Abstract
Herpes simplex virus type 1 (HSV-1) plasmid vectors have a number of attractive features for gene transfer into neurons. In particular, the large size of the HSV-1 genome suggests that HSV-1 vectors might be designed to co-express multiple genes. Here, we report a general strategy for constructing large HSV-1 plasmid vectors that co-express multiple genes. Each transcription unit is linked to an antibiotic resistance gene, and genetic selections are used to assemble large vectors. Using this strategy, we constructed large (26 or 31 kb) HSV-1 vectors that contain two transcription units and two or three genes. These vectors were efficiently packaged into HSV-1 particles using a helper virus-free packaging system. The resulting vector stocks supported the expression of two or three genes in both cultured cells and the rat brain. Potential applications of HSV-1 vectors that co-express multiple genes are discussed.
INTRODUCTION
Gene transfer into neural cells may support both gene therapy of neurological disorders and analyses of neuronal physiology. Many of these applications require specific properties of a virus vector system including high titers, long-term expression, minimal side effects, and, in particular, co-expression of multiple genes. Herpes simplex virus type 1 (HSV-1) plasmid vectors (6) have a number of attractive features, and a growing number of investigators have used this vector system to modify neuronal physiology (5). Gene transfer with helper virus vector systems results in significant cytopathic effects and an immune response (8); therefore, we developed a helper virus-free HSV-1 system that produces substantially less cytopathic effects and cell infiltration (4). One of the principal problems with all HSV-1 vectors has been the lack of long-term expression; thus, we recently reported a tyrosine hydroxylase (TH)-neurofilament heavy subunit (NFH) chimeric promoter that enhances long-term expression in forebrain neurons (21).
Although a number of virus vector systems can accommodate only relatively small inserts, one of the potential advantages of HSV-1 plasmid vectors is that the large size of the HSV-1 genome [approximately 152 kb (15)] suggests that large vectors co-expressing multiple genes might be developed. HSV-1 plasmid vectors are packaged as head-to-tail concatamers to achieve a genome-sized DNA molecule. Thus, large vectors that can form concatamers of approximately 150 kb might be correctly packaged. S. Wang et al. (19) have shown that a 30-kb vector can be packaged using a helper virus system, and X. Wang et al. (20) have shown that a 50-kb vector can be packaged using a helper virus-free system. There are a few reports of specific HSV-1 vectors that co-express two genes; New and Rabkin (14) have reported a HSV-1 vector that expresses two histological markers (LacZ and alkaline phosphatase), and Ho et al. (7) and Fotaki et al. (3) have reported vectors that express two genes—a tetracycline-regulated transcription factor and a reporter gene.
In this study, we describe a general strategy for constructing large HSV-1 vectors that co-express multiple genes. Each transcription unit is constructed separately and is linked to an antibiotic resistance gene. Genetic selections for antibiotic resistance are first used to link individual transcription units together and then to insert an array of transcription units into an HSV-1 vector. The resulting vectors support expression of multiple genes in both cultured cells and the rat brain.
MATERIALS AND METHODS
Plasmid Constructions
We constructed (Figure 1) a plasmid for linking a single transcription unit to a specific antibiotic resistance gene and flanked by sites for a specific restriction endonuclease with an 8-bp recognition sequence. Two oligonucleotides, 5′- AATTGGGCCGGCCGATATCCTCGAGCAGTCGGAATTCGGAAGGAGATCTGTTAACGGCCGGCCA-3′ and 5′-GGCCTGGCCGGCCGTTAACAGATCTCCTTCCGAATTCCGACTGCTCGAGGATATCGGCCGGCCC-3′, were inserted into the EagI and EcoRI fragment of pBR322 to yield pBRindi-linkerI. An EcoRI and BamHI fragment from pINS-NFHlac [mouse α-globin second intron and simian virus 40 (SV40) early region polyadenylation site (poly A); (21)] was inserted into the EcoRI and BglII fragment of pBRindi-linkerI to yield pBRindi-linker-intronA+. A 63-bp linker (Promega, Madison, WI, USA) (from pSP73; XhoI-EcoRI) was inserted into the XhoI and EcoRI fragment of pBRindi-linker-intronA+ to yield pBRindi-73linker-intronA+. A BamHI and NdeI fragment from pACYC177 (New England Biolabs, Beverly, MA, USA) [kanamycin-resistance gene (Knr)] was treated with Klenow enzyme and was then inserted into pBRindi-73linker-intronA+, which had been digested with EcoRV and treated with calf intestinal phosphatase (CIP) to yield pBRindi-73linker-intronA+-Knr-FseI.
Figure 1. Diagram of the strategy for constructing HSV-1 vectors that contain multiple genes.

An individual gene plasmid contains an EcoRV site for inserting an antibiotic resistance gene, a polylinker (XhoI, PvuII, HindIII, SphI, PstI, AccI, SalI, XbaI, BamHI, SmaI, KpnI, SacI, and EcoRI) for inserting a promoter and gene, an intron and poly A site, a HpaI site for inserting boundary elements such as a MAR, and the entire array is flanked on each end by an 8-bp site for an endonuclease (FseI in the example shown). An 8-cutter plasmid contains four sites (FseI, PmeI, SwaI, and SgrAI) to receive individual transcription units (inserted using a selection for the specific antibiotic resistance gene), a polylinker (XhoI, PvuII, HindIII, SphI, PstI, AccI, SalI, XbaI, BamHI, HindIII, and BglII) for the direct insertion of a transcription unit, and this array is flanked on each end by PacI, AscI, and NotI sites to excise the array of transcription units. An HSV-1 vector backbone plasmid contains an HSV-1 a sequence (packaging site), PacI, AscI, and NotI sites to insert an array of transcription units using a selection for a specific antibiotic resistance gene, and an HSV-1 origin of DNA replication HSV-1 oris). The HSV-1 oris fragment contains an HSV-1 IE 4/5 promoter that is separated from the PacI, AscI, and NotI sites by three poly A sites (TriA) (12). The pBR sequences (horizontal lines) in each plasmid contain the ampr gene and the ColE1 origin of DNA replication. F, FseI; R, EcoRV; H, HpaI; P, PacI; A, AscI; N, NotI; Pm, PmeI: Sw, Swa1: and Sg, SgrAI.
To allow a transcription unit and the Knr gene to be excised using a different restriction enzyme, we added PmeI sites to the ends of the polylinker in pBRindi-7 linker-intronA+-Knr-FseI. Two oligonucleotides, 5′-AATTGGTTTAAACGGCCGGCCGTTTAAACA-3′ and 5′-GGCCTGTTTAAACGGCCGGCCGTTTAAACC-3′ (PmeI, FseI, and PmeI sites), were inserted into the EagI and EcoRI fragment of pBR322 to yield pBR-PmeI-linker The FseI fragment from pBRindi-73linker-intronA+-Knr-FseI was inserted into pBR-PmeI-linker that had been digested with FseI and treated with CIP to yield pBRindi-73linker-intronA+-Knr-PmeI.
To allow a transcription unit to be linked to a second antibiotic resistance gene, we constructed a plasmid that contains the chloramphenicol-resistance gene (Cmr). A PshAI and BsaAI fragment from pACYC184 (New England Biolabs) was inserted into pBRindi-73linker-intronA+ that had been digested with EcoRV and treated with CIP to yield pBRindi-73linker-intronA+-Cmr-FseI.
Two oligonucleotides, 5′-CGGAGAAGGCCGGCCGTTTAAACATTTAAATCACCGGTGC-3′ and 5′-TCGAGCACCGGTGATTTAAATGTTTACGGCCGGCCTTCTCCGGGCC-3′, were inserted into the ApaI and XhoI fragment of pBRlinker (20) to yield pBR-8cutter-linkerI (i.e., a polylinker with PacI, AseI, and NotI sites on each end; 8-cutter plasmid) (Figure 1).
We constructed a transcription unit that contains the TH-NFH promoter (21), the TH gene, an internal ribosome entry site (IRES), and the aromatic amino acid decarboxylase gene (AADC) (13). A SalI and HindIII fragment from pINS-TH-NFHlac [chicken β-globin insulator (INS) and TH-NFH promoter (2,21)], a FseI and HindIII fragment from p747 [part of TH gene (13)], and a FseI and EcoRI fragment from p747 (remainder of TH gene, IRES, and AADC gene) were inserted into the SalI and EcoRI fragment of pBRindi-73linker-intronA+-Knr-PmeI to yield pBR-TH-NFHth/ires/aadc-Knr-PmeI.
We constructed a transcription unit that contains the TH-NFH promoter and a constitutively active protein kinase C (pkcΔ) [from rat PKC βII isoform; (16)]. A SalI and HindIII fragment from pINS-TH-NFHlac and a HindIII and BglII fragment from pHSVpkcΔ were inserted into the SalI and BamHI fragment of pBRindi-73linker-intronA+-Cmr-FseI to yield pBR-TH-NFHpkcΔ-Cmr-FseI.
We constructed a transcription unit that contains the TH-NFH promoter and the LacZ gene. A SalI and HindIII fragment from pINS-TH-NFHlac and a HindIII and BamHI fragment from pINS-NFHlac [LacZ, mouse α-globin second intron, and SV40 early region poly A site (21)] were inserted into the XhoI and BamHI fragment of pBR-8cutter-linkerI to yield pBR-8cutter-linkerI/TH-NFHlac.
Transcription units were excised by digestion with the appropriate enzyme (PmeI or FseI) and were inserted into pBR-8cutter-linkerI/TH-NFHlac, which had been digested with either PmeI or FseI and treated with CIP. Colonies were selected on DH5α, HB101. SURE, or JM109 cells in the presence of either Kn or Cm.
The arrays of transcription units were excised by digestion with PacI and were inserted into pHSVpUC-linkerI (HSV-1 vector backbone plasmid) (Figure 1) (20) that had been digested with PacI and treated with CIP.
Vector Packaging and Titering
Vectors were packaged into HSV-1 particles using the helper virus-free packaging system (4) as modified to improve the titers (18). Vector stocks were purified and concentrated (11). Vector stocks were titered by counting the number of expressing cells obtained one day after the infection of baby hamster kidney fibroblast cells (BHK21). E. coli β-galactosidase (β-gal) was detected using X-gal (17), TH, AADC, and PkcΔ were detected by immunocytochemistry using the procedure described by Song et al. (16). The TH was fused to the HA epitope tag and was detected using a rabbit anti-HA antibody in a dilution of 1:1000 (CRP, Richmond, CA, USA), human AADC was detected using a rabbit anti-human AADC antibody in a dilution of 1:500 (9), and PkcΔ was fused to the flag epitope tag and was detected using a mouse monoclonal anti-flag antibody in a dilution of 1:1000 (M5: Sigma, St. Louis, MO, USA).
Stereotactic Injections, Histology, and Cell Counts
Male Sprague Dawley rats (striaturn) or male Long Evans rats (postrhinal cortex) (100–125 g) were used for these experiments. The procedures for the stereolactic injections, including the injection coordinates, have been described by Zhang et al. (21). These studies were approved by the Children’s Hospital IACUC.
Procedures for perfusions, X-gal staining, and immunohistochemistry have been previously described (21). β-gal was also detected using a rabbit anti-E. coli β-gal antibody in a 1:500 dilution (ICN Biomedicals. Costa Mesa, CA, USA).
For each recombinant protein, every fourth section {25-μm sections) was analyzed, and approximately 10 of these sections contained the positive cells. Positive cells were identified under low power (10x), and morphology was verified under high power (40x). Each section was counted at least two times on different days: the results differed by less than 10%.
RESULTS AND DISCUSSION
General Strategy for Constructing Vectors that Contain Multiple Transcription Units
The construction strategy (Figure 1) is to link each transcription unit to an antibiotic resistance gene, use genetic selections to isolate a plasmid that contains multiple transcription units, and then insert the array of transcription units into an HSV-1 vector using a genetic selection. Initial attempts to construct these vectors without using genetic selections were not successful, possibly because of the large size of the vectors.
Individual gene plasmids support the routine construction of a transcription unit and subsequent insertion of the transcription unit into an HSV-1 vector. These plasmids (Figure 1) contain a polylinker for inserting a promoter and gene proximal to an intron and poly A site. These plasmids also contain an EcoRV site for the insertion of an antibiotic resistance gene; as EcoRV yields blunt ends, virtually any antibiotic resistance gene or other selectable marker may be inserted. We derived plasmids that contain either the Knr or Cmr genes because the use of multiple antibiotic resistance genes may be useful for constructing arrays of multiple transcription units. For the insertion of a boundary element, such as a matrix attachment region (MAR), there is an Hpa1 site (blunt ends) at one end of the polylinker to accept a transcription unit. In these plasmids, the transcription unit and antibiotic resistance gene are flanked by sites for a specific endonuclease with an 8-bp recognition sequence, which is used to excise the transcription unit and antibiotic resistance gene.
To construct arrays of multiple transcription units or to insert a gene or promoter that contains a specific site, it may be useful to flank specific transcription units (and antibiotic resistance genes) with sites for different endonucleases. A second set of flanking sites can be added, by isolating a plasmid that contains the first site flanked on each side by the second site and then by inserting the polylinker and antibiotic resistance gene at the first site. Using this procedure, we added PmeI sites to a polylinker and antibiotic resistance gene that is flanked by FseI sites.
An 8-cutter plasmid for accepting the transcription units (Figure 1) contains a polylinker with a number of 8-bp sites and additional sites (6-bp recognition sequences) for directly inserting a transcription unit. To enable the excision of the resulting array of transcription units, the polylinker is flanked on each end by sites for three enzymes with 8-bp recognition sequences (PacI, AscI. and NotI). The order of these sites allows the array of transcription units to be excised using any enzyme alone or any combination of two enzymes.
An HSV-1 vector backbone plasmid was constructed to accept an array of transcription units {Figure 1). This vector contains the two sequences required to package a vector into HSV-1 particles (i.e., HSV-1 origin of DNA replication and HSV-1 packaging site) and the three 8-bp sites that flank the array of transcription units in the 8-cutter plasmid.
The resulting HSV-1 vectors contain the antibiotic resistance gene(s) used in the construction, but, because antibiotic resistance genes are prokaryotic, they are unlikely to be expressed in mammalian cells.
HSV-1 Vectors that Co-Express Two or Three Genes
All of the transcription units use the TH-NFH promoter that supports long-term expression, and the TH-NFH promoter is preceded by an insulator (INS) that further enhances long-term expression (21). We used a construct (13) that contains a TH gene, an IRES, and an AADC gene to co-express TH and AADC. The second transcription unit in these vectors expresses the LacZ gene. Alternatively, we derived vectors in which one transcription unit expresses PkcΔ (16) and the second transcription unit contains the LacZ gene. We obtained vectors in which the transcription units were in either a sequential or a divergent orientation, and vectors in which the transcription units had different orientations relative to the vector backbone (Table 1). The vectors that contain the TH, AADC, and LacZ genes are 31.3 kb, and the vectors that contain the pkcΔ and LacZ genes are 26.2 kb. Attempts to obtain vectors with three transcription units were unsuccessful possibly because of the large amount of repeated sequence in each of the three copies of the 7.8-kb INS-TH-NFH promoter fragment.
Table 1.
Titers (IVP/mL) of Vectors that Co-Express Two or Three Genes
| Vector | TH | AADC | PkcΔ | LacZ |
|---|---|---|---|---|
| pTH-NFHth/ires/aadc\TH-NFHlac (S+)a | 3.7×106 | 7.3 × 106 | 6.0 × 106 | |
| pTH-NFHth/ires/aadc\TH-NFHlac (D;l+) | 3,8 × 106 | 5.5 × 106 | 8.4 × 1 06 | |
| pTH-NFHth/ires/aadc\TH-NFHIac (D;l−) | 6.1 × 106 | 7.8 × 106 | 1.1 × 107 | |
| pTH-NFHpkcΔ\TH-NFHlac (S+) | 1.6 × 106 | 1.4 × 107 | ||
| pTH-NFHpkcΔ\TH-NFHlac (S−) | 8.4 × 105 | 6.6 × 106 | ||
| pTH-NFHpkcΔ\TH-NFHlac (D;l−) | 1.1 × 106 | 5.0 × 1 06 |
The two transcription units are transcribed in the same (S) or divergent (D) direction, and the LacZ gene (l) is transcribed away from (+) or toward (−) HSV-1 oris.
These vectors were packaged into HSV-1 particles using a helper virus-free packaging system (4,18). For titering, immunocytochemical or enzymatic assays (see Materials and Methods) were performed one day after infection of BHK cells with these vector stocks. These stocks showed high titers of each of the two or three genes in a specific vector (Table 1). Note that the titers of each of the two or three genes in a specific vector were similar, which was consistent with the co-expression of the genes. Comparisons of the titers of each of the genes in these vectors show that the orientation of the transcription units relative to each other or to the vector backbone did not appear to affect the titers. The small differences in the titers among the different genes might reflect differences in either the sensitivities of the assays or in expression of the different genes. In particular, the slightly higher titers of the LacZ gene observed in some vector stocks may be because the X-gal assay is more sensitive than the immunocytochemical assays and these slightly higher titers were not specific to one orientation of the LacZ gene, relative to either the other transcription unit or the vector backbone. These titers (Table 1) are similar to those obtained using smaller vectors that express only one gene (4,18,20,21).
Vector stocks were microinjected into either the striatum or the postrhinal cortex, and the rats were sacrificed four days later. In the striatum (Figure 2), we observed similar numbers of TH [HA-immunoreactivity (IR)], AADC-IR, or X-gal-positive cells in adjacent sections (Table 2). The slightly lower number of AADC-IR cells may be because this assay is not as sensitive as the other assays. In the postrhinal cortex (Figure 3), we observed 96% co-localization of β-gal-IR and PkcΔ (flag-IR) to the same cells (Table 2). Thus, it appears that the repeated sequences in these vectors are relatively stable, which is consistent with the presence of both inverted and direct repeats in the genome of wild-type HSV-1. Many of the positive cells in the striatum or postrhinal cortex displayed neuronal morphology. Consistent with this neuronal morphology, using pTH-NFHlac, we have shown (21) that approximately 90% of the β-gal-IR positive cells contain a neuronal marker. In the present study, the rats were sacrificed four days after gene transfer; however, we have shown that pTH-NFHlac supports expression for six months in the striatum (21) and that the vector that co-expresses PkcΔ and LacZ supports expression for three weeks in the postrhinal cortex (unpublished data).
Figure 2. TH-IR, AADC-IR, and X-gal-positive striatal cells in a rat sacrificed four days after microinjection of pTH-NFHth/ires/aadc\TH-NFHlac(D;l-).
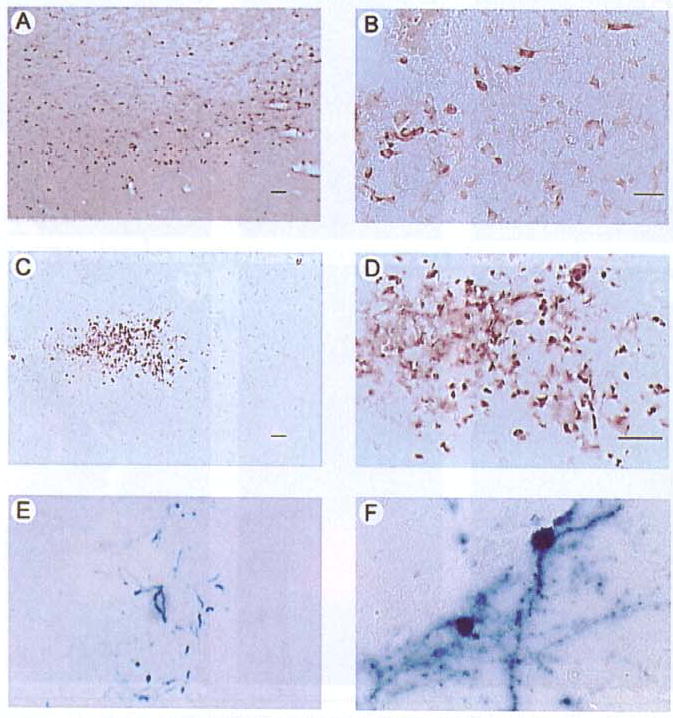
This TH contains the HA tag (13) and was detected using an anti-HA antibody. AADC was detected using an anti-human AADC antibody, β-gal was detected with X-gal (16). (A and B) Low- and high-power views of TH-IR. Numerous positive cells are visible, and many display neuronal morphology. (C and D) Low- and high-power views of AADC-IR. (E and F) Low- and high-power views of X-gal-positive cells. Scale bars: A and E, 50 μm; B and F, 25 μm; C, 25 μm; and D, 25 μm.
Table 2.
The Numbers of TH, AADC, PkcΔ, or β-Gal-Positive Cells in Rats Sacrificed Four Days after Gene Transfer
| Vector | TH (HA-IR) | AADC-IR | X-gal | β-gal-IR Only | PkcΔ (flag-IR) Only | Double Labled |
|---|---|---|---|---|---|---|
| PTH-NFHth/ires/aadc\TH-NFHlac (D;l-) | 553 ± 65 | 441 ±13 | 599 ± 53 | |||
| PTH-NFHpkcΔ\TH-NFHlac (D;l-) | 9 | 3 | 260 (96%) |
In the striatum, HA-IR, AADC-IR, or X-gal were assayed in adjacent sections (mean ± sem; n = three rats). In the postrhinal cortex, β-gal-IR and PkcΔ were assayed in the same sections; one section from each of the three rats was examined.
Figure 3. Flag-IR and β-gal-IR are present in the same postrhinal cortex cells in a rat sacrificed four days after microinjection of pTH-NFHpkΔ\TH-NFHlac (D;l-).
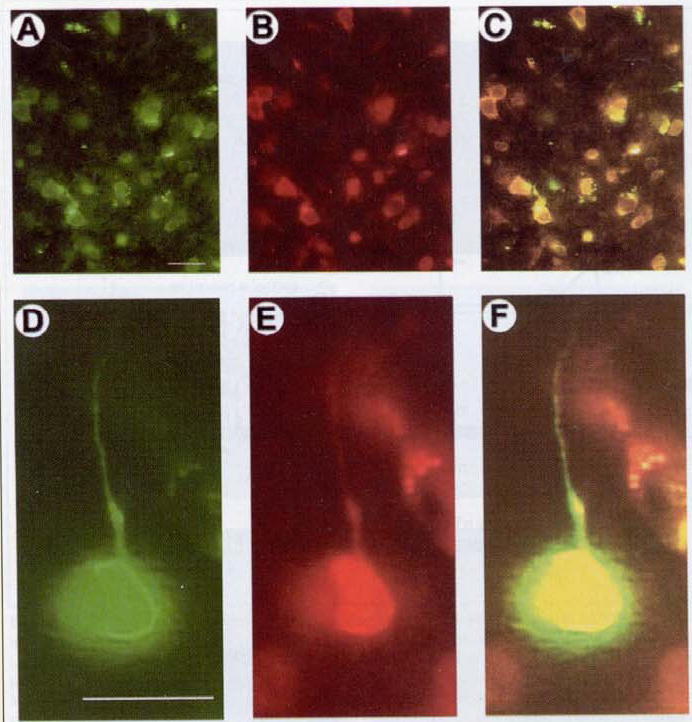
PkcΔ contains the flag tag (16) and was detected using an anti-flag antibody that was visualized using a rhodamine-conjugated secondary antibody. β-gal was detected using an anti-β-gal antibody that was visualized using a fluorescein-conjugated secondary antibody. (A–C) Low-power views of the same field showing a number of cells that contain (A) β-gal-IR and (B) flag-IR. (C) A double exposure shows co-stained cells. (D–F) High-power views of the same field showing one large cell with a pyramidal-shaped cell body and a process that resembles an apical dendrite. This cell contains (D) β-gal-IR and (E) flag-IR. (F) A double exposure shows that this cell is co-stained. Scale bars: A–C, 25 μm and D–F, 25 μm.
Potential Applications
Co-expression of multiple genes might support gene transfer of the multiple subunits of a protein or a relatively complete biosynthetic pathway, signal transduction pathway, or other pathway. For example, regarding gene therapy for Parkinson’s disease. TH and GTP cyclohyrolase have been shown to support the efficient production of L-DOPA (1); AADC converts L-DOPA to dopamine, and a vesicular monoamine transporter sequesters dopamine into synaptic vesicles, both enabling the regulated release of dopamine and relieving end-product (dopamine) inhibition of TH (10). Thus, co-expression of all four genes in striatal neurons might be an effective approach for correcting the symptoms of Parkinson’s disease.
Acknowledgments
We gratefully acknowledge Drs. W. Schlaepfer for the NFH promoter, K. O’Malley for the TH promoter and the TH/ires/AADC cassette, G. Felsenfeld for the INS, R. Sandri-Goldin for 2-2 cells, A. Davison for HSV-1 cosmids, and J. Haycock for anti-AADC antibody. We were supported by Neurovir grant nos. AG16777, NS34025, and NS42016 to A.G.
References
- 1.Bencsics C, Wachtel SR, Milstien S, Hatakeyama K, Becker JB, Kang UJ. Double transduction with GTP cyclohydrolase I and tyrosine hydroxylase is necessary for spontaneous synthesis of L-DOPA by primary fibroblasts. J Neurosci. 1996;16:4449–4456. doi: 10.1523/JNEUROSCI.16-14-04449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung JH, Whiteley M, Felsenfeld G. A 5′ element of the chicken β-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 3.Fotaki ME, Pink JR, Mous J. Tetracycline-responsive gene expression in mouse brain after amplicon-mediated gene transfer. Gene Ther. 1997;4:901–908. doi: 10.1038/sj.gt.3300487. [DOI] [PubMed] [Google Scholar]
- 4.Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geller AI. Genetic analysis of the role of protein kinase C signaling pathways in behaviors by direct gene transfer with HSV-1 vectors. Rev Neurosci. 1999;10:1–13. doi: 10.1515/revneuro.1999.10.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Geller AI, Breakefield XO. A defective HSV-1 vector expresses E. coli β-galactosidase in cultured peripheral neurons. Science. 1988;241:1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho DY, McLaughlin JR, Sapolsky RM. Inducible gene expression from defective herpes simplex virus vectors using the tetracycline-responsive promoter system. Mol Brain Res. 1996;41:200–209. doi: 10.1016/0169-328x(96)00097-6. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PA, Miyanohara A, Levine F, Cahill T, Friedmann T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kish SJ, Zhong XH, Hornykiewicz O, Haycock JW. Striatal 3,4-dihydroxyphenylalanine decarboxylase in aging: disparity between postmortem and positron emission tomography studies? Ann Neurol. 1995;38:260–264. doi: 10.1002/ana.410380220. [DOI] [PubMed] [Google Scholar]
- 10.Lee WY, Chang JW, Nemeth NL, Kang UJ. Vesicular monoamine transporter-2 and aromatic L-amino acid decarboxylase enhance dopamine delivery after L-3, 4-dihydroxyphenylalanine administration in Parkinsonian rats. J Neurosci. 1999;19:3266–3274. doi: 10.1523/JNEUROSCI.19-08-03266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim F, Hartley D, Starr P, Lang P, Song S, Yu L, Wang Y, Geller AI. Generation of high-titer defective HSV-1 vectors using an IE 2 deletion mutant and quantitative study of expression in cultured cortical cells. BioTechniques. 1996;20:460–469. doi: 10.2144/19962003460. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell IH, Harrison GS, Wood WM, Maxwell F. A DNA cassette containing a trimerized SV40 polyadenylation signal which efficiently blocks spurious plasmid-initiated transcription. BioTechniques. 1989;7:276–280. [PubMed] [Google Scholar]
- 13.Moffat M, Harmon S, Haycock J, O’Malley KL. L-Dopa and dopamine-producing gene cassettes for gene therapy approaches to Parkinson’s disease. J Neurochem. 1997;68:1792–1803. doi: 10.1006/exnr.1996.6390. [DOI] [PubMed] [Google Scholar]
- 14.New KC, Rabkin SD. Co-expression of two gene products in the CNS using double-cassette defective herpes simplex virus vectors. Mol Brain Res. 1996;37:317–323. doi: 10.1016/0169-328x(95)00262-q. [DOI] [PubMed] [Google Scholar]
- 15.Roizman, B. and A.E. Sears. 1993. Herpes simplex viruses and their replication, p. 11–68. In B. Roizman, R.J. Whitley, and C. Lopez (Eds.). The Human Herpesviruses. Raven Press, New York.
- 16.Song S, Wang Y, Bak SY, During MJ, Bryan J, Ashe O, Ullrey DB, Trask LE, et al. Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons. J Neurosci. 1998;18:4119–4132. doi: 10.1523/JNEUROSCI.18-11-04119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song S, Wang Y, Bak SY, Lang P, Ullrey D, Neve RL, O’Malley KL, Geller AI. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J Neurochem. 1997;68:1792–1803. doi: 10.1046/j.1471-4159.1997.68051792.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun M, Zhang GR, Yang T, Yu L, Geller AI. Improved titers for helper virus-free herpes simplex virus type 1 plasmid vectors by optimization of the packaging protocol and addition of noninfectious herpes simplex virus-related particles (previral DNA replication enveloped particles) to the packaging procedure. Hum Gene Ther. 1999;10:2005–2011. doi: 10.1089/10430349950017365. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Di S, Young WB, Jacobson C, Link CJ., Jr A novel herpesvirus amplicon system for in vivo gene delivery. Gene Ther. 1997;4:1132–1141. doi: 10.1038/sj.gt.3300523. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zhang G, Yang T, Zhang W, Geller AI. Fifty-one kilobase HSV-1 plasmid vector can be packaged using a helper virus-free system and supports expression in the rat brain. BioTechniques. 2000;27:102–106. doi: 10.2144/00281st05. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Wang X, Yang T, Sun M, Zhang W, Wang Y, Geller AI. A tyrosine hydroxylase-neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Mol Brain Res. 2000;84:17–31. doi: 10.1016/s0169-328x(00)00197-2. [DOI] [PubMed] [Google Scholar]


