Abstract
Genetic instability has been implicated as a causal factor in cancer and aging. Caloric restriction (CR) and suppression of the somatotroph axis significantly increase life span in the mouse and reduces multiple symptoms of aging, including cancer. To test if in vivo spontaneous mutation frequency is reduced by such mechanisms, we crossed long-lived Ames dwarf mice with a C57BL/6J line harboring multiple copies of the lacZ mutation reporter gene as part of a plasmid that can be recovered from tissues and organs into E. coli to measure mutant frequencies. Four cohorts were studied: (1) ad lib wild-type; (2) CR wild-type; (3) ad lib dwarf; and (4) CR dwarf. While both CR wild-type and ad lib dwarf mice lived significantly longer than the ad lib wild-type mice, under CR conditions dwarf mice did not live any longer than ad lib wild-type mice. While this may be due to an as yet unknown adverse effect of the C57Bl/6 background, it did not prevent an effect on spontaneous mutation frequencies at the lacZ locus, which were assessed in liver, kidney and small intestine of 7- and 15-month old mice of all four cohorts. A lower mutant frequency in the ad lib dwarf background was observed in liver and kidney at 7 and 15 months of age and in small intestine at 15 months of age as compared to the ad lib wild-type. CR also significantly reduced spontaneous mutant frequency in kidney and small intestine, but not in liver. In a separate cohort of lacZ-C57BL/6J mice CR was also found to significantly reduce spontaneous mutant frequency in liver and small intestine, across three age levels. These results indicate that two major pro-longevity interventions in the mouse are associated with a reduced mutation frequency. This could be responsible, at least in part, for the enhanced longevity associated with Ames dwarfism and CR.
1. Introduction
A gradual loss of genome integrity has long been implicated in aging and may play an especially prominent role in aging-related diseases, such as cancer (DePinho, 2000; Vijg, 2000). Our laboratory has previously demonstrated that genomic mutations increase with age in an organ and tissue-dependent manner (Dollé et al., 1997; Dollé et al., 2000; Vijg et al., 2002; Vijg, 2002). While these results demonstrated that mutations increase with chronological age, they did not establish that spontaneous mutations accumulate as a function of biological age. One strategy for testing such a potential causal relationship is to compare spontaneous mutation frequencies between normal mice and mice of increased longevity as a possible consequence of delayed aging. There are currently two major interventions that have been convincingly demonstrated in many laboratories to increase life span of mice and delay many symptoms of aging: (1) limiting food, i.e., caloric restriction (CR) and (2) inhibiting the growth hormone/pituitary axis (Bartke, 2005). To test for a possible major role of genomic instability as a cause of aging it would be important to demonstrate that in such mouse models of extended life span, mutation accumulation is retarded together with symptoms of aging.
The oldest model for extending life span is the reduction of caloric intake (Masoro, 2005). Caloric restriction typically refers to a diet in which calories are limited by 30–40 % compared with animals fed ad lib. Caloric restriction extends life span in multiple species, including rodents, flies and worms, but its mechanism of action remains unclear. One hypothesis is that CR affects basal metabolism, slowing the production of toxic reactive oxygen species, which in turn may slow aging. There is evidence that CR reduces spontaneous mutation frequency at the Hprt locus in T lymphocytes in mice (Dempsey et al., 1993) and rats (Aidoo et al., 2003). Evidence has also been obtained that CR reduces the number of mutations in T lymphocytes of rats induced by environmental mutagens (Casciano et al., 1996). However, the Hprt assay does not allow the direct analysis of different organs and tissues. In one study of caloric restriction in 6 and 12 month old mice harboring the lacI reporter gene as part of a bacteriophage lambda vector, no CR-related changes in mutant frequency were observed in liver (Stuart et al., 2000).
Ames dwarf mice are homozygous for a mutation in the Prop-1 gene and live 50–70% longer than wild-type mice (Brown-Borg et al., 1996). The Prop-1 mutants are deficient in growth hormone, thyroid stimulating hormone and prolactin, and show reduced levels of plasma insulin, IGF-1 and glucose. Like mice subjected to CR, Ames dwarf mice show a delayed occurrence of neoplastic lesions compared with their normal siblings, which may contribute significantly to the extended life span in these mice (Ikeno et al., 2003). It has been hypothesized that the beneficial effects of the Prop-1 mutation are due to the reduction in plasma IGF-1, which may also underlie the beneficial effects of caloric restriction (Bartke, 2002).
To study the effects of both CR and Ames dwarfism on spontaneous mutation frequencies in different tissues we crossed Ames mutant mice with mice harboring lacZ reporter genes, and measured mutant frequencies in liver, kidney and small intestine of 7 and 15 month old animals of the following 4 cohorts: (1) ad lib wild-type mice; (2) CR wild-type mice; (3) ad lib dwarf mice; and (4) CR dwarf mice. The results indicate a generally strong effect of the Ames dwarf mutation in reducing spontaneous mutations at the lacZ locus with a somewhat smaller effect of CR. The latter was confirmed in an independent lacZ C57BL/6J cohort for studying CR alone. These results point towards a possible role of reduced mutagenesis in increasing life span.
2. Materials and Methods
2.1. Mouse breeding and maintenance and tissue collection
C57BL/6J lacZ-plasmid transgenic mice, homozygous for two lacZ-plasmid clusters, one on chromosome 3 (A) and one on chromosome 4 (B) (Dollé et al., 1997) were crossed to heterozygous Ames dwarf (Prop1df/Prop1+, lacZ−/−,−/−) mice in a heterogeneous background (Brown-Borg et al., 1996). The resulting F1 mice were further crossed for obtaining the 4 study cohorts as described in the Results section and shown in Fig. 1. The Prop-1 mutation or the lacZ cluster was identified from tail DNA using PCR-based tests described previously (Dollé et al., 2001; Garcia et al., 2007). Animals were sacrificed by CO2 inhalation followed by cervical dislocation and tissues excised and snap-frozen in liquid nitrogen for DNA isolation. In addition to the dwarf-lacZ hybrids, one CR and one ad lib cohort of homozygous C57Bl/6J lacZ- pUR288-plasmid transgenic mice were also initiated. The animals were maintained either fed ad libitum (ad lib) or under CR conditions in the Animal Facility of the University of Texas Health Science Center as described previously (Ikeno et al., 2005). Mice were kept pathogen-free in micro-isolator units on Tek FRESH ultra laboratory bedding (Harlan Tekland, Madison, WI) and all procedures followed the guidelines approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System. At 21 days after birth, animals were weaned and housed 4 animals/cage. Ad lib diet was provided for all animals until 2 months of age when the caloric restriction started. After 2 months, wild-type animals were fed 60 % of the ad lib intake until sacrificed. Since Ames dwarf mice were more susceptible to an early death, the animals were first fed 80 % of the Ames dwarf ad lib intake for a month and after this period they were switched to a 60 % calorie restricted diet. Measurement of food intake was done weekly on 10 % of the ad lib fed control animals matched for age as described previously (Ikeno et al., 2005). For the dwarf-lacZ hybrids, separate cohorts of 28 animals each were established to determine their survival; date of death was recorded when the mice died spontaneously. All studies were done exclusively with male animals.
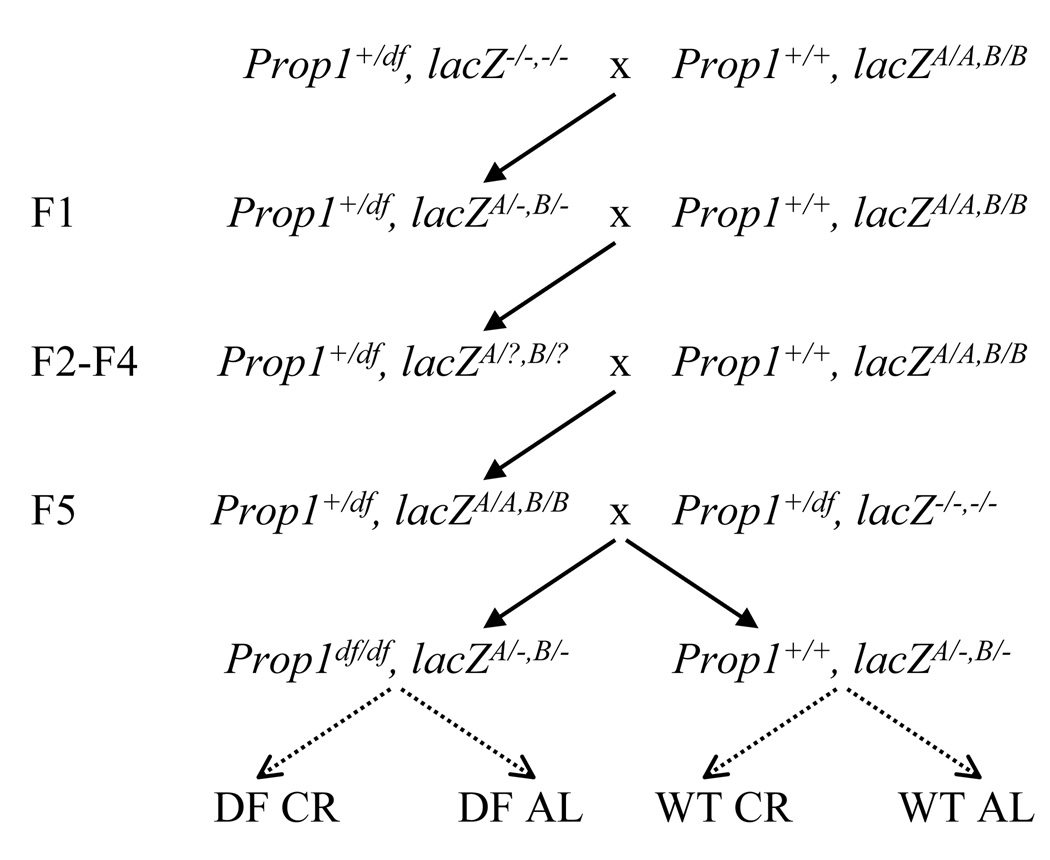
Figure 1.
Breeding scheme. Homozygous C57BL/6J lacZ-plasmid transgenic mice (Prop1+/Prop1+, lacZA/A,B/B), carrying two integration sites, one on chromosome 3 (A) and one on chromosome 4 (B) were crossed to heterozygous Ames dwarf (Prop1df/Prop1+, lacZ−/−,−/−) mice. The heterozygous Ames dwarf F1 (Prop1df/Prop1+, lacZA/−,B/−) were backcrossed with homozygous C57BL/6J lacZ-plasmid transgenic mice (Prop1+/Prop1+, lacZA/A,B/B) up to F5 generation. The F5 heterozygous Ames were backcrossed to the original Ames background and the offspring were used in the experiment. Both homozygous dwarf (DF; Prop1df/Prop1df, lacZA/−,B/−) and wild-type (WT; Prop1+/Prop1+, lacZA/−,B/−) animals were ad lib (AL) fed or calorie-restricted (CR).
2.2. Plasmid rescue and mutant frequency determination
DNA was extracted by routine phenol/chloroform extractions. Detailed protocols for plasmid rescue and mutant frequency determinations for this model are provided elsewhere (Garcia et al., 2007). Briefly, 10 to 20 µg genomic DNA was digested with Hind III for 1 h in the presence of magnetic beads (Dynal) precoated with lacZ-lacI fusion protein. The beads were washed three times to remove the unbound mouse genomic DNA. Plasmids were subsequently eluted from the beads by IPTG. After circularization of the plasmids with T4 DNA ligase they were ethanol-precipitated and used to electrotransform E.coli C (ΔlacZ, galE−) cells. One thousandth of the transformed cells was plated on the titer plate (with X-gal) and the remainder on the selective plate (with p-gal). The plates were incubated for 15 h at 37 °C. Mutant frequencies were determined as the number of colonies on the selective plates versus the number of colonies on the titer plate (times the dilution factor of 1000). Each mutant frequency is based on at least 300,000 recovered plasmids. The background mutant frequency of this system is about 1 × 10−5, as determined in E. coli, and consists mostly of false positive size-change mutants at Hind III star-activity sites (Dollé et al., 1999).
2.3. Statistical analysis
Survival curves were compared using the log-rank test. The number of mutations was analyzed for effects of age, genotype and diet using a generalized linear model analysis (McCullagh et al., 1989). The logarithm was used as the link function and the distribution was negative binomial. The ratios of mutant frequencies were used to compare experimental groups (antilogs of differences in the logarithm of the mutant frequencies). A full model was used, that is, a model that included main effects, two-factor interactions, and the three-factor interaction. The estimated dispersion parameters (liver, kidney and small intestine) were significantly different from zero. All calculations were carried out using PROC GENMOD of SAS v9.1. Statistical significance was P≤0.05.
3. Results
The breeding scheme for crossing the lacZ-plasmid reporter construct into the Ames background is shown in Fig. 1. While Ames dwarf mice have a heterogeneous genetic background, the lacZ-plasmid animals were in C57BL/6J, with the transgene cluster at two chromosomal locations, one on chromosome 3 and the other on chromosome 4. We initially decided to cross the Ames dwarf mutation as far as possible into the lacZ-C57BL/6J background. This would have had the advantage of simultaneously homogenizing the Ames background into mostly C57BL/6J, thereby reducing heterogeneity, and generating homozygosity for the lacZ loci, thus increasing the number of lacZ-plasmids that can be recovered for mutation analysis. However, with an increased contribution of the C57BL/6J background, mortality of homozygous dwarf mice between weaning and adulthood was found to rise appreciably. Although unknown to us at the time, similar results for Ames dwarf mice were obtained by Nasonkin et al. who found that a fraction of pups with a higher contribution of the C57BL/6J background display lethargy and die precipitously between weaning and adulthood (Nasonkin et al., 2004). For this reason we decided to cross the F5 Prop1df/Prop1+,lacZA/A/lacZB/B back to the original heterozygous dwarf animals (Prop1df/Prop1+, lacZ−/−,−/−). This reduction in C57BL/6J background proved to be sufficient to reduce the early mortality to a normal level.
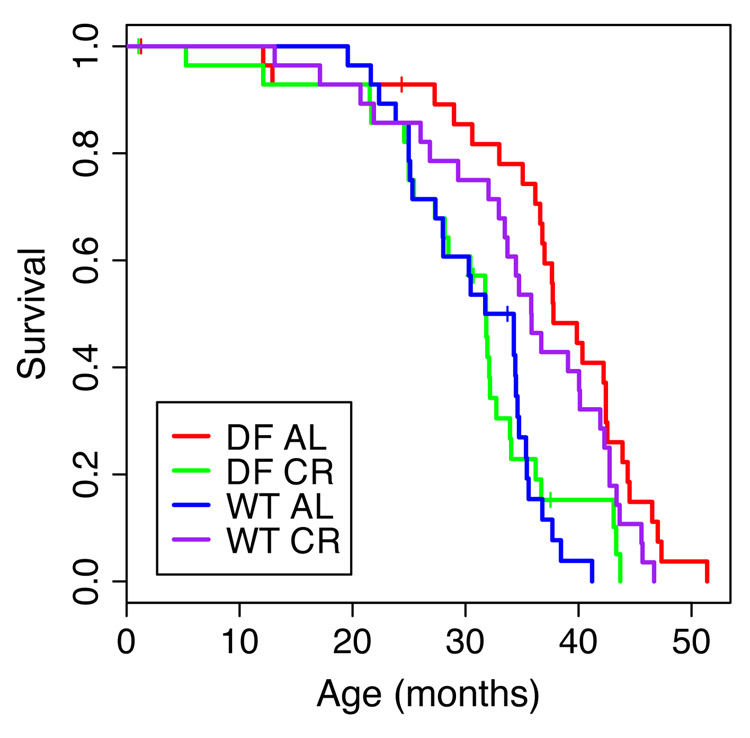
Using this strategy we established four cohorts: (1) ad lib wild-type; (2) CR wild-type, (3) ad lib dwarf and (4) CR dwarf. The survival of animals under all four conditions was determined in parallel (Fig. 2). As expected, calorie restriction extended the longevity of wild-type mice compared to that in ad lib wild-type mice (P=0.002), and ad lib Ames dwarf mice outlived ad lib wild-type animals (p=6.35e-06). However, we did not observe the further increase in longevity for the Ames dwarf mice under CR as reported previously (Bartke et al., 2001). Indeed, CR appeared to obliterate the gain in life-span of the dwarf cohort! Although we were careful to gradually calorie restrict the dwarf mice (20 % for the first month and then the 40 % reduction of food intake that is commonly applied for CR by the animal facility in San Antonio) we did see excess mortality in the CR dwarf cohort. Reasoning that 40% CR might be too severe for these fragile dwarf mice we reduced this to 30% after 19 months. However, even after censoring the cohorts for mortality before that age, we did not see any appreciable improvement of the survival curve. Indeed, the earliest age at which censoring deaths improved survival of the CR dwarf cohort was 34 months. It was only at that late age that the survival curve began to parallel the ones for the ad lib dwarf and CR wild type cohorts (results not shown).
Figure 2.
Survival plots of Ames dwarf (DF) and wild-type (WT) mice fed ad lib (AL) or calorie restricted (CR).
In view of the high costs associated with this type of study we limited our comparison of spontaneous mutation frequency at the lacZ locus to two, relatively early time points. For each cohort, between 5 and 8 mice were sacrificed at 7 and 15 months of age. Tissues were frozen and DNA extracted following a schedule in which always all four conditions were handled side-by-side. For example, liver DNAs for one time point were extracted simultaneously for all four conditions. All samples were coded and mutant frequency determinations performed blindly. For mutant frequency determinations we selected liver, kidney and small intestine on the basis of the robust age-related increases previously observed in these organs (Dollé et al., 2006; Dollé et al., 1997; Dollé et al., 2000).
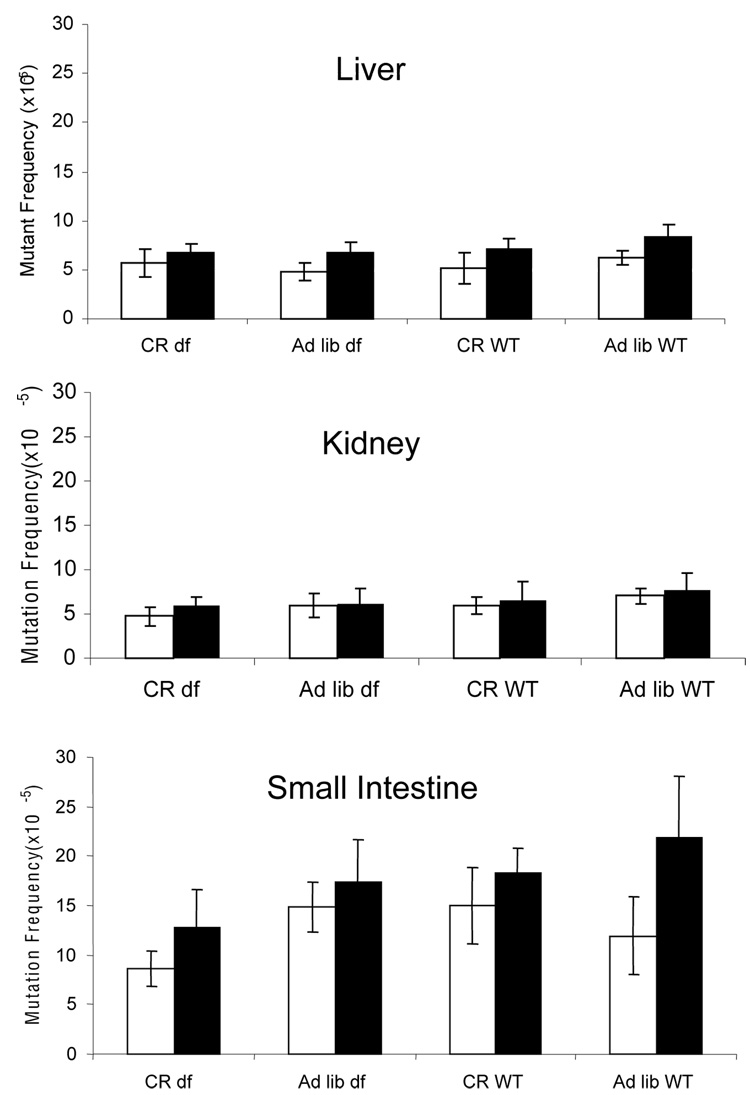
Spontaneous mutant frequencies for liver, kidney and small intestine of the lacZ-Ames dwarf hybrids at the two ages are shown in Fig. 3. All raw data are available from Supplementary Table 1–Supplementary Table 5. The lacZ mutant frequency data were subjected to statistical analysis by comparing ratios of mutant frequencies in dwarf versus wild type by diet, CR versus ad lib by genotype (Table 1) and ad lib dwarf versus CR wild type, CR dwarf versus combined CR wild type and ad lib dwarf, and ad lib wild type versus combined CR wild type and ad lib dwarf (Table 2).
Figure 3.
Mutant frequency at the lacZ reporter locus of Ames dwarf mice and their wild-type littermate controls at 7-month old (white bars) and 15-month old (black bars) animals after caloric restriction or fed ad lib. Error bars represent 95% confidence intervals.
Table 1.
Ratio of mutant frequency observed in Ames dwarf mice to mutant frequency observed in wild-type mice by dietary condition, tissue and age; and ratio of mutant frequency observed in CR mice to mutant frequency observed in Ad lib mice by genotype, tissue and age
| Tissue | Age (months) | Ratio Ames dwarf / Wild-type |
Ratio CR / Ad Lib |
||
|---|---|---|---|---|---|
| Ad Lib | CR | Ames dwarf | Wild-type | ||
| Liver | 7 | 0.76 ** | 1.06 | 1.20 † | 0.86 † |
| 15 | 0.84 † | 0.98 | 1.02 | 0.87 | |
| Kidney | 7 | 0.82 * | 0.79 * | 0.81 * | 0.83 † |
| 15 | 0.77 ** | 0.93 | 0.99 | 0.82 † | |
| Intestine | 7 | 1.34 * | 0.57 ** | 0.59 ** | 1.37 ** |
| 15 | 0.79 † | 0.70 * | 0.74 * | 0.83 | |
Bold typeface indicates ratio different from 1.00 (P ≤ 0.10)
Ratio different from 1.00 (P ≤ 0.01)
Ratio different from 1.00 (P ≤ 0.05)
Ratio different from 1.00 (P ≤ 0.10)
Table 2.
Ratio of mutant frequency observed in Ad lib Ames dwarf mice to mutant frequency observed in CR wild-type mice by tissue and age; ratio of mutant frequency observed in CR Ames dwarf mice to mutant frequency observed in combined CR wild-type mice and Ad lib Ames dwarf mice by tissue and age; and ratio of mutant frequency observed in Ad lib wild-type mice to mutant frequency observed in combined CR wild-type mice and Ad lib Ames dwarf mice by tissue and age
| Tissue | Age (months) | Ratio | Ratio | Ratio |
|---|---|---|---|---|
| Ad Lib Ames dwarf / CR Wild-type | CR Ames dwarf / (CR Wild-type and Ad Lib Ames dwarf) | Ad Lib Wild-type / (CR Wild-type and Ad Lib Ames dwarf) | ||
| Liver | 7 | 0.89 | 1.13 | 1.23 ** |
| 15 | 0.96 | 1.00 | 1.17 † | |
| Kidney | 7 | 0.98 | 0.80 ** | 1.21 * |
| 15 | 0.94 | 0.97 | 1.27 ** | |
| Intestine | 7 | 0.97 | 0.58 ** | 0.74 ** |
| 15 | 0.95 | 0.72 ** | 1.24 * |
Bold typeface indicates ratio different from 1.00 (P ≤ 0.10)
Ratio different from 1.00 (P ≤ 0.01)
Ratio different from 1.00 (P ≤ 0.05)
Ratio different from 1.00 (P ≤ 0.10)
As expected, all three organs experienced an increase of the spontaneous mutant frequency between 7 and 15 months (although for kidney this was not statistically significant). With the exception of small intestine at 7 months, there was a significantly lower mutant frequency in the dwarf background as compared to the ad lib wild-type (Table 1), as indicated by the ratio of mutant frequencies. Of note, while it was clear that overall there was a significant protective effect of the Ames dwarf mutation against spontaneous mutations in the small intestine, 7 month-old Ames dwarf animals showed a higher mutant frequency (14.10 × 10−5) than their wild-type littermate controls (10.55 × 10−5). In our experience, the spontaneous mutant frequency at the lacZ locus in small intestine at this age level is generally around 15 × 10−5. After re-confirming the genotypes we decided to re-extract DNA from the remaining tissues and repeat the experiment. Since essentially the same results were obtained we conclude that these observations were part of the natural variation, which is not unusual for an end point such as mutation frequency. It is also not impossible that animal-to-animal variation is higher for animals in a mixed genetic background, although that was not obvious in this case (compare Fig. 3 and Fig. 4).
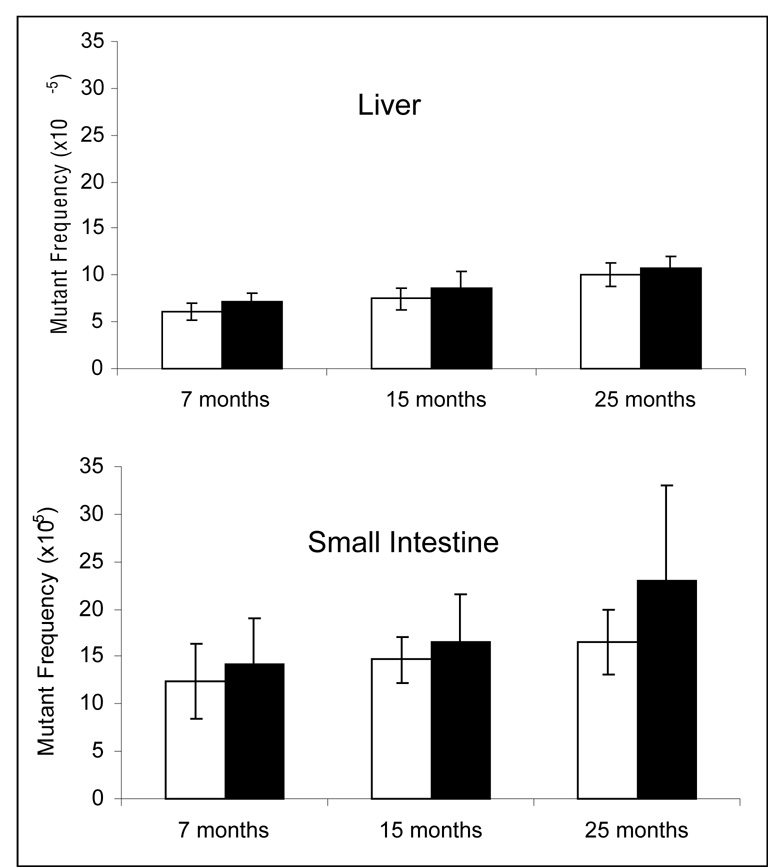
Figure 4.
Mutant frequency at the lacZ reporter locus of C57BL/6J mice at 7-, 15- and 25-months of age after caloric restriction (white bars) or fed ad lib (black bars). Error bars represent 95% confidence intervals.
For the CR diet, there was a lower mutant frequency in Ames dwarf mice compared to wild-type in kidney and small intestine at 7 months and small intestine at 15 months (indicated by ratios less than 1.00; Table 1, left columns). This can also be concluded from the ratios CR versus ad lib in the dwarf. In addition to the Ames dwarf mutation, CR in this mixed background significantly reduced spontaneous mutant frequency in kidney at 7 months and small intestine at 7 and 15 months (Table 1; right columns). In wild-type mice, CR reduced mutant frequency compared to ad lib in all tissues and at both ages except for small intestine at 7 months (Table 1).
In all tissues and at both ages, there was no significant difference in mutant frequency between ad lib Ames dwarf mice and CR wild-type mice (ratios not significantly different from 1.00; Table 2, left columns). For comparison with other groups we then combined the ad lib Ames dwarf mice and CR wild-type mice. The mutant frequency in CR Ames dwarf mice was significantly lower than in the combined ad lib Ames dwarf and CR wild-type mice in kidney and small intestine at 7 months and in small intestine at 15 months. Mutant frequency was higher in ad lib wild type than in combined ad lib Ames dwarf and CR wild-type mice in all tissues at both ages except small intestine at 7 months (Table 2).
Except for the results for small intestine at 7 months, the mutant frequency results are consistent across the different tissues and the two ages. Except for CR Ames dwarf mice, the longevity and mutant frequency results overall are consistent; ad lib wild type mice have the highest mutant frequency and the poorest survival while ad lib Ames dwarf and CR wild type have similar mutant frequencies and survival.
Since the observed CR effect was not as strong as the effect of the dwarf mutation, we decided to separately test for CR in a lacZ-C57BL/6 cohort, which would allow us to compare the effects of caloric restriction alone in a homogeneous genetic background. In this approach, animals were housed under caloric restriction or ad lib conditions and samples were collected at 7, 15 and 25 months of age. Mutant frequencies at the lacZ reporter locus of C57BL/6J mice by age and dietary condition are shown in Fig. 4 for liver and small intestine. Mutant frequency increased with age in both liver (p=0.0001, Fig. 4) and small intestine (p=0.0016; Fig 4). In liver, CR mice had lower mutant frequencies than ad lib mice at all ages but this difference approached statistical significance only at 15 months (Table 3). In small intestine, the mutant frequency in calorie-restricted mice was lower than ad lib controls at every age but the difference was statistically significant only at 25 months of age (Table 3). Hence, the results from this separate cohort essentially confirmed the results obtained for CR with the Ames dwarf mutant cohort.
Table 3.
Ratio of mutant frequency observed in CR C57BL/6 mice to mutant frequency observed in Ad lib C57BL/6 mice by tissue and age
| Tissue | Age (months) | Ratio |
|---|---|---|
| CR C57BL/6 / Ad Lib C57BL/6 |
||
| Liver | 7 | 0.88 |
| 15 | 0.87 † | |
| 25 | 0.96 | |
| Intestine | 7 | 0.88 |
| 15 | 0.88 | |
| 25 | 0.72 * |
Bold typeface indicates ratio different from 1.00 (P ≤ 0.10)
Ratio different from 1.00 (P ≤ 0.01)
Ratio different from 1.00 (P ≤ 0.05)
Ratio different from 1.00 (P ≤ 0.10)
4. Discussion
There is increasing evidence that life extension through caloric restriction, at least in rodents, exerts its beneficial effect in delaying multiple symptoms of aging by suppressing the growth hormone/insulin-like growth factor 1 (GH/IGF1) somatotroph axis (Bartke, 2005). In turn, this may lead to the activation, possibly mediated by Forkhead box O (FoxO) transcription factors (Carter et al., 2007), of various cellular defense systems, including antioxidant defense and DNA repair (Brown-Borg et al., 2000). Indeed, recent results obtained with mice harboring defects in various pathways for repairing DNA damage, indicate that suppression of the GH/IGF1 axis is part of a DNA damage response involving metabolic changes that shift energy usage from growth and proliferation to protective maintenance (Niedernhofer et al., 2006; Vijg, 2006). Our present results are in keeping with this concept. Indeed, the overall pattern emerging from our present work is that both CR and the Ames dwarf mutation reduce somatic mutation accumulation in different tissues of the mouse.
The concept of a general reduction of cellular mutation load in mice subjected to CR is in keeping with results obtained by Dempsey et al. (Dempsey et al., 1993) and Aidoo et al (Aidoo et al., 2003), for somatic mutant frequency in T lymphocytes in mice and rats, respectively. Of note, Stuart et al., also using a mouse model harboring a mutational reporter gene, found no significant reduction in the spontaneous mutant frequency in liver of CR mice as compared with ad lib fed animals (Stuart et al., 2000). Interestingly, also in our present study, it is in liver where the reduction in spontaneous mutant frequency is weakest, either in the combined CR/dwarf cohort or the C57BL/6J cohort alone. Hence, it is possible that Stuart et al. lacked the power to detect a small difference that may actually have been present. The aforementioned HPRT assay is generally more sensitive than the fully neutral, transgenic reporter assays in detecting small differences, due to the relatively low background mutation frequency of this assay, which in turn is likely to be due to in vivo selection against cells with an inactivated HPRT gene (Vijg et al., 1998). It is therefore reasonable to interpret our present results as indicative for a relatively small but significant reduction in spontaneous mutation frequency. This raises the question of the underlying causes of such a reduction and its potential impact in explaining the increased longevity of these mice. As mentioned, while the mechanisms by which reduced GH/IGF1 signals can lead to increased longevity are not clear as yet, several studies have shown that this condition is associated with an upregulation of antioxidant defense capacity. It is known that factors that promote the generation of cellular reactive oxygen species (ROS) and/or impair anti-oxidative processes contribute to oxidative damage which accumulates with age and might be responsible for the progressive decline in physiological systems that occurs in virtually all organisms. There is abundant evidence that both in dwarf mice and mice kept under CR conditions antioxidant defense and stress responses are increased (Brown-Borg et al., 2000; Romanick et al., 2004). The importance of the antioxidant defense system in the maintenance of genome stability was demonstrated by Busuttil et al., who demonstrated greatly accelerated mutation accumulation in liver of SOD1-null mice (Busuttil et al., 2005). This indicates that oxidative stress is a causal factor of the increased incidence of liver cancer in this mutant mouse model (Elchuri et al., 2005). Hence, the lower spontaneous somatic mutation accumulation observed for the GH/IGF1-deficient mice in our present study could be a result of a decreased susceptibility to oxidative damage-induced mutagenesis. This effect is likely to be stronger in the dwarf mice than in CR because of the stronger reduction in GH/IGF1 in these animals and/or because the downregulation of the somatotroph axis under CR conditions only occurred after 2 months. However, the possibility cannot be ruled out that the mechanism by which the Ames mutation and caloric restriction affect longevity is different (Bartke et al., 2001).
Alternatively, the reduced mutant frequency in CR and dwarf mice could be due to decreased cell proliferative activity. CR in the mouse has been found associated with a reduced BrdU labeling index in all of six different cell types in five different organs analyzed, up until 13 months (Wolf et al., 1995). This would reduce the chance of replication errors as a source of spontaneous mutations. In addition, CR (in rats) has been associated with an increase in spontaneous apoptosis in the liver (James et al., 1994). This is also expected to reduce mutation load of the tissue as a whole, due to the preventive removal of damaged cells.
One thing that is not clear from our studies is how to reconcile the observed synergistic effect of dwarfism and CR on spontaneous mutant frequency. Indeed, while this effect confirms previous work of a synergistic effect of CR and the Ames dwarf mutation on life span (Bartke et al., 2001), this was not reproduced in this present study (Fig. 2A). Hence, formally there is no association between the observed reduced mutant frequency and the life span effect. However, we believe that a more likely explanation is that the increased mortality in the CR dwarf cohort is independent of the effect on mutant frequency. Most likely, the increased deaths are due to the C57BL/6 background parts of which still remained in the hybrid animals that made up the cohort. It is possible that the C57BL/6J background under CR conditions, together with the naturally rather frail dwarf mice lead to increased mortality during the entire life span. The observed late longevity effect (i.e., after 34 months) in the CR dwarfs may involve the survivors, possibly those with minimal adverse C57BL/6J background. It is possible that a relatively low IGF-1 serum level in this mouse strain as compared to others (J.N.; unpublished) increases their frailty, especially in combination with other stressors such as CR or dwarfism. Of note, mice carrying mutations in the Ercc1 DNA repair gene show premature aging and a short life span, but also a reduced activity of IGF-1 signals (Niedernhofer et al., 2006). Other likely explanations for differential effects of CR on longevity of Ames dwarf mice in this as compared to the earlier study (Bartke et al., 2001) include differences in husbandry (particularly in age of weaning) and details of the CR protocol (40% until the age of 19 months vs. 30% throughout). Whatever the explanation for the increased mortality of the CR dwarf cohort, their survival benefit does appear to manifest as reduced spontaneous genome instability, albeit not as increased life span.
What could be the possible consequences of a reduced spontaneous mutation frequency in dwarf mice or mice subjected to CR? While it has been hypothesized that somatic mutation accumulation could be responsible for aging-related cell and tissue degeneration in general (Vijg, 2002), the most likely effect of the reduction in spontaneous mutation frequency in the CR and dwarf mice would be reduced cancer. Indeed, cancer is the phenotype most often found to be delayed and even reduced in the spectrum of aging-related pathology. Ikeno et al demonstrated that Ames dwarf mice show a delayed occurrence of neoplastic lesions compared with their normal siblings, a difference that likely contributed importantly to the extended life span in these mice (Ikeno et al., 2003). However, genome instability has also been implicated in other age-related diseases and the possibility cannot be ruled out that reduced spontaneous mutation rates also explain, in part, the delayed appearance of other forms of pathology in dwarf mice or CR conditions.
In summary, the results of this study provide evidence for delayed spontaneous mutation accumulation in dwarf mice and in mice subjected to caloric restriction. The protective effect was stronger in dwarf mice than in CR but seems to be present in multiple tissues. The magnitude of the effect was small, i.e., not more than an approximate 20% reduction in mutation frequency was observed in most cases, i.e., three tissues at two age levels. However, even such a small reduction in spontaneous mutagenesis could explain the reduction in spontaneous tumors that is a hallmark of both CR and dwarf mice.
Supplementary Material
Acknowledgements
This work was supported by NIH grant AG20438. We thank Armando Rodriguez for technical assistance in excising tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aidoo A, Mittelstaedt RA, Bishop ME, Lyn-Cook LE, Chen YJ, Duffy P, Heflich RH. Effect of caloric restriction on Hprt lymphocyte mutation in aging rats. Mutat Res. 2003;527:57–66. doi: 10.1016/s0027-5107(03)00072-1. [DOI] [PubMed] [Google Scholar]
- Bartke A. Insulin-like growth factor 1 and mammalian aging. Sci Aging Knowledge Environ. 2002;2002 doi: 10.1126/sageke.2002.16.vp4. vp4. [DOI] [PubMed] [Google Scholar]
- Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Busuttil RA, Garcia AM, Cabrera C, Rodriguez A, Suh Y, Kim WH, Huang TT, Vijg J. Organ-specific increase in mutation accumulation and apoptosis rate in CuZn-superoxide dismutase-deficient mice. Cancer Res. 2005;65:11271–11275. doi: 10.1158/0008-5472.CAN-05-2980. [DOI] [PubMed] [Google Scholar]
- Carter ME, Brunet A. FOXO transcription factors. Curr Biol. 2007;17:R113–R114. doi: 10.1016/j.cub.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Casciano DA, Chou M, Lyn-Cook LE, Aidoo A. Calorie restriction modulates chemically induced in vivo somatic mutation frequency. Environ Mol Mutagen. 1996;27:162–164. doi: 10.1002/(SICI)1098-2280(1996)27:2<162::AID-EM10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Dempsey JL, Pfeiffer M, Morley AA. Effect of dietary restriction on in vivo somatic mutation in mice. Mutat Res. 1993;291:141–145. doi: 10.1016/0165-1161(93)90153-q. [DOI] [PubMed] [Google Scholar]
- DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- Dollé ME, Busuttil RA, Garcia AM, Wijnhoven S, van Drunen E, Niedernhofer LJ, van der Horst G, Hoeijmakers JH, van Steeg H, Vijg J. Increased genomic instability is not a prerequisite for shortened lifespan in DNA repair deficient mice. Mutat Res. 2006;596:22–35. doi: 10.1016/j.mrfmmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Dollé ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet. 1997;17:431–434. doi: 10.1038/ng1297-431. [DOI] [PubMed] [Google Scholar]
- Dollé ME, Snyder WK, Gossen JA, Lohman PH, Vijg J. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc Natl Acad Sci U S A. 2000;97:8403–8408. doi: 10.1073/pnas.97.15.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé ME, Snyder WK, van Orsouw NJ, Vijg J. Background mutations and polymorphisms in lacZ-plasmid transgenic mice. Environ Mol Mutagen. 1999;34:112–120. [PubMed] [Google Scholar]
- Dollé ME, Snyder WK, Vijg J. Genotyping the Prop-1 mutation in Ames dwarf mice. Mech Ageing Dev. 2001;122:1915–1918. doi: 10.1016/s0047-6374(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Busuttil RA, Rodriguez A, Cabrera C, Lundell M, Dolle ME, Vijg J. Detection and analysis of somatic mutations at a lacZ reporter locus in higher organisms: application to Mus musculus and Drosophila melanogaster. Methods Mol Biol. 2007;371:267–287. doi: 10.1007/978-1-59745-361-5_20. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- James SJ, Muskhelishvili L. Rates of apoptosis and proliferation vary with caloric intake and may influence incidence of spontaneous hepatoma in C57BL/6 × C3H F1 mice. Cancer Res. 1994;54:5508–5510. [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- Nasonkin IO, Ward RD, Raetzman LT, Seasholtz AF, Saunders TL, Gillespie PJ, Camper SA. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet. 2004;13:2727–2735. doi: 10.1093/hmg/ddh311. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev. 2004;125:269–281. doi: 10.1016/j.mad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Stuart GR, Oda Y, Boer JG, Glickman BW. No change in spontaneous mutation frequency or specificity in dietary restricted mice. Carcinogenesis. 2000;21:317–319. doi: 10.1093/carcin/21.2.317. [DOI] [PubMed] [Google Scholar]
- Vijg J. Somatic mutations and aging: a re-evaluation. Mutat Res. 2000;447:117–135. doi: 10.1016/s0027-5107(99)00202-x. [DOI] [PubMed] [Google Scholar]
- Vijg J, Dollé ME. Large genome rearrangements as a primary cause of aging. Mech Ageing Dev. 2002;123:907–915. doi: 10.1016/s0047-6374(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Vijg J, Dollé ME. Large genome rearrangements as a primary cause of aging. Mech Ageing Dev. 2002;123:907–915. doi: 10.1016/s0047-6374(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Vijg J, Suh Y. Ageing: chromatin unbound. Nature. 2006;440:874–875. doi: 10.1038/440874a. [DOI] [PubMed] [Google Scholar]
- Vijg J, van Steeg H. Transgenic assays for mutations and cancer: current status and future perspectives. Mutat Res. 1998;400:337–354. doi: 10.1016/s0027-5107(98)00030-x. [DOI] [PubMed] [Google Scholar]
- Wolf N, Penn P, Jiang D, Fei RG, Pendergrass WR. Caloric restriction: conservation of in vivo cellular replicative capacity accompanies life-span extension in mice. Exp Cell Res. 1995;217:317–323. doi: 10.1006/excr.1995.1092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.