Abstract
A disulphide relay system mediates the import of cysteine-containing proteins into the intermembrane space of mitochondria. This system consists of two essential proteins, Mia40 and Erv1, which bind to newly imported proteins by disulphide transfer. A third component, Hot13, was proposed to be important in the biogenesis of cysteine-rich proteins of the intermembrane space, but the molecular function of Hot13 remained unclear. Here, we show that Hot13, a conserved zinc-binding protein, interacts functionally and physically with the import receptor Mia40. It improves the Erv1-dependent oxidation of Mia40 both in vivo and in vitro. As a consequence, in mutants lacking Hot13, the import of substrates of Mia40 is impaired, particularly in the presence of zinc ions. In mitochondria as well as in vitro, Hot13 can be functionally replaced by zinc-binding chelators. We propose that Hot13 maintains Mia40 in a zinc-free state, thereby facilitating its efficient oxidation by Erv1.
Keywords: disulphide relay, Erv1, Mia40, mitochondria, protein translocation
Introduction
Many proteins of the intermembrane space (IMS) of mitochondria are imported by a distinct machinery that mediates protein import by an oxidation-driven process (Tokatlidis, 2005; Koehler et al, 2006; Herrmann & Köhl, 2007; Hell, 2008; Stojanovski et al, 2008). Two components of this machinery were recently identified: Mia40 and Erv1. Mia40 functions as a receptor protein in the IMS. In its oxidized form, it binds to incoming polypeptides by the formation of transient disulphide bonds (Chacinska et al, 2004; Naoe et al, 2004). As a result of isomerization, the imported proteins are released presumably in an oxidized, stably folded conformation (Allen et al, 2005; Mesecke et al, 2005; Müller et al, 2008). Erv1 is a sulphhydryl oxidase that interacts directly with Mia40 and maintains it in an oxidized and active conformation (Lange et al, 2001; Mesecke et al, 2005).
In addition to Mia40 and Erv1, the soluble IMS protein Helper of Tim of 13 kDa, Hot13, is important in the biogenesis of the small Tim proteins (Curran et al, 2004). In Saccharomyces cerevisiae, the deletion of Hot13 does not cause severe growth defects on fermentable or non-fermentable carbon sources. However, mutants show an increased sensitivity to the oxidizing agent t-butyl hydroperoxide. It was suggested that Hot13 is important in redox regulation in the IMS (Curran et al, 2004).
Here, we show that Hot13 is a zinc-binding protein that influences the redox state, and hence the activity, of Mia40. Our data suggest that Hot13 facilitates demetalation of Mia40 to improve the oxidation of Mia40 by Erv1.
Results
Hot13 is conserved among eukaryotes
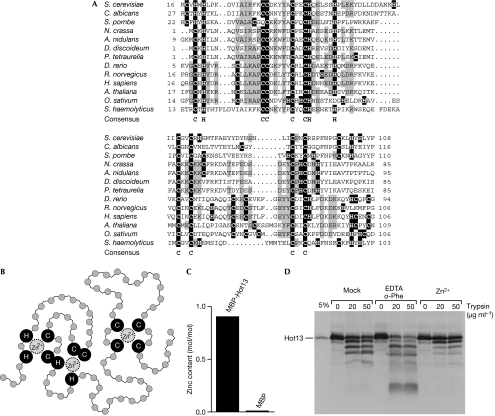
BLAST searches showed the presence of homologues of Hot13 that are ubiquitous in eukaryotes and in many bacteria. All homologues have a highly conserved cysteine-rich core domain of about 90 amino-acid residues (Fig 1A). This domain is characterized by the presence of nine invariant cysteine and three invariant histidine residues, and is referred to as the CHY zinc-finger domain (pfam05495; Marchler-Bauer et al, 2007). The domain is predicted to bind to up to three zinc ions (Fig 1B).
Figure 1.
Hot13 proteins form a ubiquitous protein family conserved among fungi, plants and animals. (A) Alignment of the conserved domain of Hot13 proteins. Cysteine and histidine residues are highlighted in black and conserved residues are shown in grey. The consensus line shows residues that are invariant among all Hot13 proteins. The proteins used here are: Saccharomyces cerevisiae (Hot13, AAS56591), Candida albicans (EAL02591), Schizosaccharomyces pombe (NP_596663), Neurospora crassa (EAA28094), Aspergillus nidulans (XP_001400209), Dictyostelium discoideum (EAL72440), Paramecium tetraurelia (XP_001445542), Danio rerio (AAH78283), Rattus norvegicus (AAH83739), Homo sapiens (AAH47393), Arabidopsis thaliana (BAE98773), Oryza sativum (NP_001050105) and Staphylococcus haemolyticus (YP_254069). The last protein sequence belongs to a Gram-positive bacterium and homologues of Hot13 are widely distributed in prokaryotes. (B) Model of zinc-ion coordination by conserved cysteine and histidine residues within Hot13 (pfam05495; Marchler-Bauer et al, 2007). (C) Zinc content in recombinant MBP-Hot13 and MBP as measured by inductively coupled plasma atomic emission spectroscopy. (D) Radiolabelled Hot13 was synthesized in reticulocyte lysate and pretreated in the absence or presence of 5 mM EDTA, 1 mM o-phenanthroline or 10 μM zinc acetate. Trypsin was added at the indicated concentrations for 30 min on ice. Hot13 was visualized by autoradiography. MBP, maltose-binding protein.
To test whether Hot13 can bind to zinc ions, we purified recombinant Hot13 from Escherichia coli and analysed the metal content by inductively coupled plasma atomic emission spectroscopy (Fig 1C). We found significant amounts of zinc in these samples (about 0.8 zinc ions per Hot13 molecule) but virtually no copper (data not shown), thus confirming the zinc-binding capacity of Hot13. The zinc-binding ability of Hot13 was confirmed by a trypsin accessibility assay with in vitro synthesized, radiolabelled Hot13 (Fig 1D). In the presence of trypsin, Hot13 was partly degraded. The addition of metal-binding chelators considerably increased the protease sensitivity of Hot13, leading to the formation of several specific fragments. By contrast, the addition of zinc acetate stabilized Hot13.
Hot13 influences the redox state of Mia40
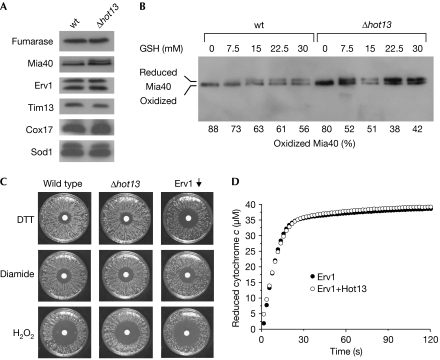
Hot13 was reported to be necessary for the import of small Tim proteins into the IMS (Curran et al, 2004), which is a process that relies on the redox state of Mia40 (Mesecke et al, 2005). HOT13 deletion mutants showed unaltered levels of Mia40 and Erv1 (Fig 2A), but when compared with wild type, the reduced form of Mia40 was more abundant. To determine more explicitly the relevance of Hot13 for the redox state of Mia40, we incubated wild-type and Δhot13 mitochondria with increasing concentrations of the physiological reductant glutathione. In Δhot13 mitochondria, but not in wild type, low concentrations of glutathione (7.5 mM) led to considerable reduction of Mia40 (Fig 2B).
Figure 2.
Hot13 modulates the redox state of Mia40. (A) Proteins in 100 μg of wild-type (wt) and Δhot13 mitochondria were resolved on a non-reducing SDS gel. Steady-state levels of the matrix protein fumarase, of the inner membrane protein Mia40, and the intermembrane space (IMS) proteins Erv1, Sod1, Cox17 and Tim13 were detected by western blotting. (B) Mitochondria were incubated with various concentrations of glutathione (GSH) for 10 min before free thiols were trapped with N-ethylmaleimide. The redox state of Mia40 was analysed by non-reducing SDS–PAGE. (C) Wild-type, Δhot13 and GAL-ERV1 cells were grown to log phase. Equal amounts of cells were spread onto glucose-containing plates. Filter discs were placed onto the cell lawn that was soaked with 10 μl of 3 M DTT, 500 mM diamide or 10 M hydrogen peroxide, respectively. The plates were grown at 30°C for 2 days. (D) Oxidized cytochrome c (40 μM) was incubated with DTT (2 mM) and recombinant Erv1 (10 μM) in a cuvette in the presence or absence of 10 μM Hot13. The reduction of cytochrome c was monitored over time by absorbance spectroscopy at 550 nm. DTT, dithiothreitol; SDS–PAGE, SDS–polyacrylamide gel electrophoresis.
Next, we tested whether the hypo-oxidation of Mia40 in Δhot13 mitochondria is due to the decreased activity of Erv1. As depletion of Erv1 activity leads to a diminished tolerance to reducing agents such as dithiothreitol (DTT; Mesecke et al, 2005), we tested the DTT resistance of Hot13-deficient cells. However, we observed no changes either in the DTT sensitivity or in the sensitivity against oxidizing agents such as diamide or hydrogen peroxide (Fig 2C). Furthermore, Hot13 did not influence the Erv1-catalysed reduction of cytochrome c by DTT (Fig 2D). Thus, Hot13 is not important for DTT resistance and activity of Erv1, which suggests that Hot13 is generally not required for the oxidation of thiol groups in the IMS. Instead, Hot13 might interact more directly with Mia40 to counteract its reduction or to increase its reoxidation.
Hot13 interacts with Mia40 and stimulates its oxidation
To test whether Hot13 interacts physically with Mia40, we constructed a yeast strain expressing Hot13 with an amino-terminal octahistidinyl tag. On affinity chromatography, a small fraction of Mia40 was copurified with this His8-Hot13 variant (Fig 3A, arrows). Similarly, a fraction of Mia40 could be co-isolated with His8-Erv1, an established interaction partner of Mia40. However, Erv1 binds to Mia40 through disulphide bonds, which explains why some of the Mia40 proteins are shifted to a larger form on an SDS gel (Fig 3A, asterisk). When the lysis of mitochondria was performed in the presence of 10 mM β-mercaptoethanol, the disulphide bonds between Erv1 and Mia40 are broken and the amounts of co-isolated Mia40 are diminished. It should be noted that the interaction of Mia40 with Hot13 is not influenced significantly by the addition of reductant, suggesting that Mia40 and Hot13 do not interact through disulphide bonds. Control proteins of the IMS or the matrix, such as cytochrome c peroxidase or Mba1, were not copurified with Hot13 or Mia40 (Fig 3A, lower panels).
Figure 3.
Hot13 promotes oxidation of Mia40 in a direct and metal-dependent manner. (A) Mitochondria (500 μg) expressing untagged or octahistidinyl-tagged variants of Hot13 and of Erv1 were lysed with 1% Triton X-100, 300 mM NaCl, 10 mM imidazol, 1 mM phenylmethanesulphonyl fluoride and 50 mM sodium phosphate, pH 8.0. For the samples shown in the right panel, 10 mM β-mercaptoethanol was added to the extract to reduce disulphide bonds. The extract was cleared by centrifugation and applied to nickel affinity chromatography. After extensive washing, bound proteins were eluted and analysed by western blotting for the presence of Mia40. Western blots of the intermembrane space (IMS) protein cytochrome c peroxidase (CCPO) and of the matrix protein Mba1 acted as controls. Ten per cent of the extract used for the His8-Hot13 pulldown was loaded for control (T, total). The asterisk indicates mixed disulphides of Mia40 and Erv1 due to incomplete reduction by the SDS sample buffer. (B) Radiolabelled Hot13 was incubated with mitoplasts from a GAL-MIA40 strain grown either in the presence of galactose (Mia40↑) or glucose (Mia40↓). The mitoplasts were washed, and bound Hot13 protein was detected by autoradiography. (C) Wild-type and Δhot13 mitoplasts were incubated with 67 μM recombinant MBP-Hot13 or 25 mM EDTA and 10 mM o-phenanthroline (o-Phe) in the presence or absence of 100 μM zinc acetate (ZnAc) for 10 min at 25°C. Free thiols were trapped and the redox state of Mia40 was determined. (D) The influence of glutathione (GSH) on the redox state of Mia40 was assessed as described in Fig 2B with or without 25 mM EDTA and 10 mM o-Phe. (E) The graph shows the mean values and standard deviations of four independent glutathione titration experiments. MBP, maltose-binding protein.
A direct interaction of Hot13 with Mia40 was also supported by a second experiment: Hot13 bound efficiently to mitoplasts—that is, mitochondria in which the outer membrane was opened by hypotonic swelling (Fig 3B). Binding was partly dependent on Mia40 as only low amounts of Hot13 were recovered with mitoplasts in which Mia40 was downregulated (Fig 3B; Mia40, downward arrow). Although significant, the interaction of Hot13 with Mia40 in both pulldown assays is relatively weak. This suggests that Hot13 and Mia40 do not form a permanent complex but rather bind transiently to each other.
Next, we tested whether Hot13 influences the redox state of Mia40. To this end, we pretreated mitoplasts with glutathione to convert Mia40 to its reduced form. After removal of the glutathione, the mitoplasts were incubated in the absence or presence of purified Hot13. In the wild type, Hot13 did not affect the redox state of Mia40, but in Δhot13 mitochondria, it shifted Mia40 to its oxidized form, suggesting a direct function of Hot13 in the oxidation of Mia40 (Fig 3C). In this in vitro assay, the addition of zinc ions led to a significant reduction of Mia40, which was counteracted by purified Hot13 or the zinc-binding chelators EDTA and o-phenanthroline.
To test more carefully the effect of metal ions on the redox state of Mia40, we exposed mitochondria to various concentrations of glutathione in the presence or absence of chelators and analysed the redox state of Mia40 (Fig 3D,E). Again, the removal of metal ions restored the oxidation of Mia40 in Δhot13 mitochondria, suggesting a function of Hot13 in metal homeostasis in the IMS. Mia40 had been reported to bind to zinc ions (Terziyska et al, 2005), and it is conceivable that the binding of zinc ions to the thiol groups of Mia40 stabilizes the reduced form of the protein. Hot13 might influence the zinc binding of Mia40 and hence facilitate its reoxidation.
Hot13 stimulates oxidation of Mia40 in vitro
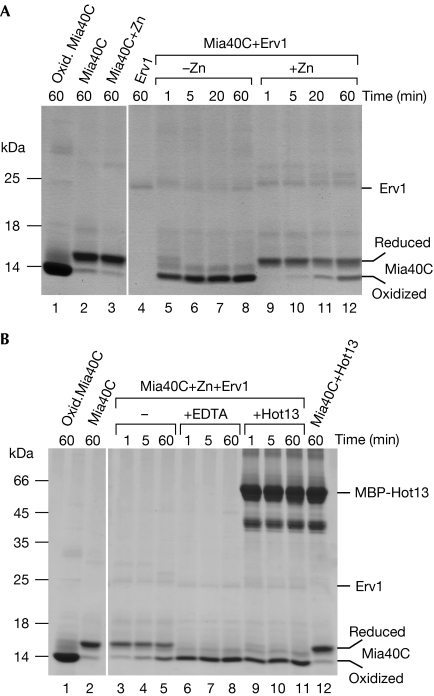
Recently, a reconstitution assay was developed that allowed the oxidation of Mia40 by Erv1 to be monitored (Grumbt et al, 2007). In this assay, purified Erv1 was incubated with the recombinantly expressed carboxy-terminal domain of Mia40 (Mia40C). The redox state of Mia40 was assessed by incubation of the protein with the alkylating agent 4-acetamido-4′-maleimidylstilbene-2,2′-disulphonic acid (AMS), which caused a size shift of the derivatized protein. As AMS reacts only with the reduced form of the redox-active cysteine pair of Mia40C, migration of the oxidized form of Mia40 was not affected (Fig 4A, lanes 1 and 2). On incubation with Erv1, Mia40C was rapidly oxidized (Fig 4A, lanes 5–8). By contrast, when Mia40C was incubated with zinc acetate before repurification by gel filtration, its oxidation by Erv1 was diminished and, even after 60 min, most of the Mia40C remained reduced (Fig 4A, lanes 9–12). We conclude that zinc binding stabilizes the reduced form of Mia40.
Figure 4.
Hot13 suppresses the inhibitory effect of zinc ions on the Erv1-mediated oxidation of Mia40. (A) The carboxy-terminal domain of Mia40 (Mia40C) was purified in its oxidized (Oxid.) form. The protein was reduced by incubation with 10 mM DTT and further incubated without (−Zn) or with (+Zn) 50 μM zinc acetate for 20 min at 25°C. DTT and free zinc ions were removed by gel filtration. Samples shown in lanes 5–12 were further incubated with purified Erv1 at 25°C for the time points indicated. In all samples, free thiol groups were derivatized with 15 mM 4-acetamido-4′-maleimidylstilbene-2,2′-disulphonic acid (AMS). Proteins were stained with Coomassie blue. (B) Mia40C was reduced and incubated with zinc as in (A) and re-isolated by gel filtration. It was further incubated in the presence or absence of 1 mM EDTA or purified maltose-binding protein (MBP)-Hot13. Erv1 was added at the indicated time points. All samples were treated with AMS and analysed as in (A). The concentrations of Mia40C, Hot13 and Erv1 were 50, 50 and 7 μM, respectively. DTT, dithiothreitol.
To test the influence of Hot13 on the oxidation of Mia40C, zinc-bound Mia40C was incubated with Erv1 in the absence or presence of EDTA or recombinant Hot13 (Fig 4B). Removal of the zinc ions from Mia40C by EDTA stimulated its Erv1-dependent oxidation (lanes 6–8 compared with lanes 3–5). Similarly, the presence of Hot13 allowed the rapid oxidation of Mia40C (lanes 9–11). As this assay contains only the three purified components, we consider it most likely that Hot13 interacts directly with Mia40 and converts it into the zinc-free form, which can then be rapidly oxidized by Erv1.
Hot13 promotes import in the presence of zinc ions
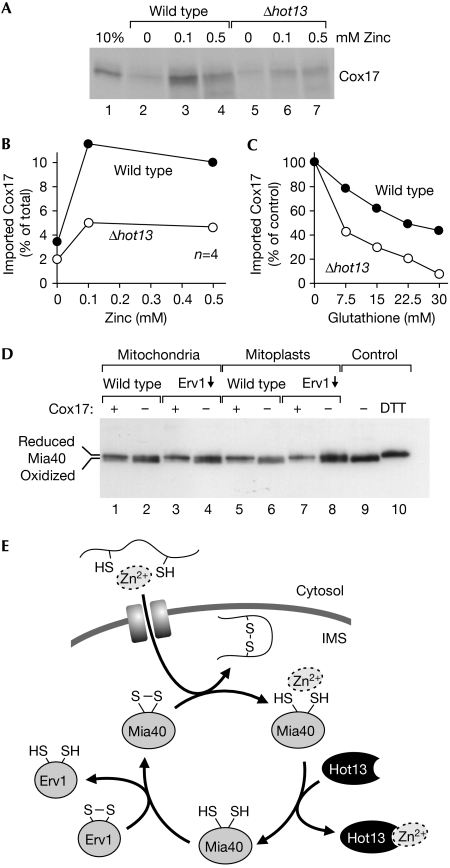
Next, we tested whether zinc ions affect the import of Cox17. To this end, we prepared radiolabelled recombinant Cox17 to challenge the import capacity with these ‘chemical amounts' of protein. Under these conditions, the amount of oxidized Mia40 is limiting for protein import (Mesecke et al, 2005). As shown in Fig 5A and B, the addition of zinc ions significantly improved the import efficiency: in the absence of zinc, only about 3% of the Cox17 protein was taken up by wild-type mitochondria, whereas in the presence of 100 μM zinc, more than 10% were imported. By contrast, import into Δhot13 mitochondria was significantly weaker and stimulated to a much lesser degree by zinc ions (Fig 5A, lanes 5–7). As we found that, especially in the presence of zinc ions, Mia40 is rapidly reduced by glutathione in Δhot13 mitochondria, we tested the effect of glutathione on the import of Cox17. As shown in Fig 5C, glutathione reduced the import of Cox17 considerably more in mutant mitochondria than in wild-type mitochondria. From this, we conclude that zinc has a stimulating effect on the import of Cox17 into mitochondria and, in parallel, increases the relevance of Hot13 for the import reaction.
Figure 5.
The import of purified Cox17 shifts Mia40 into its reduced conformation. (A) Recombinant Cox17 was expressed in the presence of 35S-sulphate and purified. Cox17 (12.5 pmol) was incubated with isolated wild-type or Δhot13 mitochondria in the presence of 14 mM glutathione and the indicated zinc concentrations at 25°C. After 10 min, the mitochondria were treated with proteinase K to remove non-imported protein, re-isolated, washed and subjected to SDS–PAGE. For a control, 10% of the Cox17 used per import reaction was loaded in lane 1. (B) The experiment shown in (A) was repeated four times. The radioactive signals were quantified and expressed in relation to the total Cox17 protein added per reaction. The mean values are shown. (C) Recombinant Cox17 was imported into mitochondria in the presence of 0.5 mM zinc and increasing concentrations of glutathione. After autoradiography, the imported protein was quantified and expressed in comparison with the amount of protein imported in the absence of glutathione. (D) Recombinant Cox17 was expressed in the presence of 200 μM zinc acetate and purified. The protein was incubated with mitochondria or mitoplasts from wild-type or Erv1-depleted yeast strains for 10 min. For a control, the samples were incubated with elution buffer. Samples were analysed as in Fig 2B. (E) Model for the influence of metal ions on protein import into the IMS. Imported proteins can be associated with zinc ions. These are passed through Mia40 to Hot13 and further to an unknown acceptor. Hot13-mediated demetalation of Mia40 presumably improves the reoxidation of the import receptor by Erv1. IMS, intermembrane space; SDS–PAGE, SDS–polyacrylamide gel electrophoresis.
Protein import into the IMS leads to a reduction of Mia40
It has been suggested that, during the import of proteins into the IMS, Mia40 is converted into its reduced form (Mesecke et al, 2005). However, the redox state of Mia40 after the import reaction had not been experimentally determined. Therefore, we performed import experiments with the Mia40 substrate Cox17. Recombinant Cox17 was purified in the presence of zinc to maintain it in a reduced import-competent state. Mitochondria of wild-type and Erv1-depleted cells were then incubated with the purified protein or mock-treated with the protein purification buffer. As shown in Fig 5D, incubation with Cox17 caused a significant shift of Mia40 to its reduced form. This shift was also observed when Cox17 was incubated with mitoplasts. These data show that the import of zinc-bound precursor proteins into the IMS leads to the reduction of Mia40, which is consistent with the proposed role of Mia40 in the oxidation of newly imported proteins.
Discussion
Hot13 was initially identified as an important component for the assembly of small Tim proteins in the IMS of mitochondria (Curran et al, 2004). Here, we show that Hot13 is a zinc-binding protein that influences the redox state of the import receptor Mia40. Hot13 interacts with Mia40 and promotes its efficient oxidation by Erv1. The observed hypo-oxidation of Mia40 in the absence of Hot13 might explain the impaired import efficiency of small Tim proteins (Curran et al, 2004).
The relevance of zinc ions for the biogenesis of small Tim proteins has been hotly debated in the past (Koehler et al, 2006). Zinc ions were shown to be important for the net translocation of small Tim proteins into the IMS (Lutz et al, 2003). Recently, it was suggested that precursors of newly synthesized, small Tim proteins bind to zinc already in the cytosol, which stabilizes the reduced import-competent conformation (Lu & Woodburn, 2005). Zinc might associate initially with precursors of small Tim proteins forming relatively stable, loosely folded intermediates (Ivanova et al, 2007). These might represent their translocation-competent state, which explains the observed stimulation of the import process by zinc ions.
From our observations, we propose a role of Hot13 in the Mia40-dependent import process, which is depicted in Fig 5E. After their import into the IMS, the precursor proteins are trapped by Mia40. During this interaction, the zinc ions are passed from the small Tim precursors to Mia40, thus stimulating the efficient oxidation of imported proteins. The zinc ions are further transferred to Hot13 to allow the efficient reoxidation of Mia40. According to this model, Hot13 functions as a metal chaperone that transfers zinc ions from Mia40 to an unknown acceptor, which might pump the zinc ions back into the cytosol or store them in the mitochondria. This model is supported by the observation that the import of chemical amounts of Cox17 is stimulated by zinc. However, in the absence of Hot13, the positive effect of zinc was much weaker, presumably because the inefficient removal of zinc from Mia40 slowed down the import process.
The role of Hot13 in demetalation of Mia40 might be specific, as we did not find altered activity of the copper zinc superoxide dismutase in the IMS of Δhot13 mitochondria (data not shown). However, the fact that homologues of Hot13 are present in bacteria that do not contain Mia40-like proteins indicates that, at least in prokaryotes, Hot13 is important in another Mia40-independent process. It will be exciting in the future to characterize the zinc transfer processes in the IMS of mitochondria in more detail.
Methods
Yeast strains. To generate the Δhot13 strain, the entire HOT13 reading frame was replaced by a KanMX6 cassette in the yeast wild-type strain W303 (Altmann et al, 2007). The His8-Hot13 strain was generated by genomic integration (Lafontaine & Tollervey, 1996). GAL-ERV1 and GAL-MIA40 mutants have been described previously (Mesecke et al, 2005). All strains were grown in liquid yeast extract-peptone (YP) or lactate medium (Altmann et al, 2007). For repression of the ERV1 or MIA40 genes, 2% or 0.1% glucose, respectively, was added to the medium. Mitochondria were isolated as described previously (Altmann et al, 2007).
Purification of recombinant proteins. The entire HOT13 sequence was cloned into the pMal-cRI vector (New England Biolabs, Ipswich, MA, USA) and was expressed as a fusion protein with maltose-binding protein in E. coli BL21 cells as described earlier (Lutz et al, 2003). Purification of Mia40C, the C-terminal fragment of Mia40 consisting of residues 284–403, and of Erv1 have been described earlier (Grumbt et al, 2007). Radiolabelled Cox17 was prepared as described previously (Mesecke et al, 2005).
Miscellaneous. The following techniques were carried out as described in previous articles: analysis of the redox state of Mia40 (Mesecke et al, 2005); Erv1 activity assays (Bihlmaier et al, 2007); determination of metal ions (Lutz et al, 2003); modification of free thiol groups by AMS (Grumbt et al, 2007). Radiolabelled proteins were synthesized in reticulocyte lysate according to the protocols of the manufacturer (Promega, Madison, WI, USA).
Acknowledgments
We thank Thomas Lisowsky for the bacterial strain expressing Erv1, Julia Zimmer and Vera Fritzinger for help with some experiments, and Helmut Hartl for metal detection assays by atomic emission spectroscopy. This study was supported by fellowships of the Bayerische Eliteförderung to N.M. and B.G., by the Fonds der Chemischen Industrie to K.B., and by grants from the Deutsche Forschungsgemeinschaft to K.H. and J.M.H. and the Stiftung für Innovation Rheinland-Pfalz to J.M.H.
Footnotes
The authors declare that they have no conflict of interest.
References
- Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K (2005) Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol 353: 937–944 [DOI] [PubMed] [Google Scholar]
- Altmann K, Dürr M, Westermann B (2007) Saccharomyces cerevisiae as a model organism to study mitochondrial biology. In Mitochondria. Practical Protocols, Leister D, Herrmann JM (eds) Vol 372, pp 81–90. Totowa, New Jersey, USA: Humana [DOI] [PubMed] [Google Scholar]
- Bihlmaier K, Mesecke N, Terzyiska N, Bien M, Hell K, Herrmann JM (2007) The disulfide relay system of mitochondria is connected to the respiratory chain. J Cell Biol 179: 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacinska A, Pfannschmidt S, Wiedemann N, Kozjak V, Sanjuan Szklarz LK, Schulze-Specking A, Truscott KN, Guiard B, Meisinger C, Pfanner N (2004) Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J 23: 3735–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Leuenberger D, Leverich EP, Hwang DK, Beverly KN, Koehler CM (2004) The role of Hot13p and redox chemistry in the mitochondrial TIM22 import pathway. J Biol Chem 279: 43744–43751 [DOI] [PubMed] [Google Scholar]
- Grumbt B, Stroobant V, Terziyska N, Israel L, Hell K (2007) Functional characterization of Mia40p, the central component of the disulfide relay system of the mitochondrial intermembrane space. J Biol Chem 282: 37461–37470 [DOI] [PubMed] [Google Scholar]
- Hell K (2008) The Erv1-Mia40 disulfide relay system in the intermembrane space of mitochondria. Biochim Biophys Acta 1783: 601–609 [DOI] [PubMed] [Google Scholar]
- Herrmann JM, Köhl R (2007) Catch me if you can! Oxidative protein trapping in the intermembrane space of mitochondria. J Cell Biol 176: 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Ball M, Lu H (2007) Zinc binding of Tim10: evidence for existence of an unstructured binding intermediate for a zinc finger protein. Proteins 71: 467–475 [DOI] [PubMed] [Google Scholar]
- Koehler CM, Beverly KN, Leverich EP (2006) Redox pathways of the mitochondrion. Antioxid Redox Signal 8: 813–822 [DOI] [PubMed] [Google Scholar]
- Lafontaine D, Tollervey D (1996) One-step PCR mediated strategy for the construction of conditionally expressed and epitope tagged yeast proteins. Nucleic Acids Res 24: 3469–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H, Lisowsky T, Gerber J, Muhlenhoff U, Kispal G, Lill R (2001) An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep 2: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Woodburn J (2005) Zinc binding stabilizes mitochondrial Tim10 in a reduced and import-competent state kinetically. J Mol Biol 353: 897–910 [DOI] [PubMed] [Google Scholar]
- Lutz T, Neupert W, Herrmann JM (2003) Import of small Tim proteins into the mitochondrial intermembrane space. EMBO J 22: 4400–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A et al. (2007) CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res 35: D237–D240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K, Herrmann JM (2005) A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121: 1059–1069 [DOI] [PubMed] [Google Scholar]
- Müller JM, Milenkovic D, Guiard B, Pfanner N, Chacinska A (2008) Precursor oxidation by mia40 and erv1 promotes vectorial transport of proteins into the mitochondrial intermembrane space. Mol Biol Cell 19: 226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoe M, Ohwa Y, Ishikawa D, Ohshima C, Nishikawa S, Yamamoto H, Endo T (2004) Identification of Tim40 that mediates protein sorting to the mitochondrial intermembrane space. J Biol Chem 279: 47815–47821 [DOI] [PubMed] [Google Scholar]
- Stojanovski D, Muller JM, Milenkovic D, Guiard B, Pfanner N, Chacinska A (2008) The MIA system for protein import into the mitochondrial intermembrane space. Biochim Biophys Acta 1783: 610–617 [DOI] [PubMed] [Google Scholar]
- Terziyska N, Lutz T, Kozany C, Mokranjac D, Mesecke N, Neupert W, Herrmann JM, Hell K (2005) Mia40, a novel factor for protein import into the intermembrane space of mitochondria is able to bind metal ions. FEBS Lett 579: 179–184 [DOI] [PubMed] [Google Scholar]
- Tokatlidis K (2005) A disulfide relay system in mitochondria. Cell 121: 965–967 [DOI] [PubMed] [Google Scholar]