Abstract
Systematic variation of the branching and basicity of the side chain of chloroquine yielded a series of new 7-chloro-4-aminoquinoline derivatives exhibiting high in vitro activity against 4 different strains of P. falciparum. Many of the compounds tested showed excellent potency against chloroquine sensitive and resistant strains. In particular 4b, 5a, 5b, 5d, 17a, and 17b were found to be significantly more potent than chloroquine against the resistant strains Dd2 and FCB.
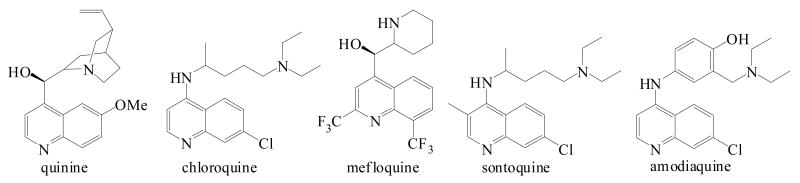
Malaria remains the world's most widespread and devastating infectious disease, with approximately 300 million annual cases and more than 2 million casualties. Among the protozoan parasites of the genus Plasmodium causing malaria in humans, Plasmodium falciparum is the most lethal species. Since the discovery of the antimalarial potency of quinine and other cinchona alkaloids, a variety of agents exhibiting a 4-substituted quinoline pharmacophore has been introduced. In particular, chloroquine (CQ), mefloquine, sontoquine, and amodiaquine have proved to be among the most effective antimalarial drugs (Figure 1).1,2,3 Aminoquinolines are known to form a complex with ferriprotoporphyrin IX (FPIX), which is generated in the food vacuole of the intraerythrocytic malaria parasite as a result of proteolysis of host hemoglobin (Hb) which serves as a major source of amino acids during the protozoan life stages within the infected red blood cell. Free FPIX is cytotoxic to Plasmodium which therefore has developed a strategy to limit the amount of free FPIX, converting it into insoluble crystalline hemozoin.4 The drug-FPIX interactions inhibit conversion of hematin to hemozoin and hence its detoxification via crystallization, and the accumulation of significant concentrations of toxic FPIX-aminoquinoline adducts is believed to be ultimately responsible for killing the parasite.5-9 It is widely accepted that the 4-aminoquinoline pharmacophore plays a crucial role in the complexation to FPIX resulting in inhibition of hemozoin formation and parasite growth,10 while the presence of a basic amino group in the side chain is generally considered essential for trapping high concentrations of the drug in the acidic food vacuole of the parasite.11
Figure 1.
Structures of antimalarial aminoquinolines.
To date, numerous isolates of P. falciparum have developed resistance against a majority of currently employed antimalarial drugs. In order to address the ever-increasing health impact of malaria, promising chloroquine resistant (CQR) reversal agents12,13 and new therapeutics14 including artemisinin and other endoperoxides have been introduced.15-20 However, the latter are less affordable in the most plagued tropical and subtropical regions and resistance to endoperoxide-derived antimalarials has already been reported.21 Arguably, quinine, chloroquine and mefloquine are among the most successful antimalarial drugs ever used, and additional lead compounds with improved activity against CQR strains have been discovered via synthetic modifications of these structures.22-24 Importantly, 4-aminoquinolines carrying an aliphatic side chain are often well tolerated and afford excellent activity-toxicity profiles.25 The evident need for safe, effective and inexpensive antimalarials that are equally active against multiple species of Plasmodia, e.g. P. falciparum and P. vivax, has therefore directed increasing efforts to the design of new CQ analogues.
Since modification of the 7-chloroquinoline ring, i.e. incorporation of other electron-withdrawing or electron-donating substituents such as amino and methoxy groups into the various positions in the quinoline ring, have generally proved detrimental to the antimalarial activity,26,27 a systematic variation of the side chain structure and basicity seems to be more promising. Although few comprehensive and methodical modifications of the CQ side chain have been reported to date, it has been established that both shortening and lengthening of the separation of the two aliphatic amino groups to either 2-3 or 10-12 carbon atoms as well as the incorporation of a phenol moiety can lead to increased activity against CQR strains.28-31 Several studies revealed that introduction of a branched dialkylamino motif at the side chain terminus of CQ, e.g. replacement of the ethyl by isopropyl or tert-butyl groups, can furnish metabolically more stable antimalarials with enhanced life-time and retained activity against drug resistant strains of P. falciparum.32,33
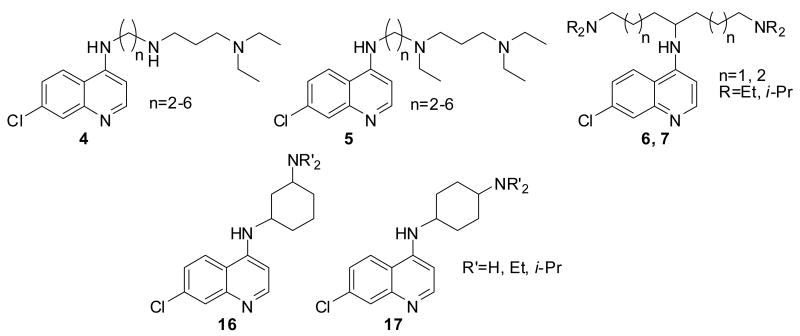
We envisioned that incorporation of an increasing number of basic amino groups along with systematic structural variations (length and branching) of the aliphatic side chain attached to the potent 4-amino-7-chloroquinoline pharmacophore would provide new candidates that overcome antimalarial drug resistance. The introduction of a highly branched tether between the two amino functions in CQ as well as the replacement of the metabolically unstable terminal diethylamino group by an isopropyl analogue were expected to enhance the life-time of CQ analogues exhibiting retained activity against CQR strains. Herein, we report the synthesis and evaluation of the antimalarial activity of a series of novel 4-amino-7-chloroquinolines carrying either a branched or a linear side chain with two or three amino functions (Figure 2).
Figure 2.
Structures of tribasic and dibasic 4-amino-7-chloroquinolines.
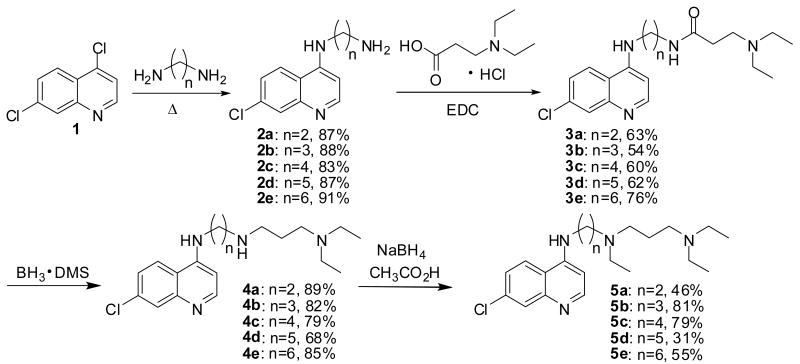
Our synthetic approach towards these heme-targeted antimalarials involved inexpensive materials and high-yielding steps in most cases. Amination of 4,7-dichloroquinoline, 1, with commercially available α,ω-diaminoalkanes gave N-(7-chloro-4-quinolyl)-1,n-diaminoalkanes 2 in 83 to 91% yield (Scheme 1). Coupling of 2 with N,N-diethylamino-3-propionic acid in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) furnished amides 3 which were reduced with borane-dimethyl sulfide to the corresponding series of secondary amines 4. Finally, tertiary amines 5 were prepared by treatment of precursors 4 with sodium borohydride in glacial acetic acid.
Scheme 1.
Synthesis of 4-amino-7-chloroquinolines 4a-e and 5a-e.
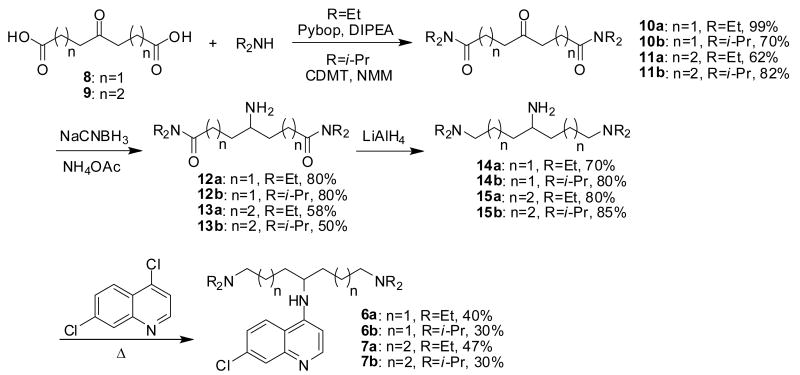
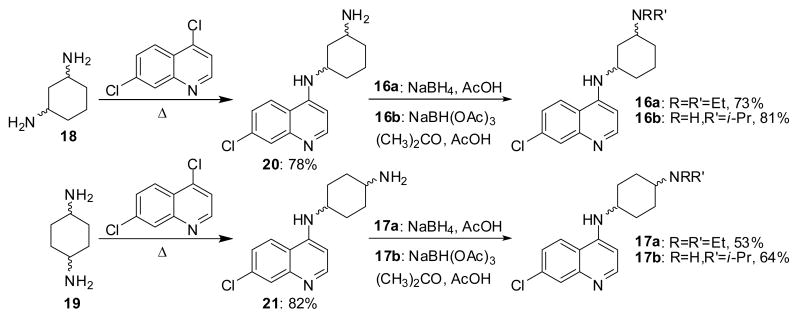
The symmetrically branched amines 6 and 7 were synthesized from 4-ketopimelic acid, 8, and 5-oxoazelaic acid, 9 (Scheme 2). Screening of different coupling conditions revealed that Pybop and CDMT allow efficient amide formation with diethylamine and diisopropylamine, respectively. The corresponding α,ω-diamides 10 and 11 were thus obtained in 62-99% yield. Reductive amination of the ketone group using ammonium acetate and sodium cyanoborohydride, and subsequent reduction of the terminal amides with lithium aluminium hydride gave triamines 14 and 15 in good yields. These amines were then employed in a carefully optimized nucleophilic aromatic substitution procedure using excess of 1 at high temperatures in a closed vessel to produce chloroquinolines 6 and 7. The 1,3- and 1,4-diaminocyclohexane-derived chloroquinolines 16a, 16b, 17a, and 17b were prepared from 4,7-dichloroquinoline and a mixture of the cis- and trans-isomers of diaminocyclohexanes 18 and 19. The nucleophilic halide displacements were followed by treatment of intermediates 20 and 21 with either NaBH4 and acetic acid or acetone and NaBH(OAc)3 (Scheme 3).
Scheme 2.
Synthesis of symmetrically branched 4-amino-7-chloroquinolines 6a,b and 7a,b.
Scheme 3.
Synthesis of 1,3- and 1,4-diaminocyclohexane-derived chloroquinolines 16a,b and 17a,b.
The antiplasmodial activity of tribasic compounds 4a-e, 5a-e, 6a, 6b, 7a, and 7b as well as the dibasic 1,3- and 1,4-diaminocyclohexane-derived chloroquinoline analogues 16a, 16b, 17a, and 17b was measured versus two CQS (HB3 and GCO3) and two CQR (Dd2 and FCB) strains using a standardized, inexpensive assay based on SYBR Green I intercalation that has recently been adopted and validated by several laboratories.34-36 The IC50 values were calculated from experiments carried out in triplicate and compared to CQ (Table 1). Many of the aminoquinolines prepared for this study showed antimalarial activity versus HB3 and GCO3 similar to that of CQ and we were pleased to find that several compounds were significantly more potent against the resistant strains Dd2 and FCB than CQ. Among the 18 compounds evaluated, 8 gave IC50's between 28.1 and 80.0 nM for Dd2 (CQ IC50 = 140 nM) and 6 showed IC50's ranging from 49.1 to 73.7 nM for FCB (CQ IC50 = 170 nM). Interestingly, the antimalarial activity of the linear tribasic aminoquinolines 4a-e and 5a-e proved to be generally superior over that of the tribasic compounds 6a, 6b, 7a, and 7b carrying a symmetrically branched side chain. Impressive antimalarial activity was also observed with the highly branched dibasic CQ analogues 16a, 16b, 17a, and 17b but these compounds possess an inherently higher selectivity index (SI, the ratio of the IC50 for a resistant versus a sensitive strain) than the linear tribasic aminoquinolines. Noteworthy, diastereomeric mixtures of 16a and 17a have previously been prepared by Drake et al. and Jensen later reported higher antimalarial activity against certain CQS and CQR strains relative to chloroquine.37-39 The selectivity index provides a quantitative measure of the antimalarial activity against CQR strains relative to that against sensitive strains and thus indicates promising drug discovery leads. The selectivity index of CQ is about 10 whereas all compounds tested have SI's between 0.68 and 4.43. In this regard, it is important that 5a and 5b combine high antimalarial activity against HB3 and GCO3 with very low SI values between 1.14 and 1.78. These new heme-targeted antiplasmodials thus show activity versus CQS strains similar to that of CQ and, more importantly, they retain their potency against CQR strains. Comparison of the antimalarial activity of 5a and 5b with the results obtained for the tribasic aminoquinolines 4a and 4b suggests that the presence of a tertiary central amino group in this series is crucial for the activity against Dd2, GCO3 and FCB but not for HB3. Similarly, the potency of 5a and 5b versus Dd2, GCO3 and FCB diminishes when the chain length is increased. The impressive SI values of all compounds tested demonstrate that systematic variations of both the CQ side chain structure and basicity provide new venues to overcome antimalarial drug resistance. This can be combined with the introduction of a third basic amino function which should further favor accumulation of the drug within the acidic food vacuole of the parasite. However, the relatively high IC50's of 6a, 6b, 7a, and 7b reveal that basicity and structure of the side chain can not be optimized independently.
Table 1.
Antiplasmodial activity.
| Strain/Experimental IC50 (nM) | ||||||
|---|---|---|---|---|---|---|
| Compound | HB3 | Dd2 | SI1 | GCO3 | FCB | SI1 |
| CQ | 13.5 | 140 | 10.4 | 16.0 | 170 | 10.6 |
| 4a | 29.2 | 129 | 4.43 | 118 | 146 | 1.24 |
| 4b | 27.3 | 56.3 | 2.06 | 64.8 | 73.7 | 1.14 |
| 4c | 72.5 | 170 | 2.34 | 139 | 189 | 1.36 |
| 4d | 46.0 | 103 | 2.25 | 68.7 | 97.3 | 1.42 |
| 4e | 82.8 | 269 | 3.25 | 129 | 216 | 1.67 |
| 5a | 27.3 | 31.2 | 1.14 | 37.7 | 52.7 | 1.40 |
| 5b | 21.2 | 28.1 | 1.33 | 27.6 | 49.1 | 1.78 |
| 5c | 24.1 | 84.6 | 3.52 | 87.4 | 156 | 1.78 |
| 5d | 15.7 | 43.4 | 2.76 | 55.2 | 66.8 | 1.21 |
| 5e | 62.9 | 274 | 4.36 | 175 | 263 | 1.50 |
| 6a | 187 | 128 | 0.68 | 96.6 | 186 | 1.92 |
| 6b | 44.1 | 99.8 | 2.26 | 42.4 | 104 | 2.45 |
| 7a | 716 | 882 | 1.23 | 517 | 1060 | 2.05 |
| 7b | 1314 | 2550 | 1.94 | 1512 | 2225 | 1.47 |
| 16a | 26.3 | 80.0 | 3.04 | 40.6 | 113 | 2.78 |
| 17a | 25.5 | 51.8 | 2.03 | 32.0 | 57.6 | 1.80 |
| 16b | 27.8 | 76.1 | 2.74 | 26.1 | 110 | 4.21 |
| 17b | 31.3 | 75.7 | 2.42 | 19.5 | 66.0 | 3.89 |
The experimental IC50's are averages of two separate determinations each conducted in triplicate.
The selectivity index (SI) is the ratio of the IC50 for the resistant versus the sensitive strain (Dd2/HB3, 4th column; FCB/GCO3, 7th column).
In conclusion, we have prepared a series of new heme-targeted antimalarials by systematically varying both the structure and basicity of the side chain attached to the 7-chloro-4-aminoquinoline pharmacophore of CQ. All 18 compounds tested show potent antiplasmodial activity against 4 different strains in vitro and can be synthesized from readily available, inexpensive starting materials through a few high-yielding steps. Comparison with CQ revealed that 4b, 5a, 5b, 5d, 16a, 16b, 17a, and 17b afford clearly superior activity against the CQ resistant strain Dd2 and 4b, 5a, 5b, 5d, 17a, and 17b proved significantly more potent against FCB. In particular, the tribasic 4-aminoquinolines 5a and 5b carrying a short linear side chain with two additional aliphatic tertiary amino functions are highly potent antimalarials and equally effective against both CQS and CQR strains. This study reveals that methodical variation of the side chain of chloroquine provides a promising entry towards affordable heme-targeted antimalarials that overcome the ever-increasing problem with worldwide drug resistance.
Supplementary Material
Detailed synthetic procedures of all compounds tested and synthetic precursors, product characterization, 1H and 13C-NMR spectra, and HPLC chromatograms obtained by two orthogonal methods. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the NIH (Grant RO1AI060792) for financial support.
Footnotes
Abbreviations: CQ, chloroquine; CQR, chloroquine resistant; CQS, chloroquine sensitive; Dd2, CQR strain; FCB, CQR strain; HB3 CQS strain; GCO3, CQS strain; FPIX, ferriprotoporphyrin IX; Hb, hemoglobin; P. falciparum, Plasmodium falciparum; IC50, 50% inhibitory drug concentration; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; Pybop, benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate; CDMT, 2-chloro-4,6-dimethoxy-1,3,5-triazine.
References
- 1.De D, Byers LD, Krogstad DJ. Antimalarials: synthesis of 4-aminoquinolines that circumvent drug resistance in malaria parasites. J Hetercyclic Chem. 1997;34:315–320. [Google Scholar]
- 2.O'Neill PM, Bray PG, Hawley SR, Ward SA, Park BK. 4-Aminoquinolines-past, present, and future: a chemical perspective. Pharmacol Ther. 1998;77:29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez JN. Chemotherapeutic agents against malaria: What next after chloroquine? Curr Topics Med Chem. 2002;2:1173–1185. doi: 10.2174/1568026023392986. [DOI] [PubMed] [Google Scholar]
- 4.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment β-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 5.Ridley RG. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature. 2002;415:686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 6.Leed A, DuBay K, Ursos LM, Sears D, de Dios AC, Roepe PD. Solution structures of antimalarial drug-heme complexes. Biochemistry. 2002;41:10245–10255. doi: 10.1021/bi020195i. [DOI] [PubMed] [Google Scholar]
- 7.Chong CR, Sullivan DJ., Jr Inhibition of heme crystal growth by antimalarials and other compounds: Implications for drug discovery. Biochem Pharmacol. 2003;66:2201–2212. doi: 10.1016/j.bcp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 8.de Dios AC, Casabianca LB, Kosar A, Roepe PD. Structure of the amodiaquine-FPIX μ-oxo dimer solution complex at atomic resolution. Inorg Chem. 2004;43:8078–8084. doi: 10.1021/ic0489948. [DOI] [PubMed] [Google Scholar]
- 9.de Dios AC, Tycko R, Ursos LMB, Roepe PD. NMR Studies of Chloroquine – Ferriprotoporphyrin IX Complex. J Phys Chem A. 2003;107:5821–5825. [Google Scholar]
- 10.Cheruku SR, Maiti S, Dorn A, Scorneaux B, Bhattacharjee AK, Ellis WY, Vennerstrom JL. Carbon isosteres of the 4-aminopyridine substructure of chloroquine: Effects on pKa, hematin binding, inhibition of hermozoin formation, and parasite growth. J Med Chem. 2003;46:3166–3169. doi: 10.1021/jm030038x. [DOI] [PubMed] [Google Scholar]
- 11.Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, Walden J. Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of β-hematin formation, and antiplasmodial activity. J Med Chem. 2000;43:283–291. doi: 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
- 12.Martin SK, Oduola AM, Milhous WK. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 13.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum. J Med Chem. 2006;49:5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisman JL, Liou AP, Shelat AA, Cohen FE, Guy RK, DeRisi JL. Searching for new antimalarial therapeutics amongst Known drugs. Chem Biol Drug Des. 2006;67:409–416. doi: 10.1111/j.1747-0285.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Dong Y, Wittlin S, Charman SA, Chollet J, Chiu FC, Charman WN, Matile H, Urwyler H, Dorn A, Bajpai S, Wang X, Padmanilayam M, Karle JM, Brun R, Vennerstrom JL. Weak base dispiro-1,2,4-trioxolanes: potent antimalarial ozonides. Bioorg Med Chem Lett. 2007;17:1260–1265. doi: 10.1016/j.bmcl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y, Tang Y, Chollet J, Matile H, Wittlin S, Charman SA, Charman WN, Tomas JS, Scheurer C, Snyder C, Scorneaux B, Bajpai S, Alexander SA, Wang X, Padmanilayam M, Cheruku SR, Brun R, Vennerstrom JL. Effect of functional group polarity on the antimalarial activity of spiro and dispiro-1,2,4-trioxolanes. Bioorg Med Chem. 2006;14:6368–6382. doi: 10.1016/j.bmc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Posner GH, Paik IH, Chang W, Borstnik K, Sinishtaj S, Rosenthal AS, Shapiro TA. Malaria-Infected Mice Are Cured by a Single Dose of Novel Artemisinin Derivatives. J Med Chem. 2007;50:2516–2519. doi: 10.1021/jm070149m. [DOI] [PubMed] [Google Scholar]
- 18.Paik IH, Xie S, Shapiro TA, Labonte T, Narducci Sarjeant AA, Baege AC, Posner GH. Second generation, orally active, antimalarial, artemisinin-derived trioxane dimers with high stability, efficacy, and anticancer activity. J Med Chem. 2006;49:2731–2734. doi: 10.1021/jm058288w. [DOI] [PubMed] [Google Scholar]
- 19.Vennerstrom L, Arbe-Barnes S, Brun R, Charman SA, Chiu FC, Chollet J, Dong Y, Dorn A, Hunziker D, Matile H, McIntosh K, Padmanilayam M, SantoTomas J, Scheurer C, Scorneaux B, Tang Y, Urwyler H, Wittlin S, Charman WN. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430(7002):900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill PM, Posner GH. A medicinal chemistry perspective on artmeisinin and related endoperoxides. J Med Chem. 2004;47:2945–2964. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- 21.Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase 6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 22.Delarue S, Girault S, Maes L, Debreu-Fontaine MA, Labaeid M, Grellier P, Sergheraert C. Synthesis and in vitro and in vivo antimalarial activity of new 4-anilinoquinolines. J Med Chem. 2001;44:2827–2833. doi: 10.1021/jm010842o. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill PM, Willock DJ, Hawley SR, Bray PG, Storr RC, Ward SA, Park BK. Synthesis, antimalarial activity, and molecular modeling of tebuquine analogues. J Med Chem. 1997;40:437–448. doi: 10.1021/jm960370r. [DOI] [PubMed] [Google Scholar]
- 24.Madrid PB, Liou AP, DeRisi JL, Guy RK. Incorporation of an intramolecular hydrogen-bonding motif in the side chain of 4-aminoquinolines enhances activity against drug-resistant P. falciparum. J Med Chem. 2006;49:4535–4543. doi: 10.1021/jm0600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riccio ES, Lee PS, Winegar RA, Krogstad DJ, De D, Mirsalis JC. Genetic toxicology testing of the antimalarial drugs chloroquine and a new analog, AQ-13. Environ Mol Mutagen. 2001;38:69–79. doi: 10.1002/em.1052. [DOI] [PubMed] [Google Scholar]
- 26.Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, Walden JC. Structure-function relationships in aminoquinolines: Effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of β-hematin formation, and antiplasmodial activity. J Med Chem. 2000;43:283–291. doi: 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
- 27.Kaschula CH, Egan TJ, Hunter R, Basilico N, Parapani S, Tarameli D, Pasini E, Monti D. Structure-activity relationships in 4-aminoquinoline antiplasmodials. The role of the group at the 7-position. J Med Chem. 2002;45:3531–3539. doi: 10.1021/jm020858u. [DOI] [PubMed] [Google Scholar]
- 28.Ridley RG, Hofheinz H, Matile H, Jacquet C, Dorn A, Masciadri R, Jolidon S, Richter WF, Guenzi A, Girometta MA, Urwyler H, Huber W, Thiathong S, Peters W. 4-Aminoquinoline analogues of CQ with shortened side chains retain activity against CQ-resistant Plasmodium falciparum. Antimicrobial Chemother. 1996;40:1846–1854. doi: 10.1128/aac.40.8.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De D, Krogstad FM, Byers LD, Krogstad DJ. Structure-activity relationships for antiplasmodial activity among 7-substituted 4-aminoquinolines. J Med Chem. 1998;41:4918–4926. doi: 10.1021/jm980146x. [DOI] [PubMed] [Google Scholar]
- 30.Madrid PB, Liou AP, DeRisi JL, Guy K. Incorporation of an intramolecular hydrogen bonding motif in the side chain of 4-aminoquinolines enhances activity against drug-resistant P. falciparum. J Med Chem. 2006;49:4535–4543. doi: 10.1021/jm0600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawley SR, Bray PG, Park BK, Ward SA. Amodiaquine accumulation in plasmodium falciparum as a possible explanation for its superior antimalarial activity over chloroquine. Mol Biochem Parasitol. 1996;80:15–25. doi: 10.1016/0166-6851(96)02655-2. [DOI] [PubMed] [Google Scholar]
- 32.Stocks PA, Raynes KJ, Bray PG, Park BK, O'Neill PM, Ward SA. Novel short chain chloroquine analogues retain activity against chloroquine resistant K1 Plasmodium falciparum. J Med Chem. 2002;45:4975–4983. doi: 10.1021/jm0108707. [DOI] [PubMed] [Google Scholar]
- 33.Madrid PB, Wilson NT, DeRisi JL, Guy RK. Parallel synthesis and antimalarial screening of a 4-aminoquinoline library. J Comb Chem. 2004;6:437–442. doi: 10.1021/cc0340473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. 2007;51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake NL, Creech HJ, Garman JA, Haywood ST, Peck RM, van Hook JO, Walton E. Synthetic antimalrials. The preparation of certain 4-aminoquinolines. J Am Chem Soc. 1946;68:1208–1213. doi: 10.1021/ja01211a021. [DOI] [PubMed] [Google Scholar]
- 38.Geary TG, Divo AA, Jensen JB. Activity of quinoline-containing antimalarials against chloroquine-sensitive and -resistant strains of Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1987;81:499–503. doi: 10.1016/0035-9203(87)90175-1. [DOI] [PubMed] [Google Scholar]
- 39.Geary TG, Jensen JB. Lack of cross-resistance to 4-aminoquinolines in chloroquine-resistant Plasmodium falciparum in vitro. J Parasitol. 1983;69:97–105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed synthetic procedures of all compounds tested and synthetic precursors, product characterization, 1H and 13C-NMR spectra, and HPLC chromatograms obtained by two orthogonal methods. This material is available free of charge via the Internet at http://pubs.acs.org.