Abstract
Both glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) can protect nigrostriatal dopaminergic neurons from neurotoxins in rodent and monkey models of Parkinson’s disease (PD). These two neurotrophic factors are usually tested individually. This study was designed to compare GDNF, BDNF, or both, for their capabilities to correct behavioral deficits and protect nigrostriatal dopaminergic neurons in a rat model of PD. Gene transfer used a helper virus-free Herpes Simplex Virus (HSV-1) vector system and a modified neurofilament heavy gene promoter that supports long-term expression in forebrain neurons. Rats received unilateral intrastriatal injections of HSV-1 vectors that express either GDNF or BDNF, or both vectors, followed by intrastriatal injections of 6-hydroxydopamine (6-OHDA). Recombinant GDNF or BDNF was detected in striatal neurons in rats sacrificed at 7 months after gene transfer. Of note, GDNF was significantly more effective than BDNF for both correcting behavioral deficits and protecting nigrostriatal dopaminergic neurons. Expression of both neurotrophic factors was no more effective than expression of only GDNF. These results suggest that GDNF is more effective than BDNF for correcting the rat model of PD, and that there are no detectable benefits from expressing both of these neurotrophic factors.
Keywords: Parkinson’s disease, Gene therapy, Glial cell line-derived neurotrophic factor, Brain-derived neurotrophic factor, Helper virus-free HSV-1 vector, Neurodegeneration
1. Introduction
Parkinson’s disease (PD) is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) [40]. The primary therapies for PD are centered on administration of l-DOPA (levodopa), which restores striatal dopamine levels and alleviates the symptoms of PD, for limited periods of time. However, levodopa treatment does not address the progressive nature of nigrostriatal dopaminergic neuron loss [40,41]. An alternative and complementary approach to treat this neurological disorder is to use neurotrophic factors to reduce the progressive neuronal loss [4,14,22]. Specific neurotrophic factors, including glial cell line-derived neurotrophic factor (GDNF) [3,18,24,27] and brain-derived neurotrophic factor (BDNF) [1,17,21], have been found to attenuate the lesion-induced loss of nigrostriatal dopaminergic neurons in animal models of PD. Of note, GDNF and BDNF can interfere with both apoptotic and necrotic forms of cell death.
GDNF is a potent survival factor for injured nigrostriatal dopaminergic neurons and is being evaluated as a potential treatment for PD [4,5,14,22]. Studies using intracerebral injection of recombinant GDNF protein in animal models of PD have shown that GDNF can efficiently protect injured nigrostriatal neurons, and stimulate dopamine turnover and release in rescued neurons [10,11]. In vivo delivery of the GDNF gene has been achieved using adeno-associated virus (AAV) [8,18,19], adenovirus [3], or lentivirus vectors [3,23,24]. Expression of GDNF from these vectors can protect nigrostriatal neurons in both rodent [3,18] and monkey [8,24] models of PD.
Another neurotrophic factor, BDNF, can protect nigrostriatal dopaminergic neurons [1,2,17]. Transplantation of modified fibroblasts that express BDNF into either the striatum or the midbrain attenuates 6-hydroxydopamine (6-OHDA)-induced losses of nigrostriatal neurons [25,43]. Also, BDNF can modulate dopaminergic neurotransmission in nigrostriatal neurons, as shown by elevated rotational behavior and increased turnover of dopamine in the striatum [1]. BDNF can promote functional recovery from 6-OHDA lesions following expression in striatal cells from an AAV vector [21].
GDNF and BDNF are usually tested individually. It is important to determine which neurotrophic factor is more effective, and if GDNF and BDNF can act synergistically to further improve behavioral performance and protection of nigrostriatal neurons.
We compared the capabilities of GDNF, BDNF, or both, to correct behavioral deficits and protect nigrostriatal dopaminergic neurons, following direct gene transfer into striatal neurons in a rat model of PD [18]. Gene transfer used a helper virus-free Herpes Simplex Virus (HSV-1) vector system and a modified neurofilament heavy gene promoter that supports long-term expression in rat striatal neurons [36,37,44]. Expression of GDNF, or BDNF, or both, supported partial behavioral correction and increased the numbers of surviving nigrostriatal neurons. GDNF was more effective than BDNF. Expression of both neurotrophic factors did not support further improvements.
2. Materials and methods
2.1. Vectors
Two vectors were constructed that contained either a GDNF or a BDNF cDNA, an ires, and the Lac Z gene. First, a plasmid containing an ires/lac cassette was constructed [28,38]. The ires (617 bp) was isolated from plasmid 747 (gift from Dr. O’Malley, [29]) by a BamHI complete, HindIII partial digestion. A 4.0-kb fragment containing the Lac Z gene and the SV40 early region polyadenylation site was isolated from pBR-INS-TH-NFH-linker [38] by a NotI complete, HindIII partial digestion. These two fragments were inserted into pcDNA1 (Invitrogen) that had been digested with BamHI and NotI to yield pcDNAires/lac.
Next, a human BDNF cDNA (770 bp) was isolated from pHSVbdnf [15] by digestion with BamHI and HindIII. The 4.7-kb fragment containing the ires/lac cassette was isolated from pcDNAires/lac by a NotI complete, BamHI partial digestion. These two fragments were inserted into pcDNA1 that had been digested with NotI and HindIII to yield pcDNAbdnf/ires/lac.
We constructed pcDNAgdnf/ires/lac using a similar strategy. A human GDNF cDNA was amplified by PCR using pGDNF3a (gift from Dr. Bohn [27]) as the template. The primers were: 5′ GGGAAGCTTTCTAAGATGAAGTTATGGGATGTCG 3′ (nucleotides 44–69) and 5′ CGCGGATCCGTCAGATACATCCACACCGTTTAGC 3′ (complimentary to nucleotides 662–687). The PCR products were digested with HindIII and BamHI, and the fragment containing the ires/lac cassette was isolated as above. These two fragments were inserted into pcDNA1 that had been digested with NotI and HindIII to yield pcDNAgdnf/ires/lac.
pINS-TH-NFHlac (Lac Z-vector) has been described [44]. We modified this vector by destroying two of the three HindIII sites to yield pINS-TH-NFHlacΔ [36]. This vector contains a unique HindIII site at the 5′ end of the Lac Z gene. pcDNAbdnf/ires/lac or pcDNAgdnf/ires/lac were digested with HindIII complete and EcoRI partial. The ~5.5-kb bdnf/ires/lac or gdnf/ires/lac cassettes were isolated and inserted into pINS-TH-NFHlacΔ that had been digested with the same enzymes to yield pINS-TH-NFHbdnf/ires/lac (BDNF-vector) or pINS-TH-NFHgdnf/ires/lac (GDNF-vector), respectively.
Each of these three vectors (GDNF-vector, BDNF-vector, Lac Z-vector) contain a modified neurofilament heavy gene promoter (INS-TH-NFH promoter) [44]. A previously published time course, using a vector that contained this promoter, showed that the numbers of expressing cells in the striatum were relatively stable between 2 weeks and 6 months after gene transfer [44]. In two other studies, expression was observed for either 8 or 14 months [36,37], the longest time points examined.
2.2. Cell culture
2–2 cells [33] and Baby Hamster Kidney (BHK) fibroblast cells [35] were maintained in Dulbecco’s modified minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and 4 mM glutamine (Invitrogen) in humidified incubators containing 5% CO2 at 37 °C. G418 (0.5 mg/ml; RPI Corp.) was present during the growth of 2–2 cells, but was removed before plating the cells for HSV-1 vector packaging.
2.3. Vector packaging and titering
Vectors were packaged into HSV-1 particles using the helper virus-free packaging system [9], as modified to improve the titers [35]. Vector stocks were purified and concentrated as described [26].
For titering, 5 × 105 BHK cells in 0.5 ml of medium were plated in a 24-well plate and 4 h later transduced with a specific amount of a vector stock, or mock transduced [9]. One day later, immunocytochemistry was performed as described [37]. The primary antibodies were rabbit anti-GDNF (1:500 dilution, Santa Cruz), mouse anti-BDNF (1:500 dilution, Sigma), or rabbit anti-E. coli β-galactosidase (β-gal, 1:500 dilution, Sigma). β-gal was also detected by 5-bromo-4-chloro-3-indoyl-d-galactopyranoside (X-Gal, Sigma) staining [34]. The titers of vector genomes were determined by extracting DNA from vector stocks and measuring the amounts of vector DNAs using a PCR assay and primers for the Lac Z gene [42].
These vectors were titered on BHK fibroblast cells as the best available assay. BHK cells form a monolayer; in contrast, most neuronal cell lines, including PC12 cells, do not form a monolayer; and the titers obtained on BHK cells are higher than the titers obtained on PC12 cells. Expression from the modified neurofilament promoter (in these vectors) in fibroblast cells represents ectopic expression, and this ectopic expression declines rapidly at longer times after gene transfer.
2.4. Neurotrophic factor production in cultured cells
7 × 105 BHK cells in 0.5 ml of medium were transduced with a specific vector [0.1 multiplicity of infection (moi)], or mock transduced. One day later, the media were collected, and cell lysates were prepared using a commercial kit (Sigma). Cell lysates were treated with 1 N HCl pH ~3 for 15 min, followed by addition of 1 N NaOH to pH 7.6. Enzyme-linked immunosorbent assays (ELISA) were performed according to manufacturer’s instructions using the BDNF Emax or GDNF Emax kits (Promega).
2.5. Gene transfer and rotational testing
All animal procedures were approved by the W. Roxbury VA Hospital IACUC. Adult male Sprague–Dawley rats (225–250 gm, Charles River) were housed 2–3 per cage in a temperature-controlled room with a 12-h light–dark cycle. The rats had access to food and water ad libitum.
To establish no preexisting rotational bias, 2–3 days after arrival from the vendor, the rats were tested for apomorphine-induced rotational behavior (1 mg/kg i.p., Sigma) using a computer-controlled rotometer (Omnitech Electronics). The rat turning responses were monitored for a 45-min period. The number of full turns to the right or left side was recorded. Rats that exhibited <2 net turns/5 min were used in this study. Rats were randomly assigned to the different groups. Other investigators have used the same dose of apomorphine that we used in this study [6].
Rats were anesthetized with 60–80 mg/kg ketamine, 5–10 mg/kg xylazine, and placed in a stereotaxic frame. A specific vector or PBS was microinjected at each of 3 sites in the right striatum [3 μl/site: anterior–posterior (AP) 1.2, medial–lateral (ML) 3.0, dorsal–ventral (DV) −5.5; AP 0.2, ML 3.4, DV −5.5; AP 0.8, ML 4.4, DV −5.5] [18]. AP is relative to bregma, ML is relative to the sagittal suture, and DV is relative to the bregma–lambda plane [31]. Starting at 2 weeks after gene transfer, apomorphine-induced rotational behavior was tested for a 60-min period once per week for 2 weeks.
2.6. 6-OHDA lesioning
6-OHDA (3.5 μg/μl, Sigma) was dissolved in saline solution containing 0.2% ascorbic acid. 6-OHDA was microinjected at each of 4 sites (2 μl/site) in the right striatum (AP 1.3, ML 2.6, DV −5.0; AP 0.4, ML 3.0, DV −5.0; AP 0.4, ML 4.2, DV −5.0; AP 1.3, ML 4.5, DV −5.0) [18]. Starting 3 weeks later, the rats were tested for apomorphine-induced rotational behavior. The rats were tested once a week for the first 2 months and then twice a month, until the rats were sacrificed. Clockwise turns (ipsilateral to the lesion) were counted as positive turns, and counterclockwise turns were counted as negative turns. The net apomorphine-induced rotations performed by each rat were calculated as the difference between the clockwise and counterclockwise turns over the 60-min period following injection of apomorphine.
In an earlier study that used the same anesthetics and injected 6-OHDA into the midbrain, we performed in vivo dialysis, in the striatum, at an average of 3 months after gene transfer. The lesioned only control rats exhibited extracellular levels of l-DOPA, dopamine, and DOPAC that were 2–12% of the levels observed in normal rats, under high K+ conditions (P < 0.05) [37]. A ≥90% reduction in striatal dopamine levels is consistent with 6-OHDA lesions that ablate ≥90% of the nigrostriatal neurons [16].
2.7. Immunohistochemistry
Rats were perfused and immunohistochemistry was performed as described [36]. Twenty-five-micrometer coronal brain sections that contained either the midbrain or the striatum were cut on a freezing microtome. The primary antibodies were anti-GDNF, anti-BDNF, anti-β-gal (see vector packaging and titering section), mouse monoclonal anti-tyrosine hydroxylase (TH; 1:200 dilution, Roche Laboratories), and mouse monoclonal anti-NeuN (1:200 dilution, Chemicon).
2.8. Cell counts
For quantitative analyses of the numbers of TH-IR neurons, 25 μm coronal brain sections from the midbrain were analyzed. Stereology was performed using the optical dissector method and the StereoInvestigator program (MicroBrightField Inc.). Every 4th section was counted, and 10–15 of these sections contained the SNc. With reference to a rat atlas [31] and known landmarks, a contour was drawn around the SNc in each of the sections that contained the TH-IR cells. Stereological cell counts were performed under 60× magnification. The counting frame area was 7800 μm2, 270 to 480 sample sites per rat were counted from the lesioned or unlesioned hemisphere, respectively, and the coefficient of error (CE) for each rat was ≤10%. For immunofluorescent costaining assays, every 6th section that contained the striatum was stained with the appropriate antibodies, and, for each assay, at least 200 positive cells were scored from 4 randomly chosen sections.
2.9. Statistical analyses
The ELISA data, the behavioral data, and the cell counts were analyzed for statistically significant differences (SigmaStat, SPSS Inc.). Between-groups analysis of variance (ANOVA) followed by pairwise multiple comparisons (Tukey test or one-way ANOVA) were performed. Significant difference was accepted at the P < 0.05 level.
3. Results
3.1. HSV-1 vectors that express GDNF or BDNF
HSV-1 vectors were constructed that expressed either GDNF (GDNF-vector) or BDNF (BDNF-vector). Both of these vectors contained a single transcription unit that included either a GDNF or a BDNF cDNA, an ires, and the Lac Z gene. β-gal was expressed from each vector to facilitate detection of cells that contained recombinant gene products. A control vector expressed only β-gal (Lac Z-vector). Vectors were packaged using a helper virus-free HSV-1 packaging system, and the vector stocks were purified. Vector stocks were titered at 1 day after transduction of BHK fibroblast cells, using X-Gal staining. Both the GDNF-vector and the BDNF-vector had similar titers as the control Lac Z-vector [3–6 × 106 infectious vector particles (IVP)/ml]. Also, the titers of vector genomes (VG/ml) were determined by extracting DNA from vector stocks and measuring the amounts of vector DNAs using a PCR assay and primers for the Lac Z gene. The titers of vector genomes (VG/ml) were 60- to 80-fold higher than the titers of infectious vector particles (IVP/ml). Additionally, at 1 day after transduction of BHK cells, either GDNF-immunoreactivity (IR) or BDNF-IR was detected (not shown).
3.2. Production of GDNF or BDNF in cultured cells
BHK cells were transduced with the GDNF-vector, the BDNF-vector, or the control Lac Z-vector. One day later, the culture media were collected, and cell lysates were prepared. The levels of GDNF or BDNF were assayed by ELISA. The Lac Z-vector did not support the production of detectable levels of either neurotrophic factor compared to mock-transduced cultures (Table 1). GDNF or BDNF was detected in both the media and the cell lysates from cells transduced with either the GDNF-vector or the BDNF-vector, respectively (Table 1). The levels of each neurotrophic factor were significantly higher than those detected in the control conditions (Lac Z-vector or mock, P < 0.01 ANOVA). Slightly higher levels of BDNF were detected in the culture media and lower levels in the cell lysates compared to GDNF. These differences were not statistically significant (P > 0.05), and might be due to differences in the efficiency of translation or the stability of either the mRNAs or the proteins, in this heterologous host cell. The purpose of this experiment was to establish that the GDNF-vector and the BDNF-vector supported production of the predicted neurotrophic factor, as shown in Table 1.
Table 1.
The GDNF-vector or the BDNF-vector supported production of either GDNF or BDNF, respectively, following gene transfer into cultured fibroblast cells
| GDNF (pg/ml)
|
BDNF (pg/ml)
|
|||
|---|---|---|---|---|
| Condition | Media | Cell lysate | Media | Cell lysate |
| GDNF-vector | 396 ± 95 | 441 ± 247 | ||
| BDNF-vector | 850 ± 420 | 207 ± 108 | ||
| Lac Z-vector | 40 ± 20 | 47 ± 33 | 25 ± 18 | 76 ± 11 |
| Mock | 50 ± 43 | 88 ± 32 | 22 ± 6 | 112 ± 18 |
BHK cells were transduced with vector stocks (0.1 moi). One day later, media were collected and cell lysates were prepared, and neurotrophic factor levels were assayed by ELISAs. Experiments were performed in duplicate and repeated 3 times, and the mean ± SD are shown.
3.3. Expression of GDNF or BDNF in the rat striatum
In initial experiments, the rats were sacrificed at 4 days after either the GDNF-vector or the BDNF-vector was microinjected into the striatum. In a rat that received the BDNF-vector, sections from the striatum proximal to the injection sites contained BDNF-IR and β-gal-IR, and costained cells were observed (Figs. 1a–c). Adjacent sections were costained for BDNF-IR and a marker for neurons, NeuN [30], and the results showed that BDNF-IR was present in neurons (Figs. 1d–f). No BDNF-IR cells were observed in the contralateral, uninjected striatum (Figs. 1g–i). In a rat that received the GDNF-vector, GDNF-IR was detected in neurons (not shown).
Fig. 1.
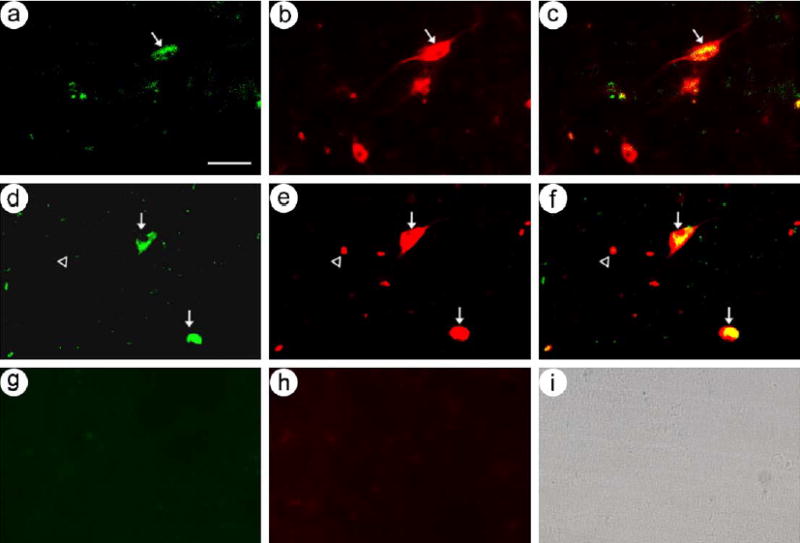
The BDNF-vector supported expression of BDNF in striatal neurons in rats sacrificed at 4 days after gene transfer. Gene products were colocalized to the same cells using rhodamine- or fluorescein-conjugated secondary antibodies. (a–c) BDNF and β-gal were present in the same cells; (a) BDNF-IR, (b) β-gal-IR, and (c) merge. The arrows point to costained cells. (d–f) BDNF was expressed in neurons; (d) BDNF-IR, (e) NeuN-IR, and (f) merge. Arrows, costained cells; arrowheads, cells that contain only NeuN-IR. (g–i) BDNF and β-gal were not expressed in the contralateral, uninjected striatum; (g) BDNF-IR, (h) β-gal-IR, and (i) a brightfield photomicrograph showing the cells in this section. Scale bar, 30 μm.
Both the GDNF-vector and the BDNF-vector contained the Lac Z gene after an ires (bdnf/ires/lac or gdnf/ires/lac cassettes), and the two genes were coexpressed in the preponderance of the transfected striatal cells. Most of the GDNF-IR or the BDNF-IR was detected in cell bodies, and some staining was observed in proximal processes. Similarly, most of the β-gal-IR was detected in the cell bodies, and, in a few cells, the β-gal-IR extended into the proximal processes. Lac Z is the second gene in these cassettes, and previous observations showed that the gene after the ires in such cassettes was expressed at a lower level than the first gene [36,37].
Some positive cells were observed near the needle tracks in neocortex, but the majority of the transfected cells was detected in the striatum, within ~2.5 mm in the anteroposterior direction. Due to retrograde transport of the vector, a small number of transfected cells were observed in distant sites, consistent with earlier studies that delivered HSV-1 vectors into the striatum [7,35,36,37,44]. The current studies focused on the transfected striatal cells near the injection sites.
3.4. Long-term, neuron-specific expression of GDNF or BDNF in the striatum
GDNF-IR or BDNF-IR was observed in striatal cells from rats that were sacrificed at 7 months after gene transfer with either the GDNF-vector (Fig. 2a) or the BDNF-vector (Fig. 2b), respectively, and no GDNF-IR was detected in the contralateral striatum (Fig. 2c). We coinjected the GDNF-vector and the BDNF-vector, the rats were sacrificed 7 months later, and GDNF-IR and BDNF-IR were detected in the same striatal cells (Figs. 2d–f). Neither GDNF-IR nor BDNF-IR cells were detected in the contralateral striatum (not shown). Cell counts showed that ~95% of the GDNF-IR cells also contained BDNF-IR (Table 2).
Fig. 2.

The GDNF-vector or the BDNF-vector supported long-term expression of GDNF or BDNF, respectively, in striatal neurons. The rats were sacrificed at 7 months after gene transfer. (a) GDNF-vector with GDNF-IR, (b) BDNF-vector with BDNF-IR, and (c) no GDNF-IR was detected in the contralateral striatum (GDNF-vector). (d–f) GDNF and BDNF were detected in the same striatal cells in a rat that received a mixture of the GDNF- and BDNF-vectors; (d) GDNF-IR, (e) BDNF-IR, and (f) merge. (g–l) GDNF or BDNF were expressed in neurons in rats that received either the GDNF-vector or the BDNF-vector, respectively. (g–i) GDNF-vector; (g) GDNF-IR, (h) NeuN-IR, and (i) merge. Arrows, costained cells; arrowheads, cells that contain only NeuN-IR. (j –l) BDNF-vector; (j) BDNF-IR, (k) NeuN-IR, and (l) merge. Scale bars: (a–c) 40 μm; (d–l) 50 μm.
Table 2.
Coinjection of the GDNF- and BDNF-vectors supported expression of GDNF and BDNF in the same striatal cells, and these vectors supported neuron-specific expression
| Comparison
|
Numbers of positive cells
|
|||
|---|---|---|---|---|
| First IR | Second IR | First IR | Second IR | % Costained |
| GDNF | BDNF | 242 | 230 | 95 |
| GDNF | NeuN | 252 | 226 | 90 |
| BDNF | NeuN | 221 | 200 | 91 |
Immunofluorescent costaining using antibodies to the indicated proteins was performed on sections that contained the striatum from rats sacrificed at 7 months after microinjection of the GDNF-vector, or the BDNF-vector, or a mixture of the GDNF- and BDNF-vectors. The positive cells were counted in 4 randomly chosen sections from each rat.
GDNF-IR or BDNF-IR were detected in neurons (NeuN-IR) in rats that were sacrificed at 7 months after receiving either the GDNF-vector (Figs. 2g–i) or the BDNF-vector (Figs. 2j–l), respectively. Cell counts showed that ~90% of the GDNF-IR or the BDNF-IR cells were neurons (Table 2).
3.5. Comparison of GDNF or BDNF for reversing behavioral impairments in the 6-OHDA rat model of PD
The GDNF-vector, the BDNF-vector, or a mixture of both the GDNF- and BDNF-vectors was microinjected unilaterally into striatum [18]. Controls included rats that received either the Lac Z-vector or PBS. Following gene transfer, the rats were tested 2 times with apomorphine to assess the impact of expression of recombinant GDNF, or BDNF, or both, on apomorphine-induced rotational behavior. A few of the rats that received the GDNF-vector displayed an increased rotational bias, but the change was not significant (Fig. 3, P > 0.05 ANOVA). None of the rats in the other groups showed a significant turning bias compared to before gene transfer (P > 0.05).
Fig. 3.

The GDNF-vector, the BDNF-vector, or coinjection of both vectors, supported behavioral correction of the 6-OHDA rat model of PD. Rats received the GDNF-vector, the BDNF-vector, a mixture of the GDNF- and BDNF-vectors, or the Lac Z-vector or PBS (control group). Following gene transfer, the rats were tested twice with apomorphine. At 4 weeks after gene transfer, the rats were lesioned by intrastriatal injection of 6-OHDA. Starting 3 weeks later, the rats were tested with apomorphine once a week for the first 2 months and then twice a month for the next 4 months. The values shown are the mean number of rotations for each group at each time point, for the 60-min period after injection of apomorphine [mean ± SEM; GDNF-vector, n = 9 rats; BDNF-vector, n = 7 rats; mixture of GDNF- and BDNF-vectors, n = 10 rats; controls, n = 12 rats (Lac Z-vector, n = 8 rats or PBS, n = 4 rats)].
At 4 weeks after gene transfer, the rats were lesioned by intrastriatal injection of 6-OHDA [18]. Starting at 3 weeks after lesioning, the rats were tested with apomorphine once a week for the first 2 months and then twice a month for the next 4 months. Significant differences gradually developed between the rats in the different groups, as shown by a between-groups ANOVA (Fig. 3, P < 0.001). Subsequent pairwise multiple comparisons (Tukey test) showed that the control group, rats that received either the Lac Z-vector or PBS, maintained high rotational rates throughout the testing period, and there was no significant difference between these treatments (Lac Z-vector vs. PBS, P > 0.05). Of note, the rats that received the GDNF-vector, or the BDNF-vector, or a mixture of the GDNF- and BDNF-vectors, displayed significant, 30% to 70%, reductions in apomorphine-induced rotational behavior compared to the control group [Fig. 3, GDNF-vector, BDNF-vector, or mixture of GDNF- and BDNF-vectors vs. controls (Lac Z-vector or PBS); P < 0.001, P < 0.05, P < 0.05, respectively]. These reductions were maintained for 6 months (Fig. 3). Importantly, the rats that received the GDNF-vector displayed a larger reduction in apomorphine-induced rotational behavior compared to the rats that received either the BDNF-vector or a mixture of the GDNF- and BDNF-vectors (GDNF-vector vs. BDNF-vector P < 0.02; GDNF-vector vs. mixture of GDNF- and BDNF-vectors P < 0.01). There was no significant difference in rotation rates between the rats that received the BDNF-vector or a mixture of the GDNF- and BDNF-vectors (P > 0.05).
The behavioral data shown in Fig. 3 appear to have three stages. During the first 8 weeks after microinjection of 6-OHDA, there was a gradual increase in the numbers of rotations in the control group, which likely reflects the gradual progression of the lesion to a steady state level. During weeks 9 to 16, the numbers of rotations were relatively stable for each group. During weeks 18 to 26, there was a large increase in the numbers of rotations by the control group, which we have not observed in other experiments. Thus, the period from weeks 9 to 16 appears to represent the steady state numbers of rotations, before the unusual increase in rotations by the control group. Therefore, we compared the numbers of rotations performed by the rats in each group, during the period from weeks 9 to 16 (Fig. 3). A between-groups ANOVA showed that there was an overall effect of group (weeks 9–16: P < 0.001). Subsequent pairwise multiple comparisons (Tukey test) showed that within the control group, rats that received either the Lac Z-vector or PBS exhibited no difference (weeks 9–16: Lac Z-vector vs. PBS, P > 0.05), and these two subgroups were combined into one control group. The rats that received the GDNF-vector, or the BDNF-vector, or a mixture of the GDNF- and BDNF-vectors, displayed significant reductions in the numbers of apomorphine-induced rotations compared to the control group (weeks 9–16: GDNF-vector, BDNF-vector, or mixture of GDNF- and BDNF-vectors vs. control group; P < 0.001, P < 0.02, P < 0.001, respectively). Of note, the rats that received the GDNF-vector displayed a larger reduction in the numbers of apomorphine-induced rotations than the rats that received either the BDNF-vector or a mixture of the GDNF- and BDNF-vectors (weeks 9–16: GDNF-vector vs. BDNF-vector or mixture of GDNF- and BDNF-vectors, P < 0.001). Additionally, there was a significant difference in the numbers of apomorphine-induced rotations between the rats that received the BDNF-vector or a mixture of the GDNF- and BDNF-vectors (weeks 9–16: P < 0.02).
Any side effects caused by the vector system [39] were not detected using the current assays. The experimental rats showed normal weights, and survived until sacrificed. Consistent with previously studies [9,36,37], there was minimal cell infiltration near the injection sites in rats sacrificed at 4 days after gene transfer, and virtually no cell infiltration was detected in rats sacrificed at 7 months after gene transfer. Also, no brain tumors were observed.
3.6. Comparison of GDNF or BDNF for protecting nigrostriatal neurons following 6-OHDA lesioning
Rats were lesioned with 6-OHDA, and the extent of the lesion was verified in selected rats that were sacrificed between 2 and 6 months after lesioning. Sections from the striatum or the midbrain of a rat sacrificed at 2.5 months after lesioning were stained for TH-IR. The unlesioned, contralateral striatum contained dense staining for TH-IR processes, but the lesioned striatum contained minimal staining for TH-IR processes (not shown). The midbrain in the contralateral, unlesioned hemisphere contained numerous TH-IR cell bodies and processes in both the ventral tegmental area (VTA) and the SNc (Fig. 4a). In contrast, in the lesioned hemisphere, the VTA contained numerous TH-IR cell bodies and processes, but the SNc contained few TH-IR cell bodies and processes (Fig. 4b).
Fig. 4.
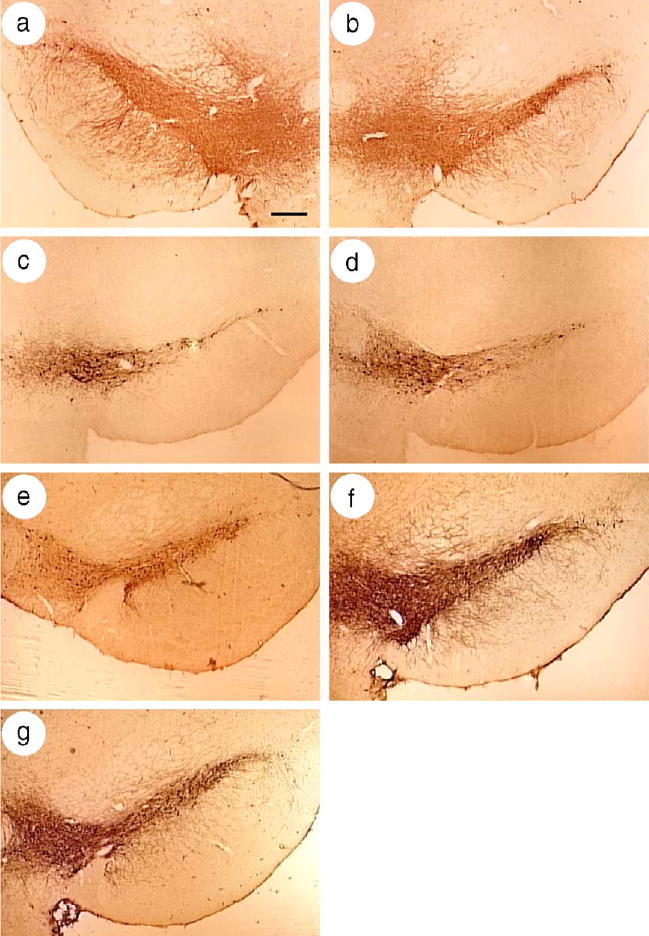
Microinjection of the GDNF-vector, or the BDNF-vector, or both vectors, protected nigrostriatal neurons from 6-OHDA. Sections from the midbrain were stained for TH-IR. (a and b) A rat was lesioned by intrastriatal microinjection of 6-OHDA, and sacrificed at 2.5 months later (no gene transfer). (a) The SNc in the unlesioned, contralateral hemisphere contained numerous TH-IR cells and processes, but (b) the SNc in the lesioned hemisphere contained few TH-IR cells and processes. The VTA contained similar numbers of TH-IR cells in both the lesioned and contralateral hemispheres. (c–g) The SNc in the ipsilateral, lesioned hemispheres from rats in the different groups that were sacrificed at 6 months after lesioning. (c) A 6-OHDA lesion reduced the number of TH-IR cells in the SNc, and (d) a similar reduction was observed in a rat that received the Lac Z-vector. (e–g) Partial protection of the TH-IR cells in the SNc was observed in rats that received (e) the BDNF-vector, or (f) the GDNF-vector, or (g) a mixture of the GDNF- and BDNF-vectors. Scale bar, 300 μm.
The rats that were tested for apomorphine-induced rotational behavior were sacrificed at 6 months after lesioning and analyzed for the numbers of TH-IR neurons in the SNc. In rats that received either lesion-only (Fig. 4c) or the Lac Z-vector (Fig. 4d), the lesioned hemispheres contained fewer TH-IR neurons in the SNc, compared to the unlesioned hemispheres (Fig. 4a). In contrast, in the lesioned hemispheres, the numbers of TH-IR neurons in rats that received the BDNF-vector (Fig. 4e), or the GDNF-vector (Fig. 4f), or a mixture of the GDNF- and BDNF-vectors (Fig. 4g) were higher than in the controls (Figs. 4c and d).
To quantify these observations, stereological cell counts were performed to determine the numbers of TH-IR cells in the SNc, in both the lesioned hemispheres and the contralateral, control hemispheres (Table 3). The results showed that the lesioned hemispheres in the control rats contained only 20% of the TH-IR neurons that were observed in the unlesioned hemispheres (Table 3, P < 0.001 ANOVA). There was no significant difference in the number of TH-IR neurons in the lesioned hemispheres between the rats that received the Lac Z-vector or lesion-only (P > 0.05). Of note, in rats that received the GDNF-vector, or the BDNF-vector, or a mixture of the GDNF- and BDNF-vectors, the lesioned hemispheres contained 53%, 33%, or 44% of the number of TH-IR cells that were found in the unlesioned hemispheres, respectively (Table 3). A between-groups ANOVA showed a significant difference in the numbers of TH-IR cells in the lesioned hemispheres of the rats in the different groups (P < 0.001). Importantly, subsequent pairwise multiple comparisons (Tukey test) showed that the GDNF-vector supported a higher number of TH-IR cells in the lesioned hemispheres compared to the BDNF-vector (P < 0.05). In the group that received a mixture of the GDNF- and BDNF-vectors, the lesioned hemispheres contained numbers of TH-IR cells that were between those in the BDNF-vector and the GDNF-vector groups, but not significantly different compared to either group (GDNF- and BDNF-vectors vs. GDNF-vector or BDNF-vector P > 0.05).
Table 3.
Stereological counts of the numbers of TH-IR neurons in the SNc from rats sacrificed at 6 months after the 6-OHDA lesion
| Numbers of TH-IR positive neurons
|
|||
|---|---|---|---|
| Condition | Lesioned hemisphere | Unlesioned hemisphere | % of surviving neuronsa |
| GDNF-vector | 4443 ± 504 | 8125 ± 856 | 53 ± 6 |
| BDNF-vector | 2735 ± 473 | 33 ± 6 | |
| GDNF- and BDNF-vectors | 3706 ± 411 | 44 ± 5 | |
| Lac Z-vector | 1704 ± 603 | 8674 ± 1622 | 20 ± 7 |
| Lesion-only | 1886 ± 556 | 8429 ± 632 | 22 ± 7 |
At 4 weeks after gene transfer, the rats were lesioned with 6-OHDA, and the rats were sacrificed 6 months later. TH-IR was detected in the midbrain, cells were counted by unbiased stereology, and the mean ± SEM are shown [GDNF-vector, n = 7 rats; BDNF-vector, n = 7 rats; mixture of GDNF- and BDNF-vectors, n = 6 rats; controls, n = 8 rats (Lac Z-vector, n = 5 rats; lesion-only, n = 3 rats)].
The mean number of TH-IR neurons (mean ± SEM) in the lesioned hemispheres for a specific condition was divided by the mean number of TH-IR neurons from all the unlesioned hemispheres.
4. Discussion
In this study, we compared GDNF, or BDNF, or both, for their capabilities to correct behavioral deficits and protect nigrostriatal dopaminergic neurons in a rat model of PD. Long-term expression (7 months) of GDNF or BDNF was observed. Of note, the GDNF-vector supported a significantly higher level of behavioral correction than either the BDNF-vector or a mixture of both the GDNF- and BDNF-vectors. Additionally, the GDNF-vector was more effective than the BDNF-vector for protecting nigrostriatal neurons.
Both the GDNF-vector and the BDNF-vector supported expression of the predicted proteins, GDNF and β-gal or BDNF and β-gal, respectively, in cultured fibroblast cells or in striatal cells from rats sacrificed at 4 days or 7 months after gene transfer. Neuronal-specific expression of GDNF or BDNF was observed in rats sacrificed at 4 days or 7 months after gene transfer. In addition, in rats sacrificed at 7 months after gene transfer with a mixture of the GDNF- and BDNF-vectors, GDNF and BDNF were detected in the same cells. These results suggest that following microinjection of these vectors, the predicted gene products were coexpressed in striatal neurons for 7 months. This result is consistent with our previous reports that the modified neurofilament heavy gene promoter used in these vectors supported long-term expression of one or multiple gene(s) [36,37,44]. In a previous study that used similar gene transfer conditions, the predicted gene products were coexpressed in an average of ~11,400 predominately GABAergic striatal neurons for 6 months, and significant levels of expression were maintained for up to 14 months [37].
GDNF and BDNF are usually tested separately, raising the questions of which factor is more effective, and if these two factors can act synergistically to increase correction of behavioral deficits and protection of nigrostriatal neurons. The results showed that the GDNF-vector supported both behavioral correction and protection of nigrostriatal neurons. In contrast, the BDNF-vector supported behavioral correction, but failed to support protection of nigrostriatal neurons. This observation is consistent with a previous study that reported that expression of BDNF in rat striatal cells, using an AAV vector, improved amphetamine-induced rotational behavior, but had no significant effect on the number of surviving nigrostriatal neurons [21]. In the absence of protection from 6-OHDA-induced neuron loss, BDNF might exert a modulating effect on the remaining nigrostriatal dopaminergic neurons, thereby increasing dopamine release in the striatum. Alternately, higher levels of BDNF than those achieved in either this study or the study with AAV vectors [21] may be required to protect nigrostriatal neurons.
In regard to potential synergistic effects from both neurotrophic factors, a lower level of behavioral correction was observed in the rats that received a mixture of the GDNF- and BDNF-vectors compared to the rats that received either the GDNF-vector or the BDNF-vector. In addition, the GDNF-vector supported a slightly higher (not statistically significant) level of protection of nigrostriatal neurons compared to the mixture of both vectors. In summary, these results suggest that GDNF is more effective than BDNF, and GDNF and BDNF do not act synergistically to increase either correction of behavioral deficits or protection of nigrostriatal neurons in the 6-OHDA rat model of PD.
GDNF supported partial behavioral correction and partial protection of nigrostriatal neurons. These results are consistent with previous studies that used AAV vectors to express GDNF in striatal cells, and observed behavioral correction and protection of nigrostriatal neurons [18,20]. In contrast, following AAV vector-mediated delivery of GDNF into the SNc, no behavioral correction was obtained despite more than 90% protection of nigrostriatal neurons [18], suggesting that sparing of nigrostriatal neurons in the absence of a functional striatal innervation is insufficient for functional recovery. These observations suggest that damaged nigrostriatal neurons may not be completely protected despite the potent effects of GDNF. It is also possible that higher levels and/or more widespread expression of GDNF in the striatum may be required to achieve a higher level of protection of nigrostriatal neurons. However, overexpression of GDNF from a lentivirus vector caused downregulation of TH activity in nigrostriatal neurons [12,13,32]. Recently, expression of a low level of GDNF, from an AAV vector, in the striatum of the monkey model of PD, was found to support neuroprotection and improve motor behavior, without downregulation of TH activity [8]. These observations indicate that when the nigrostriatal dopaminergic system is in a highly compromised state, precise control over the levels of recombinant gene expression is critical. Thus, use of an inducible promoter to regulate gene expression may be beneficial.
Acknowledgments
We gratefully thank Dr. K. O’Malley for the TH promoter, Dr. W. W. Schlaepfer for the NFH promoter, Dr. G. Felsenfeld for the β-globin insulator, Dr. A. Davison for HSV-1 cosmid set C, Dr. R. Sandri-Goldin for 2–2 cells, and Dr. M. Bohn for a GDNF cDNA. This work was supported by AG20177 and the National Parkinson Foundation/Parkinson’s Disease Foundation (MS); and AG16777, NS42016, NS045855, and AG021193 (AG).
References
- 1.Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altar CA, Boylan CB, Fritsche M, Jones BE, Jackson C, Wiegand SJ, Lindsay RM, Hyman C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal dopamine lesions. J Neurochem. 1994;63:1021–1032. doi: 10.1046/j.1471-4159.1994.63031021.x. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, Levivier M, Peschanski M, Studer L, Barker R. Neural transplantation for the treatment of Parkinson’s disease. Lancet Neurol. 2003;2:437–445. doi: 10.1016/s1474-4422(03)00442-3. [DOI] [PubMed] [Google Scholar]
- 5.Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 6.During MJ, Freese A, Deutch AY, Kibat PG, Sabel BA, Langer R, Roth RH. Biochemical and behavioral recovery in a rodent model of Parkinson’s disease following stereotactic implantation of dopamine-containing liposomes. Exp Neurol. 1992;115:193–199. doi: 10.1016/0014-4886(92)90053-s. [DOI] [PubMed] [Google Scholar]
- 7.During MJ, Naegele JR, O’Malley KL, Geller AI. Long-term behavioral recovery in parkinsonian rats by an HSV vector expressing tyrosine hydroxylase. Science. 1994;266:1399–1403. doi: 10.1126/science.266.5189.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J Neurosci. 2005;25:769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gash DM, Zhang Z, Cass WA, Ovadia A, Simmerman L, Martin D, Russell D, Collins F, Hoffer BJ, Gerhardt GA. Morphological and functional effects of intranigrally administered GDNF in normal rhesus monkeys. J Comp Neurol. 1995;363:345–358. doi: 10.1002/cne.903630302. [DOI] [PubMed] [Google Scholar]
- 11.Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 12.Georgievska B, Kirik D, Bjorklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- 13.Georgievska B, Kirik D, Bjorklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 15.Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 16.Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195:123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- 17.Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 18.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirik D, Georgievska B, Burger C, Winkler C, Muzyczka N, Mandel RJ, Bjorklund A. Reversal of motor impairments in parkinsonian rats by continuous intrastriatal delivery of l-dopa using rAAV-mediated gene transfer. Proc Natl Acad Sci U S A. 2002;99:4708–4713. doi: 10.1073/pnas.062047599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 21.Klein RL, Lewis MH, Muzyczka N, Meyer EM. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999;847:314–320. doi: 10.1016/s0006-8993(99)02116-2. [DOI] [PubMed] [Google Scholar]
- 22.Kordower JH. In vivo gene delivery of glial cell line-derived neurotrophic factor for Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S120–S132. doi: 10.1002/ana.10485. (discussion S132-4). [DOI] [PubMed] [Google Scholar]
- 23.Kordower JH, Bloch J, Ma SY, Chu Y, Palfi S, Roitberg BZ, Emborg M, Hantraye P, Deglon N, Aebischer P. Lentiviral gene transfer to the nonhuman primate brain. Exp Neurol. 1999;160:1–16. doi: 10.1006/exnr.1999.7178. [DOI] [PubMed] [Google Scholar]
- 24.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 25.Levivier M, Przedborski S, Bencsics C, Kang UJ. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J Neurosci. 1995;15:7810–7820. doi: 10.1523/JNEUROSCI.15-12-07810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim F, Hartley D, Starr P, Lang P, Song S, Yu L, Wang Y, Geller AI. Generation of high-titer defective HSV-1 vectors using an IE 2 deletion mutant and quantitative study of expression in cultured cortical cells. BioTechniques. 1996;20:460–469. doi: 10.2144/19962003460. [DOI] [PubMed] [Google Scholar]
- 27.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 28.T. Maniatis, E.F. Fritsch, J. Sambrook, Molecular Cloning, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1989.
- 29.Moffat M, Harmon S, Haycock J, O’Malley KL. l-Dopa and dopamine-producing gene cassettes for gene therapy approaches to Parkinson’s disease. J Neurochem. 1997;68:1792–1803. doi: 10.1006/exnr.1996.6390. [DOI] [PubMed] [Google Scholar]
- 30.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 31.G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, Sidney, 1986.
- 32.Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith IL, Hardwicke MA, Sandri-Goldin RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 34.Song S, Wang Y, Bak SY, Lang P, Ullrey D, Neve RL, O’Malley KL, Geller AI. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J Neurochem. 1997;68:1792–1803. doi: 10.1046/j.1471-4159.1997.68051792.x. [DOI] [PubMed] [Google Scholar]
- 35.Sun M, Zhang GR, Yang T, Yu L, Geller AI. Improved titers for helper virus-free herpes simplex virus type 1 plasmid vectors by optimization of the packaging protocol and addition of noninfectious herpes simplex virus-related particles (previral DNA replication enveloped particles) to the packaging procedure. Hum Gene Ther. 1999;10:2005–2011. doi: 10.1089/10430349950017365. [DOI] [PubMed] [Google Scholar]
- 36.Sun M, Zhang G, Kong L, Holmes C, Wang X, Zhang W, Goldstein DS, Geller AI. Correction of a rat model of Parkinson’s disease by coexpression of tyrosine hydroxylase and aromatic amino acid decarboxylase from a helper virus-free herpes simplex virus type 1 vector. Hum Gene Ther. 2003;14:415–424. doi: 10.1089/104303403321467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun M, Kong L, Wang X, Holmes C, Gao Q, Zhang W, Pfeilschifter J, Goldstein DS, Geller AI. Coexpression of tyrosine hydroxylase, GTP I. Cyclohydrolase, aromatic amino acid decarboxylase, and vesicular monoamine transporter-2 from a helper virus-free HSV-1 vector supports high-level, long-term biochemical and behavioral correction of a rat model of Parkinson’s disease. Hum Gene Ther. 2004;15:1177–1196. doi: 10.1089/hum.2004.15.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Zhang G, Sun M, Geller AI. General strategy for constructing large HSV-1 plasmid vectors that co-express multiple genes. BioTechniques. 2001;31:204–212. doi: 10.2144/01311dd05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood MJ, Byrnes AP, Pfaff DW, Rabkin SD, Charlton HM. Inflammatory effects of gene transfer into the CNS with defective HSV-1 vectors. Gene Ther. 1994;1:283–291. [PubMed] [Google Scholar]
- 40.M.D. Yahr, K.J. Bergmann, Parkinson’s Disease, Raven Press, New York, 1987.
- 41.Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM. Treatment of parkinsonism with levodopa. Arch Neurol. 1969;21:343–354. doi: 10.1001/archneur.1969.00480160015001. [DOI] [PubMed] [Google Scholar]
- 42.Yang T, Zhang G, Zhang W, Sun M, Wang X, Geller AI. Enhanced reporter gene expression in the rat brain from helper virus-free HSV-1 vectors packaged in the presence of specific mutated HSV-1 proteins that affect the virion. Mol Brain Res. 90;2001:1–16. doi: 10.1016/s0169-328x(01)00059-6. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto Y, Lin Q, Collier TJ, Frim DM, Breakefield XO, Bohn MC. Astrocytes retrovirally transduced with BDNF elicit behavioral improvement in a rat model of Parkinson’s disease. Brain Res. 1995;691:25–36. doi: 10.1016/0006-8993(95)00596-i. [DOI] [PubMed] [Google Scholar]
- 44.Zhang G, Wang X, Yang T, Sun M, Zhang W, Wang Y, Geller AI. A tyrosine hydroxylase-neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Mol Brain Res. 2000;84:17–31. doi: 10.1016/s0169-328x(00)00197-2. [DOI] [PubMed] [Google Scholar]


