geckos, Eublepharis macularius, from different incubation temperatures. Gen Comp Endocrinol. 2000;118:322–331. doi: 10.1006/gcen.2000.7477. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. Female mate choice in a neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. The Túngara Frog: a Study in Sexual Selection and Communication. University of Chicago Press; Chicago: 1985. [Google Scholar]
- Ryan MJ. Sexual selection and mate choice. In: Krebs JR, Davies NB, editors. Behavioral Ecology, An Evolutionary Approach. Blackwell: Oxford; 1997. pp. 179–202. [Google Scholar]
- Ryan MJ, Rand AS. The sensory basis of sexual selection for complex calls in the túngara frog, Physalaemus pustulosus (sexual selection for sensory exploitation) Evolution. 1990;44:305–314. doi: 10.1111/j.1558-5646.1990.tb05200.x. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Rand AS. In: Feature weighting in signal recognition and discrimination by túngara frogs. Ryan MJ, editor. Anuran Communication Smithsonian Institution; Washington, DC: 2001. pp. 86–101. [Google Scholar]
- Ryan MJ, Rand W, Hurd PL, Phelps SM, Rand AS. Generalization in response to mate recognition signals. Am Nat. 2003;161:380–395. doi: 10.1086/367588. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Mating call phonotaxis in the female American toad: induction by hormones. Gen Comp Endocrinol. 1984;55:150–156. doi: 10.1016/0016-6480(84)90139-4. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Prostaglandin-induced mating call phonotaxis in female American toad: facilitation by progesterone and arginine vasotocin. J Comp Physiol, A. 1985;156:823–829. [Google Scholar]
- Simmons LW. Courtship role reversal in bush crickets: another role for parasites? Behav Ecol. 1994;5:259–266. [Google Scholar]
- Slagsvold T, Lifjeld TJ, Stenmark G, Breiehagen T. On the cost of searching for a mate in female pied flycatchers Ficedula hypoleuca. Anim Behav. 1988;36:433–442. [Google Scholar]
- Sullivan MS. Mate choice as an information gathering process under time constraint: implications for behaviour and signal design. Anim Behav. 1994;47:141–151. [Google Scholar]
- Tetel MJ, Getzinger MJ, Blaustein JD. Estradiol and progesterone influence the response of ventromedial hypothalamic neurons to tactile stimuli associated with female reproduction. Brain Res. 1994;646:267–272. doi: 10.1016/0006-8993(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Thornhill R. Alternative female choice tactics in the scorpionfly Hylobittacus apicalis (Mecoptera) and its implications. Am Zool. 1984;24:367–383. [Google Scholar]
- Veen T, Borge T, Griffith SC, Sætre GP, Bures S, Gustafsson L, Sheldon BC. Artificial hybridization and adaptive mate choice in flycatchers. Nature. 2001;411:45–50. doi: 10.1038/35075000. [DOI] [PubMed] [Google Scholar]
- Wells KD. The social behaviour of anuran amphibians. Anim Behav. 1977;25:666–693. [Google Scholar]
- Wilczynski W, Rand AS, Ryan MJ. The processing of spectral cues by the call analysis of the túngara frog, Physalaemus pustulosus. Anim Behav. 1995;49:911–929. [Google Scholar]
- Wilczynski W, Rand AS, Ryan MJ. Female preference for temporal order of call components in the túngara frog: a Bayesian analysis. Anim Behav. 1999;58:841–851. doi: 10.1006/anbe.1999.1208. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Yang EJ, Simmons D. Sex differences and hormone influences on tyrosine hydroxylase immunoreactive cells in the leopard frog. J Neurobiol. 2003;56:54–65. doi: 10.1002/neu.10228. [DOI] [PubMed] [Google Scholar]