Abstract
A defective herpes simplex virus 1 (HSV-1) vector, pHSVlac, has been developed that contains a transcription unit that places the Escherichia coli lacZ gene under the control of the HSV-1 immediate early 4/5 promoter. The vector pHSVlac was propagated with the HSV-1 temperature-sensitive mutant ts K as helper virus. Infection of neurons from rat superior cervical ganglia and dorsal root ganglia in primary culture resulted in stable expression of high levels of β-galactosidase without cell death. These HSV-1 vectors should be useful for introducing genes into postmitotic cells, such as neurons, in vitro and in vivo.
Methods of delivering genes into the cells of the nervous system are required before the functions of cloned neuronal genes may be studied. Of the four approaches used to introduce genes into cells [the frog oocyte micro-injection system (1), transgenic mice (2), transfection of DNA directly into cells (3), and retrovirus vectors (4)], none can deliver a gene directly into nonmitotic cells. Here we report that pHSVlac, a defective HSV-1 vector that expresses the Escherichia coli lacZ gene from the HSV-1 immediate early (IE) 4/5 promoter, can infect peripheral neurons in primary culture and stably express high levels of β-galactosidase.
HSV-1 has great promise as a virus vector system (5). It has a wide host range; HSV-1 infects many cell types in mammals and birds (6). In addition, HSV-1 infects postmitotic neurons in adult animals and can be maintained indefinitely in a latent state (7). Latent HSV-1 is quiescent: HSV-1 gene expression is limited at most to the IE genes and a latency-associated transcript (8), DNA replication does not occur (7), and no progeny virus are produced (7). Electrophysiological properties are unaltered in latently infected neurons (9).
In virions, HSV-1 vectors are composed of head-to-tail repeats (10). The repeats are 5 to 15 kb in size, for up to 150 kb, which is the size of the HSV-1 genome. The vectors are maintained because of their growth advantage over the helper virus; HSV-1 contains three origins of DNA replication (ori), or one ori every 50 kb, whereas a vector contains one ori every 5 to 15 kb (10).
HSV-1 vectors have been propagated in a virus stock with wild-type HSV-1 as helper virus (10). The wild-type HSV-1 in the virus stock invariably causes cell death. However, intracerebral injection (11) and infection of mouse neuroblastoma cells (12) with HSV-1 temperature-sensitive (ts) mutants allow persistence of the virus without cell death. We obtained HSV-1 vectors that can infect cells without causing cell death by using ts mutants of HSV-1 as helper virus (13). At the restrictive temperature of 37° or 39°C the ts mutations block the lytic cycle and thereby prevent cell damage. Virus is grown at the permissive temperature of 31°C.
The 8.1-kb defective HSV-1 vector, pHSVlac, is shown in Fig. 1. The vector pHSVlac contains three kinds of genetic elements: (i) sequences that allow propagation of pHSVlac in E. coli (the ampicillin resistance gene and the col E1 ori); (ii) sequences from HSV-1 that support propagation of pHSVlac in an HSV-1 virus stock [the HSV-1 oris, an HSV-1 ori (14), and the HSV-1 “a” sequence, the packaging site (15)]; and (iii) a transcription unit. The components of the transcription unit are the HSV-1 IE 4/5 promoter (14), the intervening sequence following that promoter, the E. coli lacZ gene (16), and the SV40 early region polyadenylation site (16). The E. coli lacZ gene encodes a β-galactosidase absent from mammalian cells, thereby providing an assay for expression of the transcription unit in pHSVlac (17). pHSVlac DNA was packaged into HSV-1 virus particles, with HSV-1 strain 17 ts K (18) as helper virus. ts K has a mutation in the IE 3 gene, has an immediate early phenotype, and is not permissive for DNA replication.
Fig. 1.
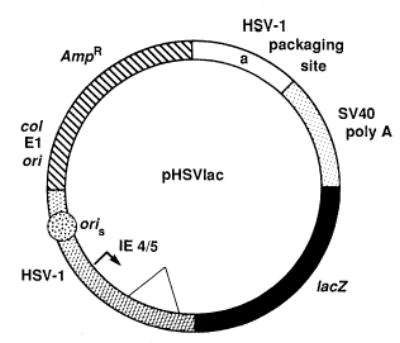
The structure of pHSVlac. The clear region contains the HSV-1 a segment, nucleotides 127 to 1132, the packaging site (15). The crosshatched region symbolizes the HSV-1 c region, nucleotides 47 to 1066 (14). pHSVlac was constructed (13) from pCH110 (16).
To determine if pHSVlac virus stock could infect neurons and express β-galactosidase, we prepared primary cultures of dissociated neurons (19) from dorsal root ganglia and superior cervical ganglia of newborn rats. Cultures were infected with pHSVlac virus stock, then incubated for 24 hours at 37°C, fixed, and assayed for β-galactosidase activity in situ (17) by using the chromogenic substrate 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal). At a multiplicity of infection (MOI) of 0.1 to 0.4 of pHSVlac about 38% of the cells in the cultures of dorsal root ganglia (Fig. 2, A to C) and 11% of the cells in the cultures of superior cervical ganglia (Fig. 2, D to F) were β-galactosidase–positive. Experiments performed at higher ratios of pHSVlac virus to cells (MOI 2) resulted in expression of β-galactosidase in virtually every cell. Cultures infected with ts K alone or mock-infected cultures contained less than 0.2% β-galactosidase–positive cells.
Fig. 2.

Expression of β-galactosidase from pHSVlac in cells from dorsal root ganglia (A to C) and superior cervical ganglia (D to F) in primary culture. Virus stock containing pHSVlac was prepared (13) with HSV-1 strain 17 ts K (18) as helper virus. The titer of the virus stock was 1 × 106 plaque-forming units (PFU) of ts K per milliter and 8 × 105 infectious particles of pHSVlac per milliter. Dissociated cell cultures (19) were prepared from newborn rat dorsal root ganglia or superior cervical ganglia and treated for 24 hours with 10−5M cytosine arabinoside. After 10 days in vitro, the cultures contained 3 to 8 × 105 cells per 35-mm plate; cultures were then infected with 0.1 ml of pHSVlac virus stock and incubated for 24 hours at 37°C. Cells were fixed with 0.5% glutaraldehyde and stained for β-galactosidase activity with X-gal (17). The width of the photomicrograph represents 230 μm.
Most of the β-galactosidase–positive cells shown in Fig. 2 have the morphological characteristics of neurons. However, β-galactosidase–positive cells that resembled glia were also observed. To determine whether some of the β-galactosidase–positive cells were indeed neurons, we performed an experiment to find out whether β-galaaosidase and a neuronal marker were present in the same cell. Primary cultures of dorsal root ganglia (19) were infected with pHSVlac virus stock, incubated for 24 hours at 37°C, fixed, and treated with a rabbit antiserum to β-galactosidase and a mouse monoclonal antibody to one of two neuronal markers, either the A2B5 antigen (20) or the 150-and 180-kD subunits of neurofilament (21). β-Galactosidase–like immunoreactivity (β-gal-IR) was visualized with a rhodamine-conjugated goat antibody to rabbit immunoglobulin G, and A2B5 or neurofilament-like immunoreactivity (A2B5-IR or Nf-IR) was visualized with a fluorescein-conjugated goat antibody to mouse immunoglobulin G. Many of the same cells with neuronal morphology contained both A2B5-IR and β-gal-IR (Fig. 3, A to C) or Nf-IR and β-gal-IR (Fig. 3, D to F). Parallel cultures treated with preimmune primary sera contained background levels of fluorescein and rhodamine epifluorescence (Fig. 3, G to F). Parallel cultures treated with antibodies against either of the neuronal markers and rabbit preimmune serum followed by the fluorescent-conjugated antibodies contained either A2B5-IR or Nf-IR but no β-gal-IR. Cultures infected with ts K alone, or mock-infected, and treated with antibodies against either neuronal marker and β-galactosidase contained either A2B5-IR or Nf-IR but no β-gal-IR. Thus, pHSVlac can infect rat sensory neurons and subsequently express β-galactosidase.
Fig. 3.

Immunofluorescent colocalization of β-gal-IR and either A2B5-IR or Nf-IR in cultured sensory neurons infected with pHSVlac. pHSVlac virus stock and cultures of dorsal root ganglia were prepared as described in Fig. 2 except that cultures were prepared on 13-mm glass cover slips coated with 0.8 μg of laminin. Cultures (5 × 104 cells in 0.5 ml) were infected with 0.1 ml of pHSVlac virus stock and incubated for 24 hours at 37°C. Fixation with 4% paraformaldehyde in 0.1M NaPO4 (pH 7.0) and immunohistochernistry were done (25) with a rabbit antibody to E. coli β-galactosidase (Cooper Biomedical, Malvern, Pennsylvania) diluted 1:800 and either mouse monoclonal to rat neurofilament (21) (SMI 33, Sternberger-Meyer) diluted 1:800 or mouse monoclonal A2B5 (20) supernatant diluted 1:2 as primary antibodies. Fluorescein isothiocyanate–conjugated goat F(ab′)2 (fragment-antigen) antibody to mouse F(ab′)2 (Cooper Biomedical) diluted 1:200 and rhodamine isothiocyanate–conjugated goat F(ab′)2 antibody to rabbit F(ab′)a diluted 1:250 (Cooper Biomedical) were used as secondary antibodies. Cover slips were mounted in phosphate-buffered saline and glycerol (1:1) containing 0.4% n-propyl gallate. The width of the photomicrograph represents 438 μm. (A) A2B5-IR. (B) β-Gal-IR. (C) Phase-contrast photomicrograph of the same field. (D) Nf-IR. (E) β-Gal-IR. (F) Phase-contrast photomicrograph of the same field. (G) Preimmune mouse serum, fluorescein isothiocyanate–conjugated second antibody. (H) Preimmune rabbit serum, rhodamine isothiocyanate–conjugated second antibody. (I) Phase-contrast photomicrograph of the same field.
We also investigated the persistence of pHSVlac DNA and stability of expression of (β-galactosidase in cultured sensory neurons. Cultures of dorsal root ganglia (19) were infected with pHSVlac virus stock; after a 2-week incubation at 37°C, 49% of the cells (Fig. 4, A to C) were β-galactosidase–positive as assayed with X-gal (17); most of these cells had neuronal morphology. Cultures infected with ts K alone or mock-infected contained less than 0.2% β-galactosidase–positive cells. The β-galactosidase–positive cells could result from either stable persistence of pHSVlac in the same cell for 2 weeks or horizontal transmission of pHSVlac. If horizontal transmission occurred at a significant rate, then all the cells would contain pHSVlac DNA and express β-galactosidase, and both ts K virus and pHSVlac virus would be present in the culture medium. In contrast, 51% of the cells are β-galactosidase–negative (Fig. 4, A to C) 2 weeks after infection. Furthermore, 2 weeks after infection the amount of virus in the culture medium was less than ten plaque-forming units (PFU) of ts K per milliliter and less than ten infectious particles of pHSVlac per milliliter, below our level of detection. We conclude that the rate of horizontal transmission of pHSVlac is very low.
Fig. 4.

Stable expression of β-galactosidase (A to C) and persistence of pHSVlac DNA (D) in cultured sensory neurons for 2 weeks. Cultures (19) of dorsal root ganglia (9 × 104 cells in 1.5 ml) were infected with 0.05 ml pHSVlac virus stock and incubated for 2 weeks at 37°C. Cultures were assayed for β-galactosidase activity as described in Fig. 2. Alternatively, cultures were infected with 5 × 105 PFU of ts K (18) and incubated for 2 days at 31°C. The resulting virus stock was passaged three times on 2 × 106 CV1 monkey fibroblasts at 31°C to yield virus stocks DRG1 and DRG2. CV1 cells (1 × 107) were infected with 5 × 107 PFU of virus stock (DRG1, DRG2, ts K alone, or mock-infected) and incubated at 31°C for 24 hours. Total cellular DNA (5 μg) was prepared (23) or 2 × 10−4 μg of pHSVlac DNA isolated from E. coli (Stds) was digested with Eco RI, resolved on 0.7% agarose gels, and transferred to Genetran (Fiasco) (24). Hybridization was performed with the 5.9-kb Eco RI fragment from the plasmid pCH110 (16) radiolabeled with 32P (26). This 5.9-kb fragment contains pBR sequences and most of the lacZ gene, lacking only 133 bp at the 3′ end (16). pHSVlac contains three Eco RI sites (13), one at each end of the pBR segment and a third in the lacZ gene 133 bp from the 3′ end of the gene. The 4.3-kb band contains most of the transcription unit in pHSVlac, and the 2.3-kb band contains the pBR sequences. The 1.5-kb fragment of pHSVlac contains the 3′ end of the lacZ gene, the SV40 early region polyadenylation site, and the a sequence; it is not homologous to the probe.
Next, an experiment was performed to show that pHSVlac DNA is present in cells 2 weeks after infection. Superinfection of a latently infected neuron results in a lytic infection; both the latent and superinfecting genomes are present in the progeny virus (22). Two weeks after infection with pHSVlac, cultures were infected with ts K alone and incubated for 2 days at 31°C to recover pHSVlac DNA in an HSV-1 virus stock. DNA isolated from the virus stocks (23) was digested with the restriction endonuclease Eco RI and subjected to DNA blot analysis (24) with a probe homologous to pHSVlac (Fig. 4D). The structure of pHSVlac DNA that persisted in sensory neurons for 2 weeks (lanes DRG1 and DRG2) is similar to the structure of pHSVlac DNA isolated from E. coli (Stds). The pHSVlac DNA is absent from a virus stock of ts K alone (ts K) and from mock-infected cells (Mock). One to 10% of PC12 cells were β-galactosidase–positive 24 hours after infection with virus stocks of pHSVlac recovered from neurons 2 weeks after infection. Thus, pHSVlac DNA can persist, unaltered, in sensory neurons for at least 2 weeks and stably express β-galactosidase from the HSV-1 IE 4/5 promoter (8).
The vector pHSVlac can be used to stably transfect postmitotic cells such as neurons and to stably express β-galactosidase. The E. coli lacZ gene in pHSVlac can be exchanged for other coding sequences. With our vector, it is now possible to introduce genes with products that affect physiology into neurons, including components of second messenger systems and neurotransmitter metabolism. Finally, HSV-1 vectors may be useful for gene therapy for neuronal diseases or for experimental modification of neuronal physiology.
References
- 1.Noda M, et al. Nature. 1983;302:818. [Google Scholar]
- 2.Palmiter RD, Norstedt G, Gelinas RE, Hammer RE, Brinster RL. Science. 1983;222:809. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- 3.Graham FL, Van der Eb AJ. Virology. 1973;52:456. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 4.Mann R, et al. Cell. 1983;33:153. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 5.Breakefield XO, Geller AI. Mol Neurobiol. 1987;1:339. doi: 10.1007/BF02935741. [DOI] [PubMed] [Google Scholar]
- 6.P. G. Spear and B. Roizman, in DNA Tumor Viruses, J. Tooze, Ed. (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1981), pp. 615–746.
- 7.Stevens JG. Curr Top Microbiol Immunol. 1975;70:31. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- 8.——— Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. Science. 1987;235:1056. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]; Deatly AM, Spivack JG, Lavi E, Fraser NW. Proc Natl Acad Sci USA. 1987;84:3204. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wagner EK, et al. J Virol. 1988;62:1194. doi: 10.1128/jvi.62.4.1194-1202.1988. RNA homologous to the IE genes was not detected in latently infected neurons by in situ hybridization. In contrast, an immediate early protein has been detected in latently infected neurons by the more sensitive technique of immunofluoresence [M. T. Green, R. J. Courtney, E. C. Dunkel, Infect. Immunol. 34, 987 1981]. Furthermore, IE promoters function constitutively in fibroblasts; transfection studies have demonstrated that transcription initiates from IE promoters in stably transformed cells [ [DOI] [PMC free article] [PubMed] [Google Scholar]; Persson RH, Bacchetti S, Smiley JR. J Virol. 1985;54:414. doi: 10.1128/jvi.54.2.414-421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mosca JD, Reyes GR, Pitha PM, Hayward GS. ibid. 1985 Our results demonstrate that the IE 45 promoter can function in persistently infected nonmitotic cells;56:867. [Google Scholar]
- 9.Fukuda J, et al. Brain Res. 1983;262:79. doi: 10.1016/0006-8993(83)90471-7. [DOI] [PubMed] [Google Scholar]
- 10.N. D. Stow and E. C. McMonagle, in Eucaryotic Viral Vectors, Y. Gluzman, Ed. (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1982), pp. 199–204; Spaete RR, Frenkel N. Cell. 1982;30:285. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- 11.Watson K, et al. J Gen Virol. 1980;49:149. doi: 10.1099/0022-1317-49-1-149. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes JG, et al. Virology. 1979;94:430. doi: 10.1016/0042-6822(79)90473-2. [DOI] [PubMed] [Google Scholar]
- 13.Geller AI. Nucleic Acids Res. 1988;16:5690. doi: 10.1093/nar/16.12.5690. manuscript in preparation. A single HSV-1 particle of pHSVlac (8.1 kb) contains about 19 copies of pHSVlac in a tandem array for 150 kb of DNA, the size of the wild-type HSV-1 genome. Three experiments demonstrate that expression of β-galactosidase from pHSVlac is independent of ts K. (i) The amount of β-galactosidase activity increases linearly with the amount of pHSVlac virus stock (0.02 to 0.30 MOI). A linear curve demonstrates that one particle (pHSVlac) is sufficient to express β-galactosidase. If two particles (pHSVlac and ts K) were required, the curve would be quadratic, (ii) Expression of β-galactosidase is observed at low MOI (0.02 to 0.10) of pHSVlac virus to cells; under these conditions most cells infected with pHSVlac are not infected with ts K. (iii) Addition of up to 10 MOI of ts K virus to such an experiment [experiment (ii)] does not increase the amount of β-galactosidase activity or the number of β-galactosidase–positive cells. Thus, pHSVlac expresses the same amount of β-galactosidase in the same number of cells in the absence or presence of ts K in these cells. It is unlikely that replication of pHSVlac DNA occurs in nonmitotic cells that contain pHSVlac but lack ts K. Therefore, infection by one pHSVlac virus particle is sufficient to render a cell β-galactosidase–positive without replication of pHSVlac DNA or coinfection by ts K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeoch DJ, Dolan A, Donald S, Brauer DHK. Nucleic Acids Res. 1986;14:1727. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison AJ, Wilkie NM. J Gen Virol. 1981;55:315. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- 16.Hall CV, Jacob PE, Ringold GM, Lee F. J Mol Appl Genet. 1983;2:101. [PubMed] [Google Scholar]
- 17.Sanes JR, Rubenstein JL, Nicolas JF. EMBO J. 1986;5:3133. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Price J, Turner D, Cepko C. Proc Natl Acad Sci USA. 1987;84:156. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davison MJ, Preston VG, McGeoch DJ. J Gen Virol. 1984;65:859. doi: 10.1099/0022-1317-65-5-859. [DOI] [PubMed] [Google Scholar]
- 19.Hawrot E, Patterson PH. Methods Enzymol. 1979;58:574. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- 20.Eisenbarth GS, Walsh FS, Nirenberg M. Proc Natl Acad Sci USA. 1979;76:4913. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sternberger LA, Sternberger NH. ibid. 1983;80:6126. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis ME, Brown SM, Warren KG, Subak-Sharpe JH. J Gen Virol. 1984;65:215. doi: 10.1099/0022-1317-65-1-215. [DOI] [PubMed] [Google Scholar]
- 23.Wigler MR, et al. Cell. 1979;16:777. [Google Scholar]
- 24.Southern EM. J Mol Biol. 1975;98:503. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 25.Huettner JE, Baughman RW. J Neurosci. 1986;6:3044. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinberg AP, Vogelstein B. Anal Biochem. 1983;132:6. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 27.Supported by NIH grants NS24279 (X.O.B.) and DK39836 (A.I.G., X.O.B., and J. F. Gusella), and an American Federation of Aging Research grant (A.I.G.). We thank J. Subak-Sharpe for providing HSV-1 strain 17 ts K; J. Ecker, R. Miller, P. Southern, L. Chun, G. Hanna, and B. Barres for helpful discussions on various techniques; J. F. Gusella for research support; and J. Martin for his support.


