Abstract
We previously reported long-term biochemical and behavioral correction of the 6-hydroxydopamine (6-OHDA) rat model of Parkinson’s disease (PD) by expression of tyrosine hydroxylase (TH) in the partially denervated striatum, using a herpes simplex virus type 1 (HSV-1) vector. This study had a number of limitations, including the use of a helper virus packaging system, limited long-term expression, and expression of only TH. To address these issues, we developed a helper virus-free packaging system, a modified neurofilament gene promoter that supports long-term expression in forebrain neurons, and a vector that coexpresses TH and aromatic amino acid decarboxylase (AADC). Coexpression of TH and AADC supported high-level (80%), behavioral correction of the 6-OHDA rat model of PD for 5 weeks. Biochemical correction included increases in extracellular dopamine and DOPAC concentrations between 2 to 4 months after gene transfer. Histologic analyses demonstrated neuronal-specific coexpression of TH and AADC at 4 days to 7 months after gene transfer, and cell counts revealed 1000 to 10,000 TH positive cells per rat at 2 months after gene transfer. This improved system efficiently corrects the rat model of PD.
OVERVIEW SUMMARY
Gene therapy has potential for treating Parkinson’s disease (PD). In this study, we used a helper virus-free herpes simplex virus type 1 (HSV-1) vector system and a modified neurofilament gene promoter that supports long-term expression in forebrain neurons. We coexpressed tyrosine hydroxylase (TH) and aromatic amino acid decarboxylase (AADC) in striatal cells in the 6-hydroxydopamine (6-OHDA) rat model of Parkinson’s disease (PD). Biochemical (2–4 months) and behavioral (5 weeks) correction was observed. TH and AADC were expressed for at least 7 months. These results indicate the promise of helper virus-free HSV-1 vectors for developing gene therapy of PD.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder that results from the progressive loss of dopaminergic neurons in the substantia nigra pars compacta that project to the corpus striatum (Yahr and Bergmann, 1987). The primary current therapy for PD is to restore striatal dopamine levels by oral administration of levodopa (L-DOPA) (Yahr et al., 1969). The exogenous L-DOPA is converted to dopamine by endogenous striatal aromatic amino acid decarboxylase (AADC), which is present in surviving dopaminergic neurons (Tashiro et al., 1989). While initially effective, L-DOPA therapy subsequently loses efficacy over time (Yahr and Bergmann, 1987).
A number of cell transplantation approaches are being explored to achieve in situ production of L-DOPA or dopamine (Yurek and Sladek, 1990). Transplantation approaches have used cells from the peripheral or central nervous system that naturally produce catecholamines (Gage et al., 1991; Gage, 1998), or genetically engineered cells transfected with specific combinations of the dopamine biosynthetic enzymes tyrosine hydroxylase (TH), GTP cyclohydroxylase (GTP CH), AADC, or a vesicular monoamine transporter (VMAT) (Kang et al., 2001). Studies with genetically engineered cells have established some critical properties of the biosynthetic pathway, including that coexpression of TH and GTP CH is sufficient to produce high levels of L-DOPA in fibroblasts (Bencsics et al., 1996), recombinant AADC can efficiently convert L-DOPA to dopamine (Kang et al., 1993; Wachtel et al., 1997), and VMAT can sequester dopamine into vesicles in heterologous cells and thereby relieve endproduct inhibition of TH by dopamine (Lee et al., 1999). Transplantation of cells that produce L-DOPA or dopamine into the striatum can correct animal models of PD (Gage et al., 1991; Gage, 1998), but cell transplantation has not yet been a successful therapy in most human trials (Lindvall et al., 1990).
An attractive alternative therapy for PD is to express dopamine biosynthetic enzymes in striatal cells using direct gene transfer technologies (Freese et al., 1990; O’Malley et al., 1992). The potential advantages of this gene therapy approach include production of catecholamines at the required site of action, so that diffusion over significant distances is not required and there are no potential problems from graft rejection or from tumor formation by the grafted cells. Potential complications include the production and release of catecholamines in cells that do not normally produce them and the limitations of the currently available gene transfer systems. We reported the first demonstration of long-term (1 year) biochemical and behavioral correction of the 6-hydroxydopamine (6-OHDA) rat model of PD by direct gene transfer (During et al., 1994). Issues that were raised about this study (Isacson, 1995) included the side effects caused by the herpes simplex virus type 1 (HSV-1) helper virus and the low levels of long-term expression (a few tens to a few hundred TH-postive striatal cells per rat) that were supported by the HSV-1 immediate early (IE) 4/5 promoter in the vector. Nonetheless, correction of the rat model of PD by direct gene transfer has by now been reported by a number of other investigators (Horellou et al., 1994; Kaplitt et al., 1994; Imaoka et al., 1998; Corti et al., 1999; Kirik et al., 2002).
To address issues raised about our initial study, we improved the vector system. We developed a helper virus-free packaging system that substantially reduces the cytopathic effects and inflammatory response previously associated with gene transfer (Fraefel et al., 1996; Sun et al., 1999; Zhang et al., 2002). We developed a promoter that supports long-term expression in forebrain neurons by adding an upstream enhancer from the TH promoter to the neurofilament heavy gene (NFH) promoter (Zhang et al., 2000), and we developed large (≥50 kb) vectors that can support coexpression of multiple genes (Wang et al., 2000; Wang et al., 2001).
Here we report results of applying this improved HSV-1 vector system to the rat model of PD. We used the helper virus-free vector system and the modified neurofilament promoter to support coexpression of TH and AADC in the partially denervated striatum of 6-OHDA lesioned rats, resulting in biochemical and behavioral correction of this rat model of PD.
MATERIAL AND METHODS
Cells
2-2 cells (Smith et al., 1992) and BHK cells were maintained in Dulbecco’s modified minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and 4 mM glutamine at 37°C in humidified incubators containing 5% CO2. G418 (0.5 mg/ml) was present during the growth of 2-2 cells but was removed before experiments.
Plasmid constructions
Constructions were performed by standard recombinant DNA procedures (Maniatis et al., 1989). pTH-NFHlac (Zhang et al., 2000) contains three Hind II sites, one of which forms the boundary between the TH-NFH promoter and the LacZ gene. To destroy the two additional HindIII sites, pTH-NFH-lac was digested partially with HindIII, linear molecules was isolated from an agarose gel, and the ends were filled in using Klenow enzyme; this procedure was repeated twice to yield pTH-NFHlac-ΔH3. A 6-kb fragment containing a human TH/ires/AADC cassette was isolated from pCMVth/ires/aadc (Moffat et al., 1997) by PvuI complete and HindIII partial digestion. This fragment was inserted into pTH-NFHlac-ΔH3 digested to completion with PvuI and HindIII to remove the LacZ gene, which was replaced with the th/ires/aadc cassette. The resulting vector was designated pTH-NFHth/ires/aadc (Fig. 1). This TH cDNA is derived from the human TH type II cDNA; the PKA phosphorylation site at the N-terminus was deleted, and the HA tag was added to assist immunohistochemical assays using rat brain sections (Moffat et al., 1997).
FIG. 1.
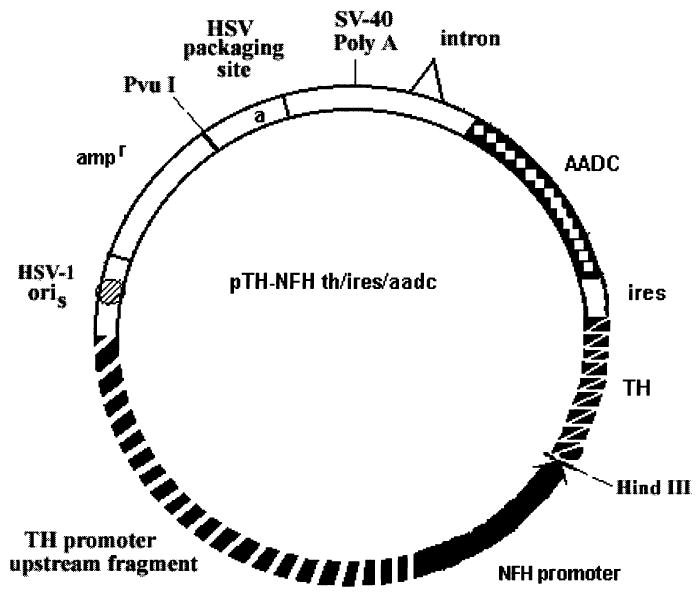
Schematic diagram of pTH-NFHth/ires/aadc. This vector contains a herpes simplex virust type 1 (HSV-1) a sequence (contains the packaging site) and an HSV-1 origin of DNA replication (oriS) to support replication and packaging of the vector into HSV-1 particles. The transcription unit in the vector contains the TH-NFH promoter, a human TH cDNA, an internal ribo-some entry site (ires), and a human AADC cDNA. TH, tyrosine hydroxylase; AADC, aromatic amino acid decarboxylase.
Vector packaging and tittering
Vectors were packaged into HSV-1 particles using the helper virus-free packaging system (Fraefel et al., 1996) as modified to improve the titers (Sun et al., 1999). Vector stocks were purified and concentrated (Lim et al., 1996). Vectors were titered by counting the numbers of positive cells observed at 1 day after the infection of BHK cells. Escherichia coli â-galactosidase was detected using X-gal (Song et al., 1997). TH and AADC were detected by immunocytochemistry (Song et al., 1997). This TH is fused to the HA tag and was detected using a rabbit anti-HA antibody (1:500 dilution, BABCO). Human AADC was detected using a rabbit anti-human AADC antibody (1:500 dilution, gift from Dr. Haycock, LSU Medical Center, New Or-leans, LA). The titers of the vector stocks were: 5 ×107 or 4 ×107 infectious particles per microliter pTH-NFHth/ires/aadc or pTH-NFHlac, respectively.
6-OHDA lesions
Male Sprague-Dawley rats (225–250 g) were obtained from Charles River (Wilmington, MA). Rats received food and water ad libitum. The rats were kept in a temperature and humidity controlled room with a 12-hr light-dark cycle. To establish that the rats had no preexisting rotational bias, 2–5 days after arrival from the vendor, the rats were tested for apomorphine-induced (1 mg/kg intraperitoneally) rotations, and rats that exhibited less than 2 net turns per 5 min were used in this study. Rats were anesthetized with 60–80 mg/kg ketamine and 5–10 mg/kg xylazine, and placed in a stereotaxic frame. 6-OHDA (2 μg/μl in ascorbate-saline) was microinjected at each of two sites (2.5 μl per site) in the substantial nigra (anterior-posterior [AP] –5.5, medial-lateral [ML] 1.9, dorsal-ventral (DV) –7.1; AP –5.5, ML 2.3, DV –6.8) (Perese et al., 1989). AP is relative to bregma, ML is relative to the sagittal suture, and DV is relative to the bregma-lambda plane (Paxinos and Watson, 1986). Starting 2 weeks later, the rats were tested for apomorphine-induced rotational behavior (once per week for at least 4 weeks), and rats that exhibited an average of 5 or more rotations per minute for the 15–20-min interval after apomorphine administration were used for gene transfer. This rotation rate is consistent with a 90% or more reduction in striatal TH activity and dopamine levels (Hefti et al., 1980). These studies were approved by the W. Roxbury VA Hospital and Children’s Hospital IACUCs.
Gene transfer
pTH-NFHth/ires/aadc, pTH-NFHlac, or PBS was injected at each of three sites in the right striatum (3 μl per site: AP 0.6, ML 2.0, DV –5.0; AP 0.6, ML 3.2, DV –5.0; AP 1.8, ML 2.6, DV –5.0) (Paxinos and Watson, 1986; During et al., 1994). Starting 2 weeks after gene transfer, apomorphine-induced rotational behavior was tested once per week. Statistical analyses of the behavioral and neurochemical data were performed using between groups or repeated measures of analysis of variance (ANOVAs; SigmaStat, SPSS Science, Chicago, IL).
Microdialysis
For selected rats, at 2–4 months after gene transfer, a guide cannula was implanted proximal to the three injection sites (AP 0.6, ML 2.6). The dialysis probes had a molecular weight cutoff of 20 kd (CMA12, CMA). Microdialysis was performed 1–2 days after insertion of the guide cannula. To insert the dialysis probe, the animal was anesthetized with isoflurane. The probe was inserted through the cannula, and the exposed 4-mm membrane spanned the dorso-ventral coordinates of the striatum. After equilibration for 2 hr, dialysate was collected in artificial CSF buffer (147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 0.85 mM MgCl2) using a micropump (CMA/100, 2 μl/min flow rate [During et al., 1994]). Catecholamines were quantitated by high-performance liquid chromatography (HPLC) using methods previously validated in our laboratory (Holmes et al., 1994; Yadid et al., 2000). The levels of L-DOPA, dopamine, and DOPAC were determined in each sample.
Immunohistochemistry
Rats were perfused with 100 ml of phosphate-buffered saline (PBS) followed by 200 ml of 4% paraformaldehyde in PBS. The brains were postfixed in 4% paraformaldehyde for an additional 4 hr and cryoprotected in 30% sucrose in PBS (4°C, 2 days). Twenty-five micrometers of coronal brain sections were cut on a freezing microtome. Immunohistochemistry was performed on free-floating sections as described (Zhang et al., 2000). The primary antibodies were mouse monoclonal antiTH antibody (1:200 dilution [Rohrer et al., 1986]), rabbit anti-HA (1:500 dilution, BABCO), rabbit anti-human AADC (1:500 dilution, gift from Dr. Haycock), mouse monoclonal anti-â-gal (1:500 dilution, Sigma, St. Louis, MO), and mouse monoclonal anti-NeuN (1:200 dilution, Chemicon, Temecula, CA). Antibodies were visualized using biotinylated secondary antibodies and the ABC reagent (Vector Laboratories, Burlingame, CA) or fluorescein or rhodamine isothiocyanate-conjugated secondary antibodies (Vector Laboratories).
Cell counts
Twenty-five micrometers of coronal brain sections were prepared from the striatum. For most rats and assays, every 4th section was analyzed, and approximately 10–15 of these sections contained the positive cells. Cells that contained recombinant gene products were located at 10× magnification and counted at 40× magnification. Each section was counted on 2 different days, and the results differed by less than 10%.
RESULTS
Long-term, neuronal-specific expression of human TH and AADC in the rat striatum
An HSV-1 vector (pTH-NFHth/ires/aadc, Fig. 1) was constructed that expresses a TH/ires/AADC cassette (Moffat et al., 1997) from a chimeric TH-NFH promoter (Zhang et al., 2000); this promoter supports long-term expression in the rat striatum. The control vector, pTH-NFHlac, expresses E. coli â-galac-tosidase (Zhang et al., 2000). Vectors were packaged using a helper virus-free system (Fraefel et al., 1996; Sun et al., 1999). The resulting vector stocks were microinjected into the striatum, and the rats were sacrificed at 4 days, 3.5 weeks, or 7 weeks after gene transfer. Immunohistochemistry was performed to detect recombinant gene expression. The HA tag is fused to this TH gene, and human TH was detected using an anti-HA antibody. Human AADC was detected using a rabbit anti-human AADC antibody that does not cross react with rat AADC. Sections from rats that received pTH-NFHth/ires/aadc contained staining for recombinant TH or recombinant AADC (Fig. 2A–2J). The staining for recombinant TH frequently filled the cell body and extended into the proximal processes. This staining often revealed a neuronal morphology, consistent with the neuronal-specific expression supported by the TH-NFH promoter (Zhang et al., 2000) in the vector, but some cells displayed a morphology that could also be interpreted as astrocytic. Staining for recombinant TH was observed at each of the three time points examined (4 days, 3.5 weeks, 7 weeks after gene transfer). The staining for recombinant AADC was punctate and was usually restricted to the cell body; staining for recombinant AADC was observed at each of the two time points examined (4 days and 3.5 weeks after gene transfer). Because AADC is the second gene in the TH/ires/AADC cassette, the level of AADC expression may be lower than that of TH, consistent with the relative intensities of the AADC and TH staining. In contrast, sections from a rat sacrificed at 7 weeks after microinjection of the control vector, pTH-NFHlac, lacked staining for either recombinant TH (Fig. 2K) or recombinant AADC (Fig. 2L). Cell counts revealed 1000 to 10,000 TH-positive striatal cells in the rats that received pTH-NFHth/ires/aadc.
FIG. 2.

pTH-NFHth/ires/aadc supports long-term expression of both TH and AADC in the striatum. A–J: Rats sacrificed at 4 days, 3.5 weeks, or 7 weeks after microinjection of pTH-NFHth/ires/aadc into the striatum. This tyrosine hydroxylase (TH) contains the HA tag and was detected using an anti-HA antibody, and aromatic amino acid decarboxylase (AADC) was detected using an anti-human AADC antibody that does not recognize rat AADC. A–D: HA-immunoreactivity (IR) (A and B) and AADC-IR (C and D) at 4 days after gene transfer, low- and high-power views. E–H: HA-IR (E and F) and AADC-IR (G and H) at 3.5 weeks after gene transfer. I and J: HA-IR at 7 weeks after gene transfer. K and L: HA-IR (K) and AADC-IR (L) from a rat sacrificed at 7 weeks after microinjection of the control vector, pTH-NFHlac, into the striatum. Scale bars: A, C, E, G, and I, 50 μm; B, D, F, H, J, K, and L, 25 μm.
We established that pTH-NFHth/ires/aadc supports coexpression of TH and AADC in striatal neurons for at least 7 months. Rats were sacrificed at 4 days or 7 months after gene transfer. Human TH and AADC were detected using anti-HA or AADC antibodies, respectively, neurons were identified using an anti-NeuN antibody, and staining was localized to the same cells using immunofluorescent secondary antibodies. Recombinant TH and AADC were detected in the same cells at 4 days (not shown) and 7 months (Fig. 3A–3C), and cell counts revealed greater than 90% colocalization at both time points (Table 1). Both recombinant TH and AADC were detected in neurons at 4 days (not shown) and 7 months (Fig. 3D–3I), and cell counts revealed greater than 90% localization to neurons for both TH and AADC at both time points (Table 1). In contrast, sections from a rat sacrificed at 7 weeks after microinjection of the control vector, pTH-NFHlac, lacked staining for either recombinant TH or recombinant AADC (Fig. 3J–3L).
FIG. 3.
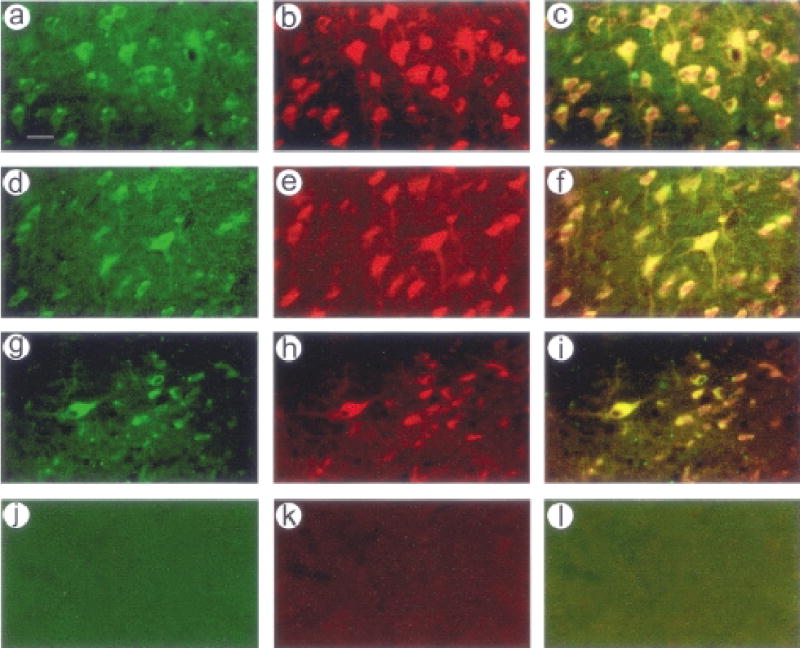
Long-term coxpression of tyrosine hydroxylase (TH) and aromatic amino acid decarboxylase (AADC) in neurons. A–I: Rats were sacrificed at 7 months after microinjection of pTH-NFHth/ires/aadc into the striatum. TH was detected using an anti-HA antibody, AADC was detected using an anti-human AADC antibody, and neurons were identified using an anti-NeuN antibody. Antibodies were visualized using rhodamine- or fluorescein-conjugated secondary antibodies. A–C: TH and AADC were expressed in the same cells; (A) HA-IR, (B) AADC-IR, and (C) costaining. D–F: AADC was expressed in neurons; (D) AADC-IR, (E) NeuN-IR, and (F) costaining. G–I: TH was expressed in neurons; (G) HA-IR, (H) NeuN-IR, and (I) costaining. (J–L) No staining for recombinant TH or AADC was observed in striatal sections from a rat sacrificed at 7 weeks after microinjection of the control vector, pTH-NFHlac, into the striatum; (J) HA-IR, (K) AADC-IR, and (L) costaining. Scale bar, 25 μm
Table 1.
Neuronal Specificity of Expression From pTH-NFHth/ires/aadc and Coexpression of TH and AADC
|
Numbers of positive cells or % costaining |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Survival time | TH-IR | NeuN-IR | Costaining | AADC-IR | NeuN-IR | Costaining | TH-IR | AADC-IR | Costaining |
| 4 days | 177 ± 2 | 171 ±4 | 97% | 111 ± 6 | 104 ± 1 | 93% | 135 ± 6 | 131 ± 1 | 97% |
| 7 months | 231 ± 9 | 223 ± 4 | 96% | 228 ± 9 | 224 ± 1 | 98% | 246 ± 4 | 243 ± 0 | 99% |
Immunofluorescence was performed on sections from 2 rats at each of the 2 time points, and positive cells were counted in at least 3 different sections. The means ± SEMs are shown.
TH, tyrosine hydroxylase; AADC, aromatic amino acid decarboxylase; SEM, standard error of the mean.
Coexpression of TH and AADC supports behavioral correction of the 6-OHDA rat model of PD
Rats were lesioned with 6-OHDA and then tested at least three times with apomorphine to identify the rats with relatively complete lesions (Perese et al., 1989). pTH-NFHth/ires/aadc or controls (pTH-NFHlac or PBS) was microinjected into three sites in the partially denervated striatum, and the rats were tested for behavioral correction at weekly intervals starting at 2 weeks after the gene transfer. The results showed that pTH-NFHth/ires/aadc can support behavioral correction of the rat model of PD (Fig. 4). The rats that received pTH-NFHth/ires/aadc showed a statistically significant (p < 0.001; ANOVA), high-level (~80%) reduction in the number of rotations. This behavioral correction was maintained for 5 weeks after gene transfer, the longest time tested in this study. Microinjection of either PBS or the control vector, pTH-NFHlac, did not cause behavioral correction (p > 0.05 compared to before gene transfer).
FIG. 4.

Delivery of pTH-NFHth/ires/aadc into the partially denervated striatum can correct the 6-hydroxydopamine (6-OHDA) rat model of Parkinson’s disease (PD). Rats were lesioned with 6-OHDA and then tested at least three times with apomorphine to identify the rats with relatively complete lesions. pTH-NFHth/ires/aadc, pTH-NFHlac, or phosphate-buffered saline (PBS) was microinjected into the partially denervated striatum. The rats were tested for behavioral correction at weekly intervals, and the values shown are the average % behavioral correction for each group at each time point (pTH-NFHth/ires/aadc n = 5 rats, pTH-NFHlac n = 7, or PBS n = 6). pTH-NFHth/ires/aadc supported an approximate 80% reduction in the number of rotations, and neither pTH-NFHlac nor PBS caused behavioral correction.
pTH-NFHth/ires/aadc supports biochemical correction of the rat model of PD
We performed in vivo microdialysis to determine if coexpression of TH and AADC would support biochemical correction of the rat model of PD. In selected rats, cannulas were implanted proximal to the three injection sites. Between 2 and 4 months after gene transfer, microdialysate samples were collected, and the levels of L-DOPA, dopamine, and DOPAC in each sample were quantified by HPLC followed by electrochemical detection. pTH- NFHth/ires/aadc directed a 160% average increase in dopamine levels compared to control conditions (pTH-NFHlac or 6-OHDA lesioning only) and a 419% average increase in DOPAC levels compared to control conditions (Table 2). These differences were statistically significant by ANOVA. pTH-NFHth/ires/aadc supported a nonsignificant, 160% increase in L-DOPA levels compared to the control group, suggesting that the recombinant AADC (coexpressed with TH) efficiently converted the L-DOPA to dopamine. Table 2 states, for each rat in each group, the time after either gene transfer or 6-OHDA lesioning at which the in vivo dialysis was performed.
Table 2.
Striatal L-DOPA, Dopamine, And Dopac Levels at two to Four Months After Gene Transfer as Measured by In Vivo Microdialysis
|
pg/ml catecholamine in dialysate |
|||
|---|---|---|---|
| Condition | L-DOPA | Dopamine | DOPAC |
| pTH-NFHth/ires/aadc | 1.6 ± 0.3 × 102 | 3.2 ± 0.2 × 102* | 1.3 ± 0.3 × 105* |
| pTH-NFHlac and 6-OHDA only | 1.0 ± 0.1 × 102 | 2.0 ± 0.4 × 102 | 3.1 ± 1.5 × 104 |
| Normal rats | 4.6 ± 0.3 × 102* | 5.8 ± 1.1 × 102* | 3.7 ± 0.3 × 105* |
The mean ± SEM of each catecholamine in each group are shown. For the pTH-NFHth/ires/aadc group (n = 5 rats), dialysate was collected from 4 rats at 3.5 months after gene transfer and from 1 rat at 4.0 months after gene transfer. For the pTH-NFH-lac and 6-OHDA lesioned only group (n = 4 rats), dialysate was collected from 2 rats that received pTH-NFHlac at 2.5 months after gene transfer, and dialysate was collected from 2 rats that received only 6-OHDA at 2.0 months after lesioning. The normal group (n = 5 rats) contained age-matched control rats that did not receive either 6-OHDA lesioning or gene transfer. The levels of L-DOPA, dopamine, and DOPAC Were determined in each dialysate sample. The asterisks indicate statistically significant differences compared to the pTH-NFHlac and lesioning only group (p < 0.05, ANOVA).
L-DOPA, levodopamine; DOPAC, ; 6-OHDA, 6-hydroxydopamine; SEM, standard error of the mean; ANOVA, analysis of variance.
DISCUSSION
In this study, we used a helper virus-free HSV-1 vector system and a modified neurofilament promoter to coexpress TH and AADC in the partially denervated striatum. Because this vector system does not express any HSV-1 genes, gene transfer with this vector system causes minimal cytopathic effects and inflammatory response compared to previous systems that used helper viruses (Fraefel et al., 1996; Zhang et al., 2002). Sections that contained the needle track and/or injection site displayed normal tissue morphology, consistent with other reports (Fraefel et al., 1996; Zhang et al., 2002). The modified neurofilament promoter supported expression of TH and AADC for 7 months, the longest time tested, and expression was principally in neurons; these results are consistent with our earlier report that showed that this promoter supports long-term expression in forebrain neurons (Zhang et al., 2000). Thus, the combination of this helper virus-free HSV-1 vector system and the modified neurofilament promoter appears to be an attractive system for supporting long-term expression in forebrain neurons.
Expression of TH was observed in approximately 1000 to 10,000 cells per striatum. In our initial study on correcting the rat model of PD, TH-immunoreactivity (IR) was observed in approximately 5–10 to 200 cells per striatum (During et al., 1994). This increase in gene transfer may, in part, explain the increase in the level of behavioral correction (approximately 65 % in our initial study versus approximately 80% in the present study). In both studies, the numbers of transfected cells in individual rats did not correlate with the amount of behavioral correction exhibited by the rats. In contrast, we have shown that delivery of a constitutively active protein kinase C into nigrostratial neurons, in normal rats, results in a correlation between the numbers of transfected cells in individual rats and the amount of apomorphine-induced rotational behavior (Song et al., 1998). Nigrostriatal neurons represent a relatively uniform population, thus different neurons may contribute similarly to rotational behavior. In contrast, the striatum is much more complex in terms of its cellular organization and neuronal populations, and this complexity may explain the lack of a correlation between numbers of cells expressing TH and the amount of behavioral correction.
The behavioral correction reported in this study is consistent with that described in previous reports that used an AAV vector to express TH (Kaplitt et al., 1994), adenovirus vectors to express TH from either a constitutive promoter or a tetracy-cline-regulated promoter (Horellou et al., 1994; Corti et al., 1999), or liposome-mediated transfection to express TH or coexpress TH and AADC (Imaoka et al., 1998). In this study, we used a helper virus-free HSV-1 vector system to coexpress TH and AADC. The biochemical data suggests that the recombinant AADC efficiently converted the L-DOPA to dopamine, resulting in elevated extracellular fluid levels dopamine and DOPAC, and no significant change in L-DOPA levels. Our initial study used a helper virus-containing HSV-1 vector system to express TH. Concerns were raised about our initial study (Isacson, 1995); specifically, it was asserted that the side effects caused by the vector system, and particularly the helper virus, might mediate sprouting of any remaining dopaminergic terminals and thereby cause behavioral correction, even though control rats that received a HSV-1 vector expressing the LacZ gene did not exhibit behavioral correction. The present study used a helper virus-free HSV-1 vector system that does not express any HSV-1 genes or cause detectable side-effects, and, again, behavioral correction was observed using a vector that coexpresses TH and AADC, but a vector that expresses Lac Z did not support behavioral correction. This study represents the first report of a long-term behavioral change using this helper virus-free HSV-1 vector system.
A number of studies, viewed collectively, suggest that at least four genes (TH, GTP CH, AADC and VMAT) may be required for the efficient production and release of dopamine from striatal cells (Kang et al., 1993, 2001; Bencsics et al., 1996; Wachtel et al., 1997; Lee et al., 1999). Coexpression of TH and GTP CH is required for the efficient production of L-DOPA in fibroblasts; GTP CH is the first and rate-limiting enzyme in the biosynthetic pathway for the cofactor for TH, tetrahydrobiopterin. Consistent with this observation, coinjection of two AAV vectors that express TH or GTP CH supported both biochemical and behavioral correction of the rat model (Kirik et al., 2002), and an earlier study reported biochemical but not behavioral correction (Mandel et al., 1998). These authors raised issues about the biochemical correction reported in our initial study in relation to both the source of cofactor for TH and the side effects of the helper virus-containing vector system. In the present study, using a helper virus-free HSV-1 vector system, we have again observed biochemical correction. Because PD is a progressive disease, in the earlier stages of the disease it may be possible to express recombinant TH and rely on endogenous sources of cofactor, while the later stages may require coexpression of both TH and GTP CH. The elevated levels of dopamine and DOPAC, but not L-DOPA, that we observed are consistent with the efficient conversion of the L-DOPA to dopamine, as has been reported in studies with fibroblasts engineered to coexpress TH and AADC (Kang et al., 1993, 2001; Wachtel et al., 1997). Additionally, fibroblast cells engineered to coexpress AADC and VMAT support the efficient conversion of L-DOPA to dopamine and improved storage of dopamine (Lee et al., 1999). The large size of HSV-1 vectors may support the coexpression of these four genes from one vector and more efficient production and regulated release of dopamine.
Acknowledgments
We thank Drs. K. O’Malley for the TH/ires/AADC cassette and the TH promoter; A. Davison for HSV-1 cosmid set C; R. Sandri-Goldin for 2-2 cells; W. Schlaepfer for the NF-H promoter; and J. Haycock for anti-AADC antibody. This work was supported by NS10805, PHARMA, AFAR, APDA, and AG20177 (M.S.), and AG16777 and NS34025 (A.G.).
References
- BENCSICS C, WACHTEL SR, MILSTIEN S, HATAKEYAMA K, BECKER JB, KANG UJ. Double transduction with GTP cyclohydrolase I and tyrosine hydroxylase is necessary for spontaneous synthesis of L-DOPA by primary fibroblasts. J Neurosci. 1996;16:4449–4456. doi: 10.1523/JNEUROSCI.16-14-04449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORTI O, SANCHEZ-CAPELO A, COLIN P, HANOUN N, HA-MON M, MALLET J. Long-term doxycycline-controlled expression of human tyrosine hydroxylase after direct adenovirus-mediated gene transfer to a rat model of Parkinson’s disease. Proc Natl Acad Sci USA. 1999;96:12120–12125. doi: 10.1073/pnas.96.21.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURING MJ, NAEGELE JR, O’MALLEY KL, GELLER AI. Long-term behavioral recovery in parkinsonian rats by an HSV vector expressing tyrosine hydroxylase. Science. 1994;266:1399–1403. doi: 10.1126/science.266.5189.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAEFEL C, SONG S, LIM F, LANG P, YU L, WANG Y, WILD P, GELLER AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE A, GELLER AI, NEVE R. HSV-1 vector mediated neuronal gene delivery. Strategies for molecular neuroscience and neurology. Biochem Pharmacol. 1990;40:2189–2199. doi: 10.1016/0006-2952(90)90711-s. [DOI] [PubMed] [Google Scholar]
- GAGE FH. Cell therapy. Nature. 1998;392(suppl):18–24. [PubMed] [Google Scholar]
- GAGE FH, KAWAJA MD, FISHER LJ. Genetically modified cells: Applications for intracerebral grafting. Trends Neurosci. 1991;14:328–333. doi: 10.1016/0166-2236(91)90156-o. [DOI] [PubMed] [Google Scholar]
- HEFTI F, MELAMED E, SAHAKIAN BJ, WURTMAN RJ. Circling behavior in rats with partial, unilateral nigrostriatal lesions: Effect of amphetamine, apomorphine, and DOPA. Pharmacol Biochem Behav. 1980;12:185–188. doi: 10.1016/0091-3057(80)90353-6. [DOI] [PubMed] [Google Scholar]
- HOLMES C, EISENHOFER G, GOLDSTEIN DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- HORELLOU P, VIGNE E, CASTEL MN, BARNEOUD P, COLIN P, PERRICAUDET M, DELAERE P, MALLET J. Direct intracerebral gene transfer of an adenoviral vector expressing tyrosine hydroxylase in a rat model of Parkinson’s disease. Neuroreport. 1994;6:49–53. doi: 10.1097/00001756-199412300-00014. [DOI] [PubMed] [Google Scholar]
- IMAOKA T, DATE I, OHMOTO T, NAGATSU T. Significant behavioral recovery in Parkinson’s disease model by direct intracerebral gene transfer using continuous injection of a plasmid DNA-liposome complex. Hum Gene Ther. 1998;9:1093–1102. doi: 10.1089/hum.1998.9.7-1093. [DOI] [PubMed] [Google Scholar]
- ISACSON O. Behavioral effects and gene delivery in a rat model of Parkinson’s disease. Science. 1995;269:856–857. doi: 10.1126/science.7638605. [DOI] [PubMed] [Google Scholar]
- KANG UJ, FISHER LJ, JOH TH, O’MALLEY KL, GAGE FH. Regulation of dopamine production by genetically modified primary fibroblasts. J Neurosci. 1993;13:5203–5211. doi: 10.1523/JNEUROSCI.13-12-05203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG UJ, LEE WY, CHANG JW. Gene therapy for Parkinson’s disease: Determining the genes necessary for optimal dopamine replacement in rat models. Hum Cell. 2001;14:39–48. [PubMed] [Google Scholar]
- KAPLITT MG, LEONE P, SAMULSKI RJ, XIAO X, PFAFF DW, O’MALLEY KL, DURING MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- KIRIK D, GEORGIEVSKA B, BURGER C, WINKLER C, MUZYCZKA N, MANDEL RJ, BJORKLUND A. Reversal of motor impairments in parkinsonian rats by continuous intrastriatal delivery of L-dopa using rAAV-mediated gene transfer. Proc Natl Acad Sci USA. 2002;99:4708–4713. doi: 10.1073/pnas.062047599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE WY, CHANG JW, NEMETH NL, KANG UJ. Vesicular monoamine transporter-2 and aromatic L-amino acid de-carboxylase enhance dopamine delivery after L-3, 4-dihydroxy-phenylalanine administration in Parkinsonian rats. J Neurosci. 1999;19:3266–3274. doi: 10.1523/JNEUROSCI.19-08-03266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIM F, HARTLEY D, STARR P, LANG P, SONG S, YU L, WANG Y, GELLER AI. Generation of high-titer defective HSV-1 vectors using an IE 2 deletion mutant and quantitative study of expression in cultured cortical cells. Biotechniques. 1996;20:460–469. doi: 10.2144/19962003460. [DOI] [PubMed] [Google Scholar]
- LINDVALL O, BRUNDIN P, WIDNER H, REHNCRONA S, GUSTAVII B, FRACKOWIAK R, LEENDERS KL, SAWLE G, ROTHWELL JC, MARSDEN CD, BJORKLUND A. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- MANDEL RJ, RENDAHL KG, SPRATT SK, SNYDER RO, COHEN LK, LEFF SE. Characterization of intrastriatal recombinant adeno-associated virus-mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydrolase I in a rat model of Parkinson’s disease. J Neurosci. 1998;18:4271–4284. doi: 10.1523/JNEUROSCI.18-11-04271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANIATIS, T., FRITSCH, E.F., and SAMBROOK, J. (1989). Molecular Cloning, 2nd ed. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY).
- MOFFAT M, HARMON S, HAYCOCK J, O’MALLEY KL. L-Dopa and dopamine-producing gene cassettes for gene therapy approaches to Parkinson’s disease. Exp Neurol. 1997;144:69–73. doi: 10.1006/exnr.1996.6390. [DOI] [PubMed] [Google Scholar]
- O’MALLEY KL, HARMON S, TANG L, TODD RD. The rat dopamine D4 receptor: Sequence, gene structure, and demonstration of expression in the cardiovascular system. Neurochem Int. 1992;20:23S–26S. [PubMed] [Google Scholar]
- PAXINOS, G., and WATSON, C. (1986). The Rat Brain in Stereotaxic Coordinates (Academic Press, Sidney).
- PERESE DA, ULMAN J, VIOLA J, EWING SE, BANKIEWICZ KS. A 6-hydroxydopamine-induced selective parkinsonian rat model. Brain Res. 1989;494:285–293. doi: 10.1016/0006-8993(89)90597-0. [DOI] [PubMed] [Google Scholar]
- ROHRER H, ACHESON AL, THIBAULT J, THOENEN H. Developmental potential of quail dorsal root ganglion cells analyzed in vitro and in vivo. J Neurosci. 1986;6:2616–2624. doi: 10.1523/JNEUROSCI.06-09-02616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH IL, HARDWICKE MA, SANDRI-GOLDIN RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- SONG S, WANG Y, BAK SY, DURING MJ, BRYAN J, ASHE O, ULLREY DB, TRASK LE, GRANT FD, O’MAL-LEY KL, RIEDEL H, GOLDSTEIN DS, NEVE KA, LA-HOSTE GJ, MARSHALL JF, HAYCOCK JW, NEVE RL, GELLER AI. Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons. J Neurosci. 1998;18:4119–4132. doi: 10.1523/JNEUROSCI.18-11-04119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG S, WANG Y, BAK SY, LANG P, ULLREY D, NEVE RL, O’MALLEY KL, GELLER AI. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J Neurochem. 1997;68:1792–1803. doi: 10.1046/j.1471-4159.1997.68051792.x. [DOI] [PubMed] [Google Scholar]
- SUN M, ZHANG GR, YANG T, YU L, GELLER AI. Improved titers for helper virus-free herpes simplex virus type 1 plasmid vectors by optimization of the packaging protocol and addition of noninfectious herpes simplex virus-related particles (pre-viral DNA replication enveloped particles) to the packaging procedure. Hum Gene Ther. 1999;10:2005–2011. doi: 10.1089/10430349950017365. [DOI] [PubMed] [Google Scholar]
- TASHIRO Y, KANEKO T, SUGIMOTO T, NAGATSU I, KIKUCHI H, MIZUNO N. Striatal neurons with aromatic L-amino acid decarboxylase-like immunoreactivity in the rat. Neurosci Lett. 1989;100:29–34. doi: 10.1016/0304-3940(89)90655-1. [DOI] [PubMed] [Google Scholar]
- WACHTEL SR, BENCSICS C, KANG UJ. Role of aromatic L-amino acid decarboxylase for dopamine replacement by genetically modified fibroblasts in a rat model of Parkinson’s disease. J Neurochem. 1997;69:2055–2063. doi: 10.1046/j.1471-4159.1997.69052055.x. [DOI] [PubMed] [Google Scholar]
- WANG X, ZHANG G, SUN M, GELLER AI. General strategy for constructing large HSV-1 plasmid vectors that coexpress multiple genes. Biotechniques. 2001;31:204–212. doi: 10.2144/01311dd05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, ZHANG G, YANG T, ZHANG W, GELLER AI. Fifty-one kilobase HSV-1 plasmid vector can be packaged using a helper virus-free system and supports expression in the rat brain. Biotechniques. 2000;27:102–106. doi: 10.2144/00281st05. [DOI] [PubMed] [Google Scholar]
- YADID G, HARVEY-WHITE JD, KOPIN IJ, GOLDSTEIN DS. Estimation of striatal dopamine spillover and metabolism in vivo. Neuroreport. 2000;11:3367–3373. doi: 10.1097/00001756-200010200-00021. [DOI] [PubMed] [Google Scholar]
- YAHR, M.D., and BERGMANN, K.J. (1987). Parkinson’s Disease (Raven Press, New York).
- YAHR MD, DUVOISIN RC, SCHEAR MJ, BARRETT RE, HOEHN MM. Treatment of parkinsonism with levodopa. Arch Neurol. 1969;21:343–354. doi: 10.1001/archneur.1969.00480160015001. [DOI] [PubMed] [Google Scholar]
- YUREK DM, SLADEK JR., JR Dopamine cell replacement: Parkinson’s disease. Ann Rev Neurosci. 1990;13:415–440. doi: 10.1146/annurev.ne.13.030190.002215. [DOI] [PubMed] [Google Scholar]
- ZHANG G, WANG X, YANG T, SUN M, ZHANG W, WANG Y, GELLER AI. A tyrosine hydroxylase-neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Mol Brain Res. 2000;84:17–31. doi: 10.1016/s0169-328x(00)00197-2. [DOI] [PubMed] [Google Scholar]
- ZHANG, W., SCHOTT, C., ZHANG, G., WANG, Y., GUERRIERE, J., LEWANDOWSKI, G., LAMPSON, L.A., and GELLER, A.I. (2002). Comparison of the inflammatory response to gene transfer into the rat brain with helper-containing or helper virus-free HSV-1 vectors. Gene Ther. (submitted). [PubMed]


