Abstract
We recently reported the use of matrix-assisted laser desorption ionization (MALDI) Fourier transformation mass spectrometry (FTMS) techniques to identify unique glycan markers in ovarian cancer cell lines which may be biomarkers for diagnosis of ovarian cancer. Glycan markers and CA125 levels are compared in a series of ovarian cancer patients and normal control subjects. Oligosaccharides (OS) were cleaved from the serum glycoproteins and isolated using solid phase extraction. MALDI–FTMS was then used to identify unique mass spectrometry (MS) peaks. Sensitivity, specificity, and the area under the receiver operating characteristic (ROC) curve were calculated to measure the test performance of glycan markers. Sixteen unique OS MS signals were identified in ovarian cancer patient sera. Their additive mass/charge intensities were used to determine their presence or absence. The ovarian cancer patients varied in their disease status, with initial cancer stages ranging from IC to IV. Forty-four of 48 patients had detectable OS signals, with CA125 values between 2 and 17,044. Four patients had undetectable signals and their CA125 ranged between 7 and 10. Twenty-three of 24 control subjects had no detectable glycan markers, with CA125 levels between 10 and 64. Sensitivity and specificity values were determined to be 91.6% and 95.8%, respectively. The area under the ROC curve for all 72 samples was 0.954 (95% CI: 0.896, 1.0) using the glycomics assay, which was superior to CA125 in discriminating between cases and controls. This preliminary study suggests that glycomics profiling may be useful for the detection of ovarian cancer.
Keywords: biomarkers, detection, glycomics, mass spectrometry, oligosaccharides, ovarian cancer
Ovarian cancer is the most deadly of the gynecological malignancies since the majority of the afflicted patients present with advanced-staged disease. There is a marked difference in the 5-year survival between patients with Stage I–II ovarian cancer (80–95%) compared to Stages III–IV (15–30%)(1). Unfortunately, there are no currently effective strategies for early detection, despite repeated efforts to use ultrasound imaging and tumor markers such as CA125, either singly or in combination(2).
The primary tumor marker for epithelial ovarian cancer (EOC) is the CA125 serum assay(3). CA125 is elevated in only 50% of Stage I cancers and about 80–90% of patients with advanced-stage disease. It is a nonspecific test, and is commonly elevated in various benign conditions such as uterine fibroids, endometriosis, and pelvic inflammatory disease. Its primary indication in clinical use is to monitor ovarian cancer patients for a response to treatment following surgery and chemotherapy and to provide surveillance for possible recurrence. Unfortunately, its lack of sensitivity and specificity has made it unsuitable for use by itself in ovarian cancer screening.
We recently reported a new glycomics assay to identify abnormal oligosaccharides (OS) present on the glycoproteins of ovarian cancer cell lines and human serum from ovarian cancer patients(4). Glycosylation of protein antigens on cancer cells is markedly different compared to normal cells(5–7). The glycoproteins are not only overexpressed but are aberrantly glycosylated. We have shown that mass spectrometry can be used to detect a unique profile of OS that have been stripped from their underlying glycoproteins in four ovarian cancer cell lines (Caov-3, OVCAR-3, SK-OV-3, and ES-2)(4). These cell lines differentially express various mucins, such as MUC 1 and CA125 (MUC 16). However, all of the ovarian cancer cell lines had similar spectra of oligosaccharides when evaluated by MALDI–FT (matrix-assisted laser desorption/ionization; Fourier transformation) mass spectrometry.
A preliminary analysis of human serum samples of 18 ovarian cancer patients and 8 healthy controls showed a similar spectrum of unique mass/charge (M/Z) peaks on mass spectrometry. Consequently, we have extended our analysis to a larger set of ovarian cancer patients and controls. We also compared this glycomics assay to the standard CA125 to evaluate which assay is better able to discriminate between ovarian cancer patients and healthy female controls.
Materials and methods
The Institutional Research Board of the University of California, Davis Medical Center gave approval for this investigation. Excess serum samples from the Clinical Laboratory in the Department of Pathology at UC Davis Medical Center were obtained for both the experimental and control arms. Frozen serum samples from female patients that had been previously assayed for the CA125 serum test were identified and collected. The medical charts of these patients were reviewed, and those with an active or previous history of either invasive EOC or primary peritoneal cancer were selected for the experimental arm. The control samples were selected frozen serum samples of female patients who had tests for other than CA125. The medical charts of these patients were also reviewed, and only those patients who did not have a history of ovarian or primary peritoneal cancers or any other malignancy and did not have significant medical or surgical problems were selected as controls. These control serum samples were subsequently tested for CA125 serum levels (Abbott Laboratories, Abbott Park, IL).
Among the experimental specimens, samples were divided into two groups based on CA125 serum levels: less than 35 IU/mL (low CA125) and greater than 150 (high CA125). The medical charts of the cancer patients were abstracted for patient age, cancer type (ovarian vs primary peritoneal), histology, grade, stage, clinical course (eg, preoperative, postoperative, receiving chemotherapy, under surveillance after treatment, and recurrent cancer).
Glycomics assay
Release of OS by β-elimination
This methodology has been previously presented(4). Frozen serum samples were thawed and 1 mL of each sample was extensively dialyzed against Milli-Q (Millipore, Billerica, MA) water at 4°C for 12–16 h. The samples were then lyophilized and kept frozen until needed. Subsequently, alkaline borohydride solution (500 μL, mixture of 1.0 M sodium borohydride and 0.1 M sodium hydroxide) was added to 2–3 mg of lyophilized serum material. The mixture was incubated at 42°C for 12 h in a water bath. After reaction, 1.0 M hydrochloric acid solution was added to the samples to stop the reaction and destroy excess sodium borohydride.
OS purification using a graphitized carbon–solid phase extraction
O-linked OS released by reductive elimination were purified by solid phase extraction using a graphitized carbon cartridge. The cartridge was washed with H2O followed by 0.05% (v/v) trifluoroacetic acid in 80% acetonitrile in water (ACN in H2O) (v/v). The solution of released OS was loaded to the cartridge. Subsequently, the cartridge was washed with nanopure water at a flow rate of about 1 mL/min to remove salts and buffer. O-linked glycans were eluted with 10% ACN in H2O, 20% ACN in H2O, and 40% ACN in 0.05% trifluoroacetic acid in H2O. Each fraction was collected and concentrated in vacuo prior to MALDI analysis.
Mass spectrometric analysis
Mass spectra were recorded on an external source HiResMALDI (IonSpec Corporation, Irvine, CA) equipped with a 7.0 T magnet. The HiResMALDI was equipped with a pulsed Nd: YAG laser (266 nm). 2,5-dihydroxy-benzoic acid and 2,5-dihydroxy-acetophenon were used as a matrix (5 mg/100 μL in 50% ACN in H2O) for positive and negative mode, respectively. A saturated solution of NaCl in 50% ACN in H2O was used as a cation dopant. The OS solution (1 μL) was applied to the MALDI probe followed by matrix solution (1 μL). For the negative ion spectra, only 2,5-dihydroxy-acetophenon matrix was used without using any dopant. The sample was dried under a stream of air prior to mass spectrometric analysis.
Unique OS markers in ovarian cancer patient sera have been previously identified and are referred to as either as “F-sugars” or “F-OS”(4). The additive intensities of all the “F-sugars” identified in the mass spectrometry analysis of the serum samples were used as a measure of the glycans biomarkers. An arbitrary intensity cutoff (0.465) was used to determine if the glycans assay was positive or negative.
Statistical analysis
The test performance of the identified OS biomarkers and CA125 serum levels for the diagnosis of ovarian cancer was evaluated by descriptive statistics such as sensitivity and specificity and receiver operating characteristic (ROC) curves. All of the statistical analyses were performed using STATA 8.0 (StataCorp LP, College Station, TX).
Results
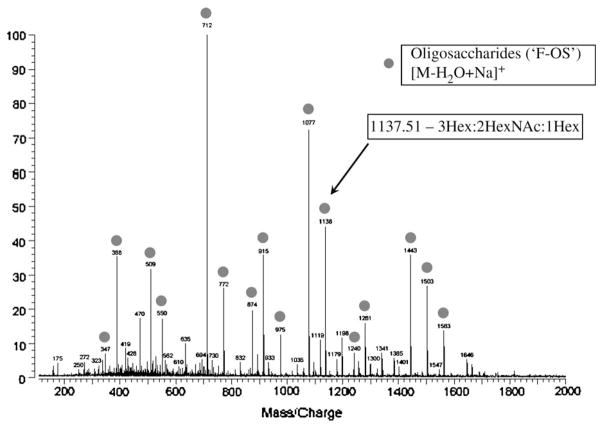
In our initial study of using glycans analysis of ovarian cancer patient sera, 16 (M/Z) values representing unique glycan species were identified on mass spectrometry based on their discriminatory pattern. The sum of the relative intensities of 16 selected M/Z values was proposed to identify patients with ovarian cancer. Table 1 shows the masses of the unique OS (“F-sugars” or “F-OS”) and their underlying structures as determined by infrared multiphoton dissociation(4). Figure 1 shows a representative example of the glycans analysis of an ovarian cancer patient serum sample using MALDI–FT with the unique F-OS’s identified by circles. Based on an earlier pilot study (An, 2006 #71), we identified a cutoff value of 0.465 for the additive intensities of the M/Z values to discriminate a positive from a negative result for the glycomics assay.
Table 1.
List of unique glycan markers in the serum of ovarian cancer patients as identified with mass spectrometrya
| Observed mass | OS composition |
|---|---|
| 347.10 | 2Hex |
| 388.14 | 1HexNAc:1Hex |
| 509.17 | 3Hex |
| 550.21 | 1HexNAc:2Hex |
| 712.28 | 3Hex:1HexNAc |
| 772.31 | 2Hex:1HexNAc:1Hex* |
| 874.36 | 4Hex:1HexNAc |
| 915.38 | 3Hex:2HexNAc |
| 975.43 | 2Hex:2HexNAc:1Hex* |
| 1077.47 | 4Hex:2HexNAc |
| 1137.51 | 3Hex:2HexNAc:1Hex* |
| 1239.57 | 5Hex:2HexNAc |
| 1280.62 | 4Hex:3HexNAc |
| 1442.72 | 5Hex:3HexNAc |
| 1502.74 | 4Hex:3HexNAc:1Hex* |
| 1562.78 | 3Hex:HexNAc:2Hex* |
Hex* represents a modified sugar on a hexose. The head groups of some of the “F-sugars” are incompletely characterized as yet.
Figure 1.
Mass spectrometry pattern of unique ovarian cancer serum glycan markers using MALDI–FT. See text for description of methodology. Each circle represents a unique glycan marker (called “F-sugars”) with the general OS structure. One peak is identified with an arrow and its specific structure is listed.
Serum samples from 48 ovarian cancer patients and 24 healthy control subjects were selected for the glycans analysis. The ovarian cancer patient samples were chosen primarily for their CA125 levels, with 23 samples that had a CA125 less than 35 U/mL (low CA125) and 25 samples greater than 150 (high CA125). The ovarian cancer patients were a very heterogeneous group at various phases in their disease course including: postoperative patients, those receiving chemotherapy, those with recurrent cancer, and those under surveillance. The initial cancer stages ranged from Stage IC to IV. Forty-four of 48 patients had “F-OS” values above an arbitrary cutoff (0.465), with corresponding CA125 values between 2 and 17,044. Four patients were below the cutoff and their CA125 ranged between 7 and 10. Twenty-three of 24 control subjects had “F-OS” below cutoff, with CA125 levels between 10 and 64.
The results of all ovarian cancer patients and healthy control subjects for the glycomics assay and CA125 serum levels were subjected to logarithmic transformation to allow for comparison. Figure 2 shows the distribution of the transformed CA125 serum values and the glycans assay by histograms for the combined ovarian cancer and normal control patients. The CA125 values show considerable overlap between the ovarian cancer patients and healthy controls, meaning that CA125 is largely unable to distinguish between the two groups. In contrast, the glycans assay results show an obvious demarcation in values between the two groups, although the separation is not perfect.
Figure 2.
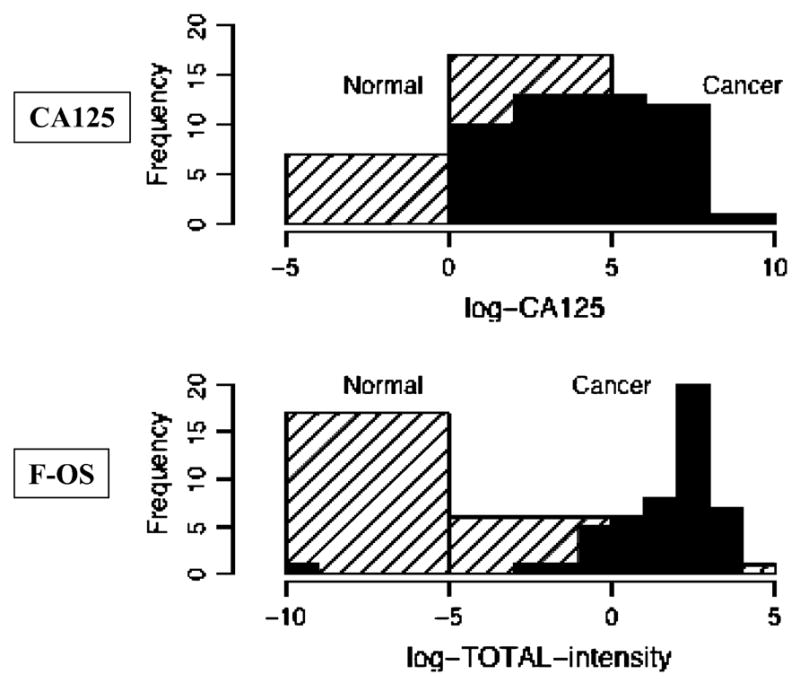
Histograms comparing the results of healthy control subjects vs ovarian cancer patients using either CA125 serum levels or the glycans assay results. Both the CA125 serum levels and the glycans assay results have undergone logarithmic transformation to allow for comparison. When the two groups are compared using the CA125 results, there is a large overlap of similar values. In contrast, when the two groups are compared using the glycans assay results the values segregate more distinctly.
A statistical tool to evaluate the test performance of biomarker in the detection of disease is the ROC curves. In Figure 3, we compared the ROC curves for the sum of the relative intensities of the selected OS and for the CA125 values. The area under the ROC curve for the F-OS is 0.95 with 95% binomial exact confidence interval (0.88, 0.99). The area under the ROC curve for the CA125 is 0.67 with 95% confidence interval (0.57, 0.79). The performance of the glycans profile is superior to the CA125 values in terms of the area under the ROC curve (P < 0.0001). For comparison, ROC curves with an area under the curve of 0.50–0.75 are considered to have fair discrimination, 0.75–0.92 good, 0.92–0.97 very good, and 0.97–1.0 excellent(8). When 0.465 is used as the cut-point for the sum of the relative intensities of the selected OS, 93.1% (67 out of 72) patients were correctly classified as either ovarian cancer or not ovarian cancer. This gives a sensitivity of 91.6% and specificity of 95.8%.
Figure 3.
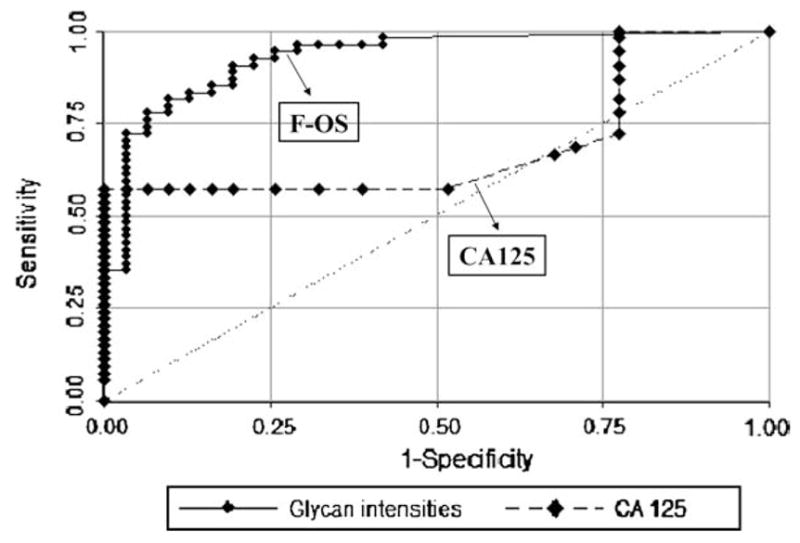
ROC curves for all samples (ovarian cancer and healthy controls) using glycomics assay and CA125 results. The ROC curve represents a trade-off between sensitivity and specificity. The greater the area under the curve, the better the test performance of the assay. The area under the curve for the glycomics assay is 0.954 with a 95% confidence interval (0.896, 1.0). The area under the ROC curve for the CA125 results is 0.67 with 95% confidence interval (0.57, 0.79). The performance of the glycans profile is superior to the CA125 values in terms of the area under the ROC curve (P < 0.0001).
Discussion
The recognition that glycoproteins are aberrantly glycosylated in ovarian cancer cells compared to normal cells provided the impetus to investigate if there was a characteristic pattern to these changes. As previously demonstrated, at least 16 unique OS peaks can be identified in the serum of ovarian cancer patients using mass spectrometry (MS) which parallel the results seen in four ovarian cancer cell lines(4). This set of glycan “fingerprints” lead to consideration for a possible ovarian cancer diagnostic test.
This preliminary study offers support for use of the glycans profiling to differentiate between ovarian cancer patients and healthy control subjects. The unique patterns of F-sugars are seen in the majority of the ovarian cancer patients (44 of 48 patients) and only 1 of 24 control patients. We were careful to select ovarian cancer patients with a spectrum of both high and low CA125 values. Our impression is that the glycans profile does identify ovarian cancer patients independent of CA125 serum levels. In fact, our results demonstrated that the glycans profile had better sensitivity and specificity to distinguish between ovarian cancer patients and controls compared to CA125, using the ROC curves.
It has been long recognized that CA125 is neither particularly sensitive nor specific for identification of ovarian cancer patients. The detection rate for ovarian cancer patients is enhanced when multiple protein tumor markers are incorporated(9–13), including CA125, M-CSF, OVX1, mesothelin marker, osteopontin, CA15-3, CA72-4, and others. This likely reflects the heterogeneity of cell surface proteins on ovarian cancer cells such that any single tumor marker will not be present on all cancer cells.
Many of these cell surface proteins are mucins. Mucins are large glycoproteins, which are known to be abnormally expressed in various adenocarcinomas(7,14–16). Ovarian cancer cell lines are known to express both MUC 1 and MUC 16(14). MUC 16 is the recently cloned gene for CA125(17,18). The differential expression of these mucins is not uniform in EOC. For example, MUC 1 was expressed in seven of seven ovarian cancer cell lines tested, but CA125 was positive in only 2(14). Complicating the detection of serum protein markers is that some antibodies do not detect all the protein epitopes. For example, antibodies against the CA125 antigen are divided into three families: OC 125-like, M11-like, and Ov197-like(18). The commercial assay CA125 II uses the epitopes OC 125 and M11(18). This may help to explain why serum CA125 fails to detect many ovarian cancer cases.
Glycosylation is the most common form of post-translational modification, occurring in at least 50% of human proteins(19). Unlike proteins, where differences in disease states may increase or decrease the protein content to some degree, changes in glycosylation are more pronounced(20). In cancer tissues, the OS are shorter and contain more sialic acids(6). The mucins from cancer cells are not only overexpressed (see above), but are also aberrantly glycosylated(5,15,21,22). Therefore, monitoring changes in glycosylation may be a more specific and sensitive method for detecting cancer than indirect methods using monoclonal antibodies that recognize a limited number of protein antigens.
The glycomics approach involves harvesting the glycans from biologic fluids without regard for the proteins involved. The glycans are harvested, preconcentrated, and fractionated into differing polarity solvents. The mixture in each fraction is mass-profiled using MALDI–FT mass spectrometry. Extensive chromatography is avoided because the glycan composition can be determined solely on the mass. As noted in Figure 1 and Table 1, the profiled OS have already been characterized. These mass profiles provide a rapid method for biomarker discovery with potential sensitivity and specificity to detect ovarian cancer.
Our study is clearly limited by the use of retrospectively collected samples and the limited clinical information available on the ovarian cancer patients. We are currently evaluating the glycomics assay using prospectively collected serum samples from well-characterized ovarian cancer patients and healthy controls. We do not yet know if the glycans profiles are altered by cancer characteristics such as grade, stage, histology, or other factors. Similarly, it is not known if nonmalignant conditions can modify the different glycans species in ways that might limit their diagnostic utility similar to problems with CA125 values (eg, endometriosis and other benign gynecological conditions). Nonetheless, this current data suggests the potential value of glycans profiling for detection of ovarian cancer and clearly warrants further investigation.
Acknowledgments
Financial support for this investigation came from a grant from the UC Davis Health Systems Research Award.
References
- 1.Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005;50:426–38. [PubMed] [Google Scholar]
- 2.Hensley ML, Castiel M, Robson ME. Screening for ovarian cancer: what we know, what we need to know. Oncology (Williston Park) 2000;14:1601–7. 1608, 1613–6. [PubMed] [Google Scholar]
- 3.Meyer T, Rustin GJ. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer. 2000;82:1535–8. doi: 10.1054/bjoc.2000.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An HJ, Miyamoto S, Lancaster KS, et al. Profiling of glycans in serum for the discovery of potential biomarkers for ovarian cancer. J Proteome Res. 2006;5:1626–35. doi: 10.1021/pr060010k. [DOI] [PubMed] [Google Scholar]
- 5.Gorelik E, Galili U, Raz A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 2001;20:245–77. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- 6.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 7.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 8.Pepe MS. The statistical evaluation of medical tests for classification and prediction. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 9.McIntosh MW, Drescher C, Karlan B, et al. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol Oncol. 2004;95:9–15. doi: 10.1016/j.ygyno.2004.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schorge JO, Drake RD, Lee H, et al. Osteopontin as an adjunct to CA125 in detecting recurrent ovarian cancer. Clin Cancer Res. 2004;10:3474–8. doi: 10.1158/1078-0432.CCR-03-0365. [DOI] [PubMed] [Google Scholar]
- 11.Woolas RP, Xu FJ, Jacobs IJ, et al. Elevation of multiple serum markers in patients with stage I ovarian cancer. J Natl Cancer Inst. 1993;85:1748–51. doi: 10.1093/jnci/85.21.1748. [DOI] [PubMed] [Google Scholar]
- 12.Mor G, Visintin I, Lai Y, et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:7677–82. doi: 10.1073/pnas.0502178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skates SJ, Horick N, Yu Y, et al. Preoperative sensitivity and specificity for early-stage ovarian cancer when combining cancer antigen CA-125II, CA 15–3, CA 72–4, and macrophage colony-stimulating factor using mixtures of multivariate normal distributions. J Clin Oncol. 2004;22:4059–66. doi: 10.1200/JCO.2004.03.091. [DOI] [PubMed] [Google Scholar]
- 14.Stimpfl M, Schmid BC, Schiebel I, et al. Expression of mucins and cytokeratins in ovarian cancer cell lines. Cancer Lett. 1999;145:133–41. doi: 10.1016/s0304-3835(99)00246-3. [DOI] [PubMed] [Google Scholar]
- 15.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 16.Hanisch FG, Stadie TR, Deutzmann F, Peter-Katalinic J. MUC1 glycoforms in breast cancer–cell line T47D as a model for carcinoma-associated alterations of 0-glycosylation. Eur J Biochem. 1996;236:318–27. doi: 10.1111/j.1432-1033.1996.00318.x. [DOI] [PubMed] [Google Scholar]
- 17.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–5. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 18.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737–40. doi: 10.1002/ijc.10250. [DOI] [PubMed] [Google Scholar]
- 19.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 20.Block TM, Comunale MA, Lowman M, et al. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci U S A. 2005;102:779–84. doi: 10.1073/pnas.0408928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dall’Olio F, Chiricolo M, Mariani E, Facchini A. Biosynthesis of the cancer-related sialyl-alpha 2,6-lactosaminyl epitope in colon cancer cell lines expressing beta-galactoside alpha 2,6-sialyltransferase under a constitutive promoter. Eur J Biochem. 2001;268:5876–84. doi: 10.1046/j.0014-2956.2001.02536.x. [DOI] [PubMed] [Google Scholar]
- 22.Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol. 1996;179:358–69. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]