Abstract
In natural situations, motor activity must often choose a single target when multiple distractors are present. The present paper asks how primate smooth pursuit eye movements choose targets, by analysis of a natural target-selection task. Monkeys tracked two targets that started 1.5° eccentric and moved in different directions (up, right, down, and left) toward the position of fixation. As expected from previous results, the smooth pursuit before the first saccade reflected a vector average of the responses to the two target motions individually. However, post-saccadic smooth eye velocity showed enhancement that was spatially selective for the motion at the endpoint of the saccade. If the saccade endpoint was close to one of the two targets, creating a targeting saccade, then pursuit was selectively enhanced for the visual motion of that target and suppressed for the other target. If the endpoint landed between the two targets, creating an averaging saccade, then post-saccadic smooth eye velocity also reflected a vector average of the two target motions. Saccades with latencies >200 msec were almost always targeting saccades. However, pursuit did not transition from vector-averaging to target-selecting until the occurrence of a saccade, even when saccade latencies were >300 msec. Thus, our data demonstrate that post-saccadic enhancement of pursuit is spatially selective and that noncued target selection for pursuit is time-locked to the occurrence of a saccade. This raises the possibility that the motor commands for saccades play a causal role, not only in enhancing visuomotor transmission for pursuit but also in choosing a target for pursuit.
Keywords: selective attention, visual motion processing, gain control, movement initiation, vector averaging, winner-take-all
In the natural world, motor systems are often faced with many choices of targets as the endpoint of movements. Once a specific target has been selected, the motor system must selectively guide movement with sensory information from that target alone and ignore all non-relevant stimuli. This process of target selection is often further complicated by the need to coordinate multiple movement systems. For example, when primates examine the fine detail of an object moving through the visual field, they make both a saccade to bring the image of the object onto the fovea and a smooth pursuit eye movement to stabilize the image on the fovea (Dodge, 1903; Robinson, 1965). Saccades are programmed primarily to correct errors between target and eye position (for review, see Sparks and Mays, 1990), whereas pursuit represents an attempt to minimize the difference between target and eye velocity (Rashbass, 1961; cf.Krauzlis and Stone, 1999) (for review, see Lisberger et al., 1987;Keller and Heinen, 1991). Thus, target selection for ocular tracking involves isolation of both the position and velocity of a moving target and coordinating the use of these signals among two different movement systems.
Saccadic eye movements have been used traditionally as a model system for analysis of target selection, and considerable information is now available about the evolution of commands for saccadic eye movements throughout the parietal and frontal cortex (Schall and Hanes, 1993; Platt and Glimcher, 1997; Gold and Shadlen, 2000) (for review, see Schall and Thompson, 1999). However, saccades are a special kind of movement in which a target can be chosen, a motor command generated, and precise motor circuits brought into play to execute the command accurately without any sensory feedback. Because a saccadic movement occurs at a single point in time, many saccades with different latencies must be examined to make an inference from the behavior as to how target selection develops over time. Numerous studies of this nature have demonstrated that, depending on the relative location and instructions given, earlier saccades tend to be made to a compromise, vector-averaged position between two targets, and target-selecting saccades generally occur with longer latencies (Becker and Jürgens, 1979; Findlay, 1982; Ottes et al., 1985;Coëffé and O'Regan, 1987; He and Kowler, 1989; Chou et al., 1999). Smooth pursuit eye movements use visual feedback to guide movement continuously and therefore offer the opportunity to directly examine the behavioral consequences of target-selection processes as they develop over time. Knowing that the initial, pre-saccadic pursuit to two identical targets (Lisberger and Ferrera, 1997) reflected a vector average of the responses to each target individually, we set out to examine whether the transition from vector-averaging to target-selecting pursuit would mirror the inferred transition of the saccadic system. We find that the transition from vector-averaging to target selection does not follow the same time course as the transition in the saccadic system, but instead is time-locked to the execution of a targeting saccade.
Our experiments were also motivated by previous results that indicate that there is a strong interaction between the saccadic and pursuit systems. The act of making a saccade to a target can activate the visuomotor pathway for the pursuit system (Lisberger, 1998). Pursuit gain (eye velocity divided by target velocity) just after a saccade is markedly enhanced compared with pursuit gain just before a saccade. This post-saccadic enhancement is unlike other enhancement effects described for ocular following (Kawano and Miles, 1986) and disparity vergence (Busettini et al., 1996), because it is provoked by the motor act of making the saccade rather than by the rapid motion of the visual scene across the retina during a saccade. The properties of post-saccadic enhancement of pursuit imply that visual motion inputs are enhanced if they come from a target that is about to be brought onto the fovea. Because previous experiments were done with single target stimuli, it is not known whether enhancement is related to cognitive functions, such as target selection, or whether it is simply a non-specific enhancement of responses to all visual motion. Our results indicate that post-saccadic enhancement is indeed a spatially selective mechanism and may therefore be comparable with attention.
MATERIALS AND METHODS
Experiments were conducted on three adult male rhesus monkeys (Macaca mulatta) weighing 7–9 kg. Experimental methods were the same as have been presented previously (Lisberger and Ferrera, 1997a; Lisberger, 1998). Briefly, monkeys were trained to fixate and track visual targets for a liquid reward (Wurtz, 1969). Eye position was monitored using a scleral search coil (Judge et al., 1980) that had been implanted with hardware for head restraint during sterile surgery under anesthesia with isoflurane. During experiments, monkeys sat in a primate chair with their heads restrained to the top of the chair. All methods had received previous approval from the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Visual stimuli were presented on a Pentium personal computer-controlled Hewlett-Packard (Palo Alto, CA) 1304A oscilloscope that subtended 36° × 24° of horizontal and vertical arc at a distance of 40 cm from the animal and had a refresh rate of 250 Hz. Targets consisted of spots of light that spanned 0.2° of visual arc. All experiments were conducted in dim light against a dark background. Experimental conditions were controlled by a custom-built UNIX application running on a DEC alpha workstation. Each daily experiment consisted of a series of trials, in which each trial presented a different target motion; because it is critical to the design of the experiment, the structure of the trials will be presented in detail at the start of Results. Trials were presented in randomized order, and the monkey was rewarded only for successfully completed trials. Any failed trial was reordered to the end of the set of trials, and a new set, in a different randomized order, did not begin until all trials had been successfully completed. Monkeys typically performed better than 85% correct on each trial set.
Eye velocity was provided by analog differentiation of the position signal by a double-pole filter with a corner frequency of 25 Hz. For a discussion of analog filtering effects on measurements of post-saccadic eye velocity, see Lisberger (1998). Position and velocity traces were sampled at 1 kHz and digitized for storage and subsequent analysis on the UNIX workstation. Saccades were then marked by visual inspection of individual trials using a custom-built program. All further analysis was done using Matlab 5.2 (MathWorks Inc., Natick, MA). All eye velocity data that occurred during saccades were excluded from analysis.
We measured the final position of each saccade, the position of both targets at the end of each saccade, and the pre-saccadic and post-saccadic eye velocity (defined below). We then used the same equation to evaluate the relative contribution of the visual signals from each target to each measure of saccade and pursuit behavior. We calculated a weight w that best satisfied the following overconstrained equation in the least-squared sense:
| Equation 1 |
For the w values associated with the post-saccadic eye position (saccade weights), is a vector representing the position of one spot at the end of the saccade (Fig.1B, arrow a), and represents the other spot. For the w values associated with eye velocity (pursuit weights), and are vectors representing the pre-saccadic or post-saccadic eye velocity (averaged over a 10 msec window) during single spot trials in which one spot or the other is presented. is a vector representing either the position or velocity of the eye (saccade and pursuit weight, respectively) in individual trials in which both spots represented by and were displayed.
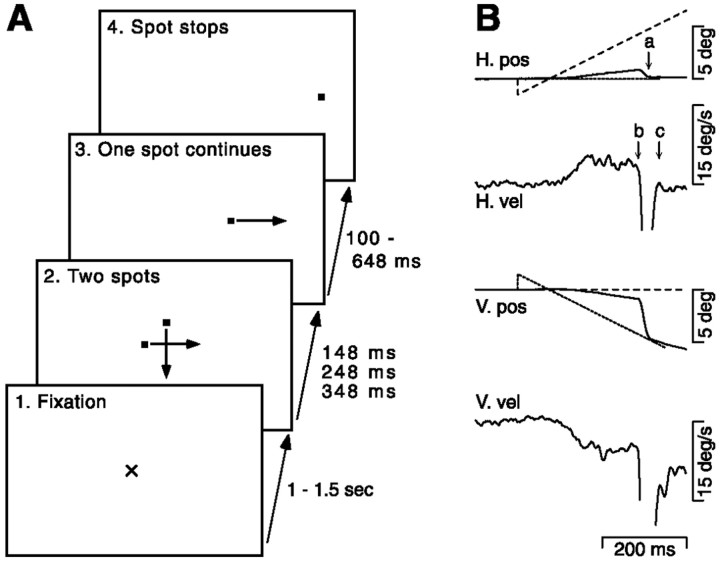
Fig. 1.
Schematic diagram showing example trial configuration and representative raw data traces. A, Representation of a two-target trial that presents a downward and a rightward moving target. The monkey was required to fixate a central fixation point for 1–1.5 sec. Two targets appeared 1.5° eccentric and moved toward the center for 148, 248, or 348 msec, after which one target vanished and the other continued. After 100–648 msec, the final target stopped and the monkey was required to fixate the target for 600 msec. B, Representative eye and target position (short-dashed lines, downward target; long-dashed lines, rightward target; solid lines, eye position) and velocity traces for the trial configuration shown inA. Arrow a marks the end of the saccade in the horizontal eye position trace. Arrows b andc mark the beginning and end of the saccade in the horizontal eye velocity trace, respectively. Positive deflections in all traces indicate rightward (horizontal traces) or upward (vertical traces) position or velocity. Negative deflections indicate leftward (horizontal traces) or downward (vertical traces) position or velocity.
As our measure of post-saccadic eye velocity, we used the mean eye velocity for the 10 msec just after a saccade. A previous report (Lisberger, 1998) conducted an extensive evaluation of the use of this epoch of eye velocity, demonstrating the validity of the filtering methods we used to obtain post-saccadic eye velocity and showing that enhancement in the 10 msec interval we used was not a motor after-effect of the saccade itself. As an additional test of the veracity of conclusions based on the first 10 msec of post-saccadic eye velocity, Figures 5 and 6 examine the time course of the effects for many subsequent 10 msec intervals as well.
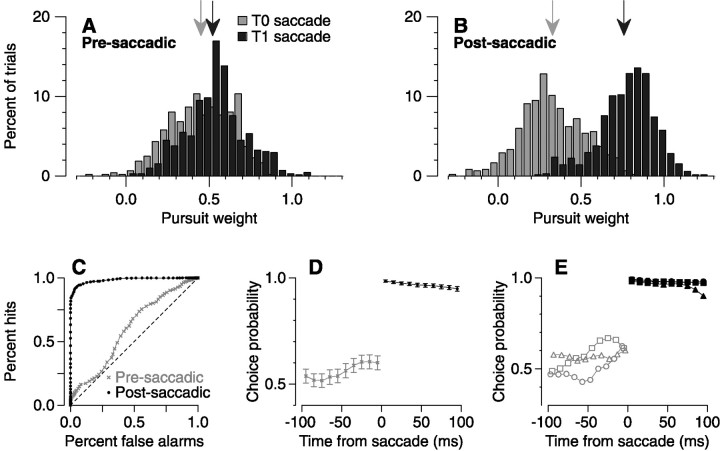
Fig. 5.
Choice probability analysis of pursuit target selection. A, Pre-saccadic distribution of pursuit weights for trials in which saccades were directed toward target 0 (gray bars) or target 1 (black bars). Targets 0 and 1 were assigned randomly for each experimental condition. Black bars are slightly offset to the right to facilitate viewing of both distributions. The means of the distribution are indicated withgray and black arrows, respectively.B, Postsaccadic distribution of pursuit weights.C, ROC curves for pre-saccadic (gray x symbols) and post-saccadic (filled black circles) pursuit weights from A andB. The area under the pre-saccadic curve (0.60) and the post-saccadic curve (0.98) are the choice probabilities calculated for eye velocity in the last 10 msec before and the first 10 msec after the saccade. D, Choice probability as a function of time in 10 msec bins before (gray x symbols) and after (filled black circles) saccade, summarizing combined data for all three monkeys. Error bars on eachpoint indicate the 95% confidence interval as estimated by bootstrapping (see Materials and Methods). E, Choice probability calculated for each monkey separately:circles, Mo; triangles, Ka;squares, Im. Confidence intervals have been left off to facilitate viewing. See Results for a description of the statistical significance of each curve.
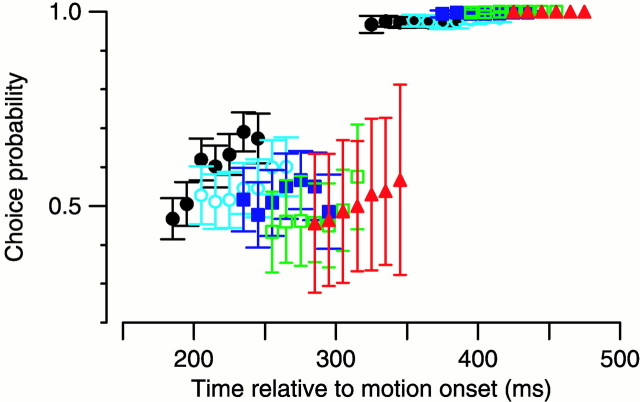
Fig. 6.
Temporal link between saccade execution and target selection for pursuit. Choice probability curves compiled separately for trials with saccade latencies between 226–275 msec (filled black circles), 251–300 msec (open cyan circles), 276–325 msec (filled blue squares), 301–350 msec (open green squares), and 326–375 msec (filled red triangles). Time points between the average saccade start and end have been omitted because there were not enough data to include in the analysis.
Even in conditions in which the targets looked identical and had equal probability of becoming the final tracking target, the monkeys sometimes had strong choice biases. For each combination of two targets in each experimental session, we calculated the choice index with the following equation:
| Equation 2 |
where nT0 andnT1 are the numbers of trials in which the monkey made a saccade in the direction of one target or the other, and every saccade was assigned to a target according to whether saccade weight was less than or greater than 0.5.
Ninety-five percent confidence intervals for choice probabilities were estimated using the percentile method from a bootstrapped estimate of the sampling distribution calculated by resampling the original distribution with replacement 1000 times and recalculating the choice probability (Mooney and Duval, 1993).
RESULTS
Monkeys were rewarded for tracking spots in a trial configuration designed to promote free choice between two equally salient targets. Trials consisted of the four segments diagrammed in Figure1A. (1) A fixation point appeared that monkeys were required to fixate for 1–1.5 sec. (2) The fixation point was extinguished. At the same time, two spots appeared 1.5° eccentric, in this case to the left and above the fixation point, and moved down and right toward the point of fixation at 20°/sec for an interval that was randomized among 148, 248, and 348 msec. (3) One spot disappeared and the other continued moving at 20°/sec for 100–648 msec. (4) The moving spot stopped and was held stationary at an eccentric position for 600 msec. This design was necessary for the success of the experiments. It allowed us to demonstrate vector-averaging in the pre-saccadic eye velocity and target choice for pursuit in the post-saccadic eye velocity in the same trials. All other experimental designs lack this internal control. Targets that move away from the position of fixation have very poor pre-saccadic pursuit, making it impossible to evaluate whether vector-averaging holds and when target selection commences. Targets that start more eccentric and move toward the position of fixation, using the standard step-ramp of Rashbass (1961), lack the saccades whose effect we wanted to study.
A crucial part of the trial was the second segment in which both targets remained visible. If the monkey made a saccade to one or the other target during this segment, then we could analyze the effect of the saccade on the sensorimotor transformation of the two target motions into pursuit movements. To encourage the monkey not to withhold saccades during this segment, we randomized its length. Monkeys then tended to make saccades while both targets were visible for the trials in which the segment lasted 348 msec (mean ± SD saccade latency was 224 ± 55 msec). We restricted our analysis to these trials. To allow the monkey to make a free choice, fixation contingencies were suspended from the onset of the second segment until 100–250 msec after the disappearance of one spot, after which monkeys were required to keep eye position within 3° of the position of the remaining spot. The last two segments of the trial were added simply to require the monkey to pursue a target until the end of the trial. Each experiment included all six possible combinations of two spots moving centripetally in the four cardinal directions, as well as randomly interspersed trials that presented the motion of single spots.
Figure 1B shows our basic finding for an individual trial in which one spot moved downward (short-dashed lines) and the other spot moved to the right (long-dashed lines). Before the saccade (arrow b), eye velocity had rightward and downward components of 3.8 and −4.4°/sec, respectively. Immediately after the saccade (arrow c), which brought eye position to the downward moving target, eye velocity was enhanced to −6.5°/sec in the downward direction but displayed only a minimal rightward component (1.3°/sec).
We analyzed these trials by relating the metrics of the saccade to the average pre-saccadic eye velocity (Fig. 1B, 0–10 msec before arrow b) and post-saccadic eye velocity (Fig.1B, 0–10 msec after arrow c). In Figure2A, for example, pre-saccadic eye position was near the position of fixation (dots), and post-saccadic eye position was either downward (red plus signs) or leftward (cyan plus signs), indicating that the monkey had made a saccade to one of the two targets. Color-coding of the time courses of associated smooth eye velocity traces (Fig. 2B) illustrates the link between the target selection for saccades and the post-saccadic pursuit. Presaccadic eye velocity included both downward and leftward components, consistent with vector-averaging. Moreover, pre-saccadic traces associated with saccades to the leftward- (blue traces) and downward- (magenta traces) moving targets primarily overlap each other, thus displaying little evidence of any bias for the spot that will be selected later for tracking. In contrast, post-saccadic eye velocity was primarily leftward or downward when the saccade selected the leftward-moving spot (cyan traces) or the downward-moving spot (red traces), respectively. A similar result appears in Figure 2, C andD, for two-spot stimuli consisting of leftward- and rightward-moving spots.
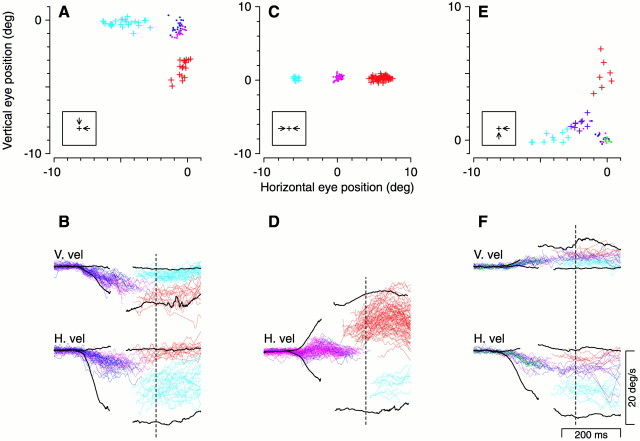
Fig. 2.
Three examples of the effect of saccadic target selection on pursuit velocity. Top row shows pre-saccadic and post-saccadic eye position for trial configurations in which the two targets moved downward and leftward (A), leftward and rightward (C), and upward and leftward (E). Insets near the origin show the stimulus configuration. Pre-saccadic eye positions are depicted asdots, and post-saccadic eye positions are depicted asplus signs. Points are colored according to which target the saccade was directed toward (see Results).Green points and purple crosses inE mark eye position for vector-averaging saccades with saccade weights between 0.35 and 0.65. Associated eye velocity for each position graph is shown in B, D, andF. Solid black lines are average eye velocity traces for trials in which one or the other targets was presented. Each velocity trace is interrupted during the rapid deflections associated with the saccades. Vertical dashed lines indicate the time at which one of the targets, not necessarily the saccade target, was extinguished. After this point in the trial, increased variability in the traces reflects the fact that the monkey was forced to track whichever target remained.
Figure 2, E and F, shows the analysis of a less common case when some saccades (Fig. 2E, purple plus signs) did not select either spot but instead took the eye to a position that represented a weighted average between the positions of the two spots (cf. Weber et al., 1993; Edelman and Keller, 1998; Chou et al., 1999). We classified these saccades as averaging if their saccade weight (see Materials and Methods and below) was between 0.35 and 0.65. For the trials with averaging saccades, the post-saccadic eye velocities (Fig. 2F, purple traces) also reflected a vector average of the responses to the two spots singly.Recanzone and Wurtz (1999) have shown that vector-averaging saccades can be elicited in a task in which monkeys are cued to track one of two moving targets if the monkey is given only a brief interval of time to identify the tracking target before initiating eye movements. In concordance with our results, they found that post-saccadic pursuit for trials with vector-averaging saccades is also vector-averaging.
To quantify the degree to which final saccadic eye position and pre-saccadic and post-saccadic pursuit eye velocity reflected averaging versus preferences for one spot or the other, we calculated a saccade weight and pre-saccadic and post-saccadic pursuit weights for each trial (see Materials and Methods). The saccade weight would be 0 or 1 if the saccade landed the eye on one or the other target, respectively. The saccade weight would be 0.5 if the eye landed equidistant from the positions of the two targets. Similarly, the pursuit weights would be 0.5 if the eye velocity reflected a vector average of the responses to the two spots singly. Pursuit weights would be 0 or 1 if eye velocity were identical to eye velocity evoked by one or the other target singly.
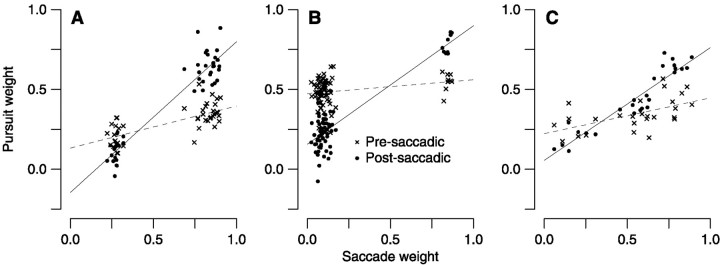
We then applied linear regression to the saccade and pursuit weights to quantify the degree to which pursuit target selection depends on saccadic target selection. Figure 3displays the result of this analysis for the data presented in Figure2. Each graph plots pre-saccadic or post-saccadic pursuit weight versus saccade weight for individual trials. If pre-saccadic pursuit does not depend on which target the saccade is directed toward, the pre-saccadic points should plot with a slope of 0. A y-intercept of 0.5 would then indicate perfectly unbiased vector-averaging pursuit. Indeed, the dashed regression lines for pre-saccadic weights (x signs) have small slopes (0.264, 0.088, and 0.225;p < 0.05) (Fig. 3A–C) andy-intercepts between 0 and 0.5 (0.133, 0.473, and 0.223) (Fig. 3A–C). In contrast, the post-saccadic pursuit weights (filled circles) all show a strong positive relationship to saccade weight (slope of 0.859, 0.744, and 0.685;p < 0.001) (Fig. 3A–C) andy-intercepts near 0 (−0.185, 0.119, and 0.035) (Fig.3A–C). Complete post-saccadic dependence of pursuit on the saccade choice would yield a slope of 1 and a y-intercept of 0.
Fig. 3.
Results of regression analysis demonstrating that pre-saccadic pursuit can be accounted for by vector-averaging and post-saccadic pursuit is dominated by the motion of the saccade target. Data are for the same experiments shown in Figure 2. Eachpoint plots pursuit weight as a function of saccade weight for a single trial; x symbols represent pre-saccadic pursuit, and filled circles represent post-saccadic pursuit. Perfect vector-averaging would yield a line with slope of 0 and a y-intercept of 0.5. Perfect target selection would yield a line with slope 1 and an intercept of 0.Dashed and solid lines show the results of linear regression for pre-saccadic and post-saccadic data, respectively.
The regression analysis is valid only when the monkeys made choices between the two spots. Although some combinations of two spots did evoke unbiased choices, others provoked idiosyncratic biases for particular spot directions. At the extreme, monkeys occasionally chose spots moving in particular directions as saccade targets to the complete exclusion of another direction, precluding analysis of the effects of saccadic target selection. Despite complete randomization of the rewarded tracking spot, these preferences were fairly consistent from one day to the next. Only 16.7% of the trial configurations showed any significant difference (p > 0.05; χ2 contingency table with Yates correction) in the choice biases of individual monkeys from one day to the following day. As a control on the validity of the regression analysis, we defined the choice index, which quantifies the degree of choice bias in each trial configuration (see Materials and Methods). The choice index would be 1 for completely unbiased choices and 0 if the monkey consistently made saccades to only one spot direction. The choice indexes calculated for the trial configurations shown in Figure2, A,B, C,D, and E,F are 0.791, 0.240, and 0.615, respectively. The full distribution of choice index is summarized in the histogram of Figure4A. Many combinations of two targets provided values of choice index below 0.5, but a substantial number had choice index near 1.0, indicating a strong preference for one target or the other.
Fig. 4.
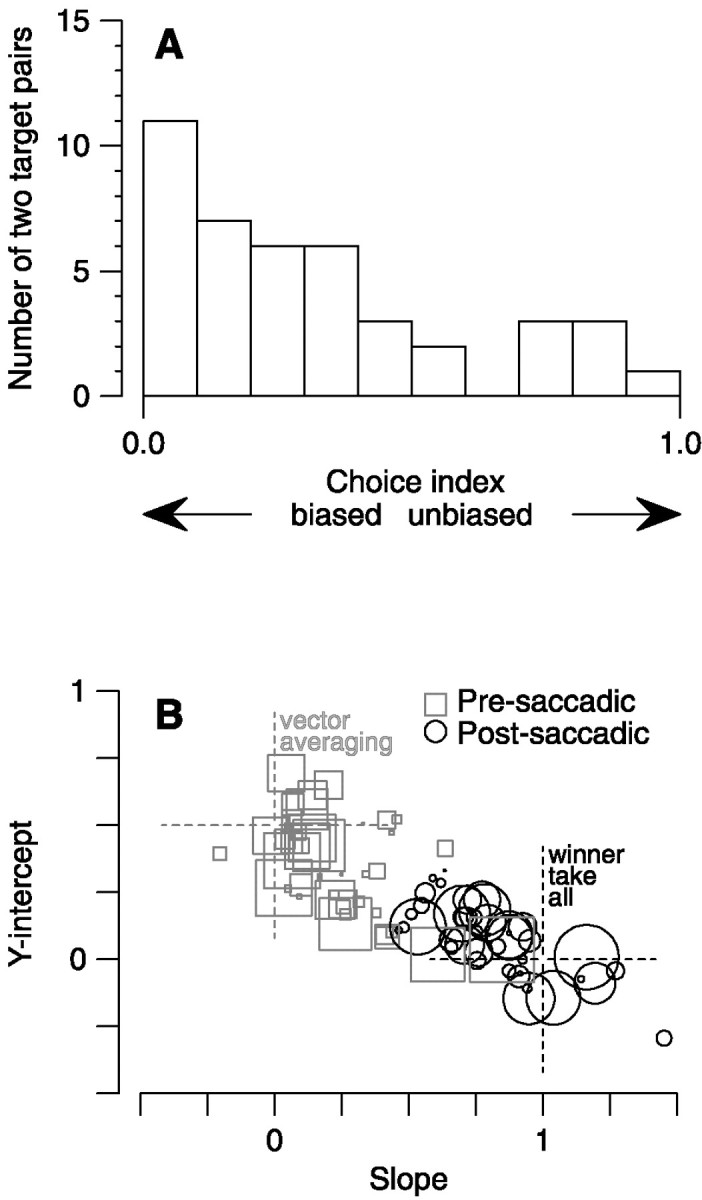
Summary data for all three monkeys tested for a total of six pairs of target motions on 8 experimental days.A is a histogram of choice indexes for all experiments. Choice indices of 0 and 1 would indicate target pairs in which the monkey always chose one target direction or in which he made equal choices to either target. B, Summary of data for all trial configurations. Slope and y-intercept values from the regression analysis are plotted as gray squares for pre-saccadic pursuit and as black circles for post-saccadic pursuit. The predicted positions for vector-averaging and winner-take-all pursuit are marked with large crosses ingray and black, respectively. Two pre-saccadic outlier points (slopes of −1.07 and 1.65;y-intercepts of 0.77 and −0.66; choice indexes of < 0.10) have been omitted.
By displaying the regression statistics and choice bias for all of our experiments, Figure 4B reveals that the target-selecting effect of saccade choice is a consistent feature of our data. Each symbol in this graph summarizes the regression statistics from one of six two-spot combinations in eight experiments on three monkeys for pre-saccadic (gray squares) and post-saccadic (black circles) eye velocity. The size of each symbol is related to the choice index for that condition. Smaller symbols indicate more biased saccade choices and, consequently, less meaningful regression coefficients. The majority of pre-saccadic points (gray squares) lie near the large gray dashed cross showing the results expected for vector-averaging (mean ± SD slope of 0.220 ± 0.342; mean ± SD y-intercept of 0.328 ± 0.228). The majority of post-saccadic points (black circles) lie near the large black dashed cross showing the results expected after pursuit target selection (mean ± SD slope of 0.771 ± 0.349; mean ± SD y-intercept of 0.077 ± 0.199). Post-saccadic eye velocity always showed a strong effect of saccadic target selection; in fact, we were never able to find a single trial in which the post-saccadic pursuit was in a direction inappropriate for the target to which the saccade was made.
Closer examination of Figure 4B reveals that the pre-saccadic slopes are, on average, slightly >0 and that a couple of slopes approach 1. This indicates that target-selection signals may bias pursuit even before the saccade. To quantify the degree to which target selection is detectable at different times during the initiation of pursuit movements, we used a method based on signal detection theory (Green and Swets, 1966) similar to that used by Britten et al. (1992,1996). For all trials taken from 32 combinations of two targets in which the choice index was >0.15, we arbitrarily assigned the two targets as target 0 and 1, combined all the data from all pairs of two target motions, and split pre-saccadic (Fig.5A) and post-saccadic (Fig.5B) pursuit weights into two groups depending on whether saccades were directed toward target 0 (gray bars) or target 1 (black bars). This reveals that the distribution of pre-saccadic pursuit weights for the two targets are primarily overlapped (Fig. 5A), whereas the post-saccadic pursuit weights are almost completely separated (Fig. 5B). In Figure5C, we calculate separate receiver operating characteristic (ROC) curves for the distributions of pre-saccadic (gray x symbols) and post-saccadic eye velocity (filled black circles). The area under these curves, which we call choice probability after Britten et al. (1996), was 0.60 for pre-saccadic and 0.98 for post-saccadic eye velocity. Choice probability can be interpreted as the probability that one could predict whether the saccade had been weighted toward target 1, given information about the weighting of smooth eye velocity relative to the two targets. A choice probability of 0.5 indicates that the prediction would be random.
One important issue is whether the after-effects of the saccade itself contaminate the first 10 msec of smooth eye velocity, making it an unacceptable index of the true post-saccadic eye velocity. Lisberger (1998) confronted this issue in two ways. First, he conducted a detailed analysis of the filtering methods and showed that the measures we have used are not contaminated. Second, he showed that post-saccadic eye velocity was enhanced in the direction of the motion of single targets, not in the direction of the saccade as might be anticipated if the measurement were contaminated by the physical after-effects of the saccade. We provide one additional demonstration of the validity of using this interval in Figure 5D, which analyzes the time course of the development of the choice probability before and after the saccade. Analysis of sequential 10 msec intervals of post-saccadic eye velocity shows that the target choice for pursuit is complete in the first 10 msec after the saccade and persists without modification for the rest of the trial.
Figure 5D also reveals the evolution of target selection for pursuit before the saccade. Approximately 45 msec before the saccade, the choice probability increases above chance probability, indicating that pre-saccadic pursuit has access to target selection signals from at least this time on. Depending on the exact timing of the internal signals for pursuit, the time course of development of choice probability indicates that target-selection signals can weakly bias pursuit even before the saccade has been executed, but that the full force of their effect is not released until immediately after the saccade occurs. Moreover, when the ROC analysis was repeated for each monkey individually (Fig. 5E), a clear pre-saccadic bias could be seen only in one monkey (squares). Another monkey had a pre-saccadic bias that only became significant in the last 10 msec before a saccade (circles), and the third monkey showed only marginally significant bias throughout the 100 msec before a saccade (triangles). The statistical significance of the choice probability was evaluated using bootstrapping (see Materials and Methods) and is displayed in Figure 5D as 95% confidence intervals.
In previous sections, we established a temporal correlation between the transition from vector-averaging to target selection in pursuit and the execution of a targeting saccade. However, this temporal correlation does not necessarily imply coordination between the two systems. For example, pursuit target selection could occur at a relatively fixed latency from target motion onset, independent of targeting saccades. If that latency were close to the average saccade latency, the temporal correlation we observe could simply be coincidental. The large variability in saccade latency in our experiments allowed us to examine this issue. We divided the trials into groups according to saccade latency and then analyzed pursuit target selection within each group as a function of the time since the onset of target motion. Figure6 plots choice probability as a function of time since the onset of target motion for trials with saccade latencies between 226–275 msec (filled black circles), 251–300 msec (open cyan circles), 276–325 msec (filled blue squares), 301–350 msec (open green squares), and 326–375 msec (filled red triangles). Time points between the average saccade start and end have been omitted because there was not enough data to include in the analysis. Comparison of the choice probability for groups of trials with different saccade latencies reveals that pursuit target selection depends on the time of occurrence of the saccade. In particular, comparison of the pre-saccadic data for saccades with the longest latencies (filled red triangles) and post-saccadic data for saccades with the shortest latencies (filled black circles) shows that choice probability does not depend simply on the time since the onset of target motion.
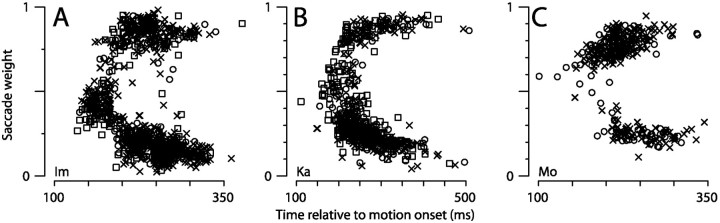
Previous experiments that have examined saccade latencies have revealed that vector-averaging saccades occur more frequently at shorter latencies and that target-directed saccades occur at longer latencies (Becker and Jürgens, 1979; Findlay, 1982; Ottes et al., 1985;Coëffé and O'Regan, 1987; He and Kowler, 1989; Chou et al., 1999). To determine whether our data follows the same pattern, we constructed a plot of saccade weight versus latency for individual saccades from each of our monkeys (Fig.7). In each monkey, averaging saccades tended to have shorter latencies, whereas targeting saccades occurred at least 200 msec after target motion onset. Thus, we infer that saccade target selection can be completed within 200 msec of the onset of the target, although saccades may occur with latencies much longer than 200 msec. Furthermore, this inferred transition from vector-averaging to target selection is in stark contrast to the pursuit transition, which is time-locked to the occurrence of a targeting saccade.
Fig. 7.
Time course of transition from averaging to targeting saccades. A, B,C, Saccade weight plotted as a function of saccade latency for monkeys Im, Ka, and Mo, respectively. Differentsymbols (squares, circles, and x symbols) represent data collected on different experimental days. Two outlier points (latency of 279 and 355 msec; saccade weight of −0.14 and −0.06) have been omitted for monkey Ka.
DISCUSSION
We used two-target stimuli to demonstrate seemingly natural and automatic selection of a pursuit target when a saccade is made to that target. Because of neural processing delays, the sensory signals driving the pursuit system just after a saccade must originate from a time before the saccade pointed the fovea at the selected target (Lisberger and Westbrook, 1985; Newsome et al., 1985; Groh et al., 1997). Thus, when two targets are present at similar eccentricities before the saccade, as in our stimuli, the two sensory signals available to drive post-saccadic pursuit are equally salient. This design allowed us to show that post-saccadic pursuit is selectively enhanced for the visual motion signals present at the endpoint of the saccade. Detailed analysis revealed that target selection for pursuit was detectable weakly 45 msec before the onset of the saccade in one monkey but is most pronounced starting immediately after the saccade. Moreover, target selection in the pursuit system is time-locked to the execution of the saccade and not to the onset of target motion. Our data provide evidence for a mechanism of target choice in which the execution of one kind of movement is time-locked to the execution of another kind of movement.
Studies on attention and target choice usually have used paradigms in which subjects were given previous information about the location or properties of targets that would be important. This has been a productive approach and has yielded many demonstrations of neural correlates of attention, including some within the visual motion system that provides inputs for pursuit (Treue and Maunsell, 1996). Similar approaches have proved useful in gaining insight into the neural mechanisms of target choice for saccades (Schall and Hanes, 1993;Schall et al., 1995). Most of the previous research on target selection for pursuit has been based on the same precepts as experiments on attention and saccades. In experiments that use the standard step-ramp target motion of Rashbass (1961) to delay saccades, subjects can be cued about which target to track based on color (Ferrera and Lisberger, 1995; 1997a), form (Krauzlis et al., 1999; Recanzone and Wurtz, 1999;Recanzone and Wurtz, 2000), or location (Krauzlis et al., 1999). Target selection in these cued paradigms tends to increase the latency of pursuit onset (Ferrera and Lisberger, 1995; Krauzlis et al., 1999), suggesting that discriminating and identifying the cued target inhibits pursuit initiation. Uncovering the neural underpinnings of target selection for these paradigms has proven difficult; only modest modulations of neural responses have been found under only a subset of conditions (Ferrera and Lisberger, 1997b; Recanzone and Wurtz, 2000). We therefore sought to find a more natural pursuit paradigm that might give insight into the time course of development of target selection in pursuit. By allowing monkeys to freely choose between two identical targets, we have revealed a target-selection mechanism that temporally links target selection for pursuit and saccades.
Previous research on the initiation of pursuit had revealed two results that were essential building blocks for the present study. First,Lisberger and Ferrera (1997) showed the use of vector-averaging to create the pre-saccadic smooth eye velocity when two targets move toward the position of fixation in different directions. Second,Lisberger (1998) demonstrated that pursuit is strongly enhanced in the wake of a saccade to the position of the tracking target. The present paper shows that this enhancement is not merely arousal but that the enhancement is selective for visual motion present at the endpoint of the saccade and may therefore be comparable with attention.
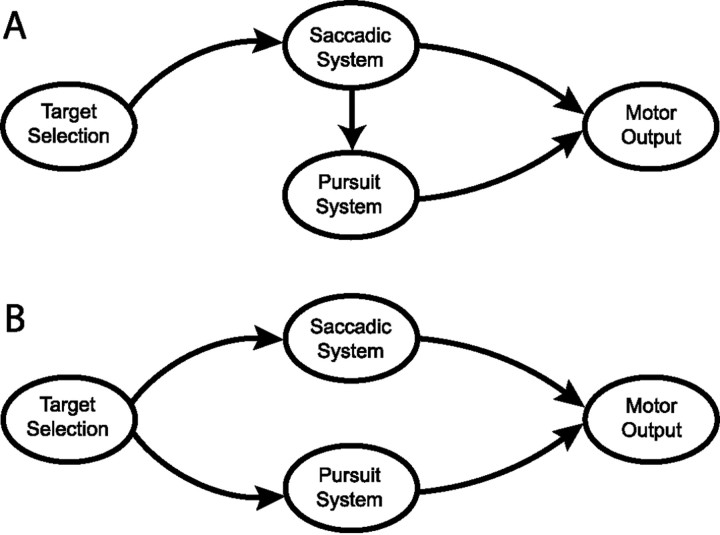
Temporal linkage of saccade and pursuit target selection could arise from at least two different underlying neural organizations. A common target-selection command could be communicated in parallel to the separate neural pathways for pursuit and saccades (Fig.8B). Alternatively, saccadic command signals could select a target for the pursuit system directly, thus coordinating target selection for saccades and pursuit via a serial communication (Fig. 8A). Our data lend support for the serial hypothesis. We suggest that the serial form of target selection involves post-saccadic enhancement, which is selective for the visual motion at the endpoint of the saccade and is accomplished by a direct influence of saccadic commands on the pursuit system. We note that the serial hypothesis does not preclude other signals directly influencing target selection for the pursuit system. In fact, other experiments have demonstrated that pursuit can select targets without a saccade (Kowler et al., 1984; Ferrera and Lisberger, 1995, 1997a; Krauzlis et al., 1999), and it is therefore clear that higher cognitive signals can also directly influence target selection for the pursuit system.
Fig. 8.
Two alternative models to explain the link between saccade and pursuit target selection. A, Serial model. Saccadic command signals control pursuit gain and target selection.B, Parallel model. A single target-selection command is distributed in parallel to the saccadic and pursuit systems.
Some evidence for the parallel hypothesis comes from the fact that pursuit and saccades show similar changes in latency in a “gap” paradigm (Krauzlis and Miles, 1996a,b). These results indicate that the release of fixation for both types of movements is programmed together and supports the idea that target selection is done once for both types of movements (Fig. 8B). However, our data show that initiation of movement is not always equivalent to target selection; in our variant of the two-target paradigms, pursuit has a normal latency, but target choice for pursuit does not occur until the time of the saccade, often hundreds of milliseconds after pursuit initiation.
A number of studies of saccadic vector-averaging in monkeys have found that saccades of shorter latencies, and especially “express” saccades (Fischer and Boch, 1983), tend to display more averaging behavior and that target selection develops later (Weber et al., 1993;Edelman and Keller, 1998; Chou et al., 1999). Our analysis of the latency of vector-averaging versus target-directed saccades corroborates these findings and may provide a way to probe the time course of target selection for saccades in the same trials used to analyze the time course of target selection for pursuit. The data showed that averaging saccades tend to have shorter latencies and that saccade endpoints come closer to one of the two targets as the latency of saccade initiation gets longer. One interpretation of these data are that the time course of the transition from averaging to targeting saccades reflects a process of target selection and that the nature of the saccade depends on when it is initiated relative to a fixed selection process. If this interpretation is correct, then our data imply that pursuit target selection is caused by the saccade itself (Fig. 8A), because the time course of pursuit target selection is linked to the execution of the saccade, which itself would not be linked to the choice of the saccade target.
We realize that all of our measures for the time at which target selection occurs are behavioral measures and that there are other interpretations of the time course of the transition from averaging to targeting saccades. If, for example, saccade target selection happens at the time of saccade execution but requires longer latencies for more complete separation of the visual inputs from two nearby targets, then our data cannot distinguish whether saccade and pursuit target selection proceed in series (Fig. 8A) or in parallel (Fig. 8B). It is tempting to infer the timing of neural selection signals from other experiments, but our task and training protocol are quite different from those studied previously and might therefore yield quite different results. Indeed, changes in the training protocol for the search array task have been shown to result in dramatic changes in the time course of selection in neural signals (Bichot et al., 1996). Nevertheless, the time course of target selection for saccades must be visible in the form of neural signals that distinguish between target and distractor sometime before changes can be observed in behavior. For a search task in which a monkey is trained to saccade to the oddball target, neural activity evolves to signal the target in the 100 msec preceding a saccade (Schall and Hanes, 1993). It would favor the hypothesis that saccade execution selects targets for pursuit if the same neurons were found to indicate the saccade target 100 msec before the execution of the saccade in our task.
We therefore suggest that pursuit target choice is accomplished partly by using the control signals for saccades to selectively enhance the strength of visual motion inputs to the pursuit system from the region of visual field at the endpoint of the saccade. Given that the saccadic and pursuit systems work in concert under natural conditions to reduce positional and velocity discrepancies between the fovea and the tracking target, it would make sense to link inextricably the choice of tracking targets for these two components of visual tracking. Our suggestion rests heavily on the tight links between saccade execution and post-saccadic enhancement of pursuit (Lisberger, 1998) and between post-saccadic enhancement and target selection (present study). Saccade control signals are available from a number of different cortical areas during the relevant interval from 45 msec before the onset to the end of a saccade and have been shown to have strong modulatory effects on receptive field properties of some neurons. For instance, in the lateral intraparietal area, neurons have been shown to dynamically remap their receptive fields in anticipation of a saccade (Duhamel et al., 1992). Post-saccade target choice for pursuit could be accomplished by an analog to this type of effect of saccade control signals but for direction-selective neurons in the parts of the visual motion pathways that provide inputs for pursuit. It might be reasonable to expect that the effect of saccadic target-selection signals on the pursuit system is mediated by cortical neurons. However, given that even premotor neurons at the furthest periphery of the saccadic motor system have been reported to convey pursuit signals (Tomlinson and Bance, 1992; Petit et al., 1999; Missal et al., 2000), sites farther down the pursuit pathway in the brainstem also remain a possibility.
The “spotlight theory” of visual attention (James, 1890) (for review, see Posner and Petersen, 1990; Desimone and Duncan, 1995) proposes attention as a limited computational resource that can be directed selectively to a part of the visual field to enhance processing. Psychophysical experiments indicate that the size of the spotlight of attention can be adjusted dependent on the specific demands of the task (Eriksen and Yeh, 1985; Eriksen and St. James, 1986) so that an adjustable zoom lens might be an appropriate analogy. Our finding that post-saccadic enhancement is selective for the motion of the saccade target suggests that the pursuit system may operate in an analogous manner, whereby target choice controls the size of an aperture through which the pursuit system views the world. We suggest that the aperture is wide open, and the gain at each site in the aperture is set low during fixation; any motion becomes a candidate for a pursuit target, and the inputs from multiple, conflicting targets are averaged. Once a saccade selects the target, then the aperture becomes much smaller; visual motion signals from the spatial location of the saccade target are enhanced selectively. We suggest that post-saccadic enhancement of pursuit provides a robust behavioral assay for studying how visual processing can be selectively enhanced in a spatially specific manner.
Footnotes
This work was supported in part by National Eye Institute Grant EY03878. S.G.L. is an Investigator of the Howard Hughes Medical Institute. J.L.G. was supported by a graduate fellowship from the National Science Foundation. We are grateful to Dr. Michael Shadlen for suggesting a key element in the experimental design and for comments on a previous version of this manuscript. We thank Dr. I-han Chou and the other members of the Lisberger laboratory for many valuable discussions and for comments on earlier drafts of this manuscript.
Correspondence should be addressed to Justin L. Gardner, Department of Physiology, 513 Parnassus Avenue, HSE-802A, University of California at San Francisco, Box 0444, San Francisco, CA 94143-0444. E-mail:justin@phy.ucsf.edu.
REFERENCES
- 1.Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vision Res. 1979;19:967–983. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- 2.Bichot NP, Schall JD, Thompson KG. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature. 1996;381:697–699. doi: 10.1038/381697a0. [DOI] [PubMed] [Google Scholar]
- 3.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 5.Busettini C, Miles FA, Krauzlis RJ. Short-latency disparity vergence responses and their dependence on a prior saccadic eye movement. J Neurophysiol. 1996;75:1392–1410. doi: 10.1152/jn.1996.75.4.1392. [DOI] [PubMed] [Google Scholar]
- 6.Chou IH, Sommer MA, Schiller PH. Express averaging saccades in monkeys. Vision Res. 1999;39:4200–4216. doi: 10.1016/s0042-6989(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 7.Coëffé C, O'Regan JK. Reducing the influence of non-target stimuli on saccade accuracy: predictability and latency effects. Vision Res. 1987;27:227–240. doi: 10.1016/0042-6989(87)90185-4. [DOI] [PubMed] [Google Scholar]
- 8.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 9.Dodge R. Five types of eye movement in the horizontal meridian plane of the field of regard. Am J Physiol. 1903;8:307–329. [Google Scholar]
- 10.Duhamel J-R, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 11.Edelman JA, Keller EL. Dependence on target configuration of express saccade-related activity in the primate superior colliculus. J Neurophysiol. 1998;80:1407–1426. doi: 10.1152/jn.1998.80.3.1407. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen C, St. James J. Visual attention within and around the field of focal attention: a zoom lens model. Percept Psychophys. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen CW, Yeh YY. Allocation of attention in the visual field. J Exp Psychol. 1985;11:583–597. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- 14.Ferrera VP, Lisberger SG. Attention and target selection for smooth pursuit eye movements. J Neurosci. 1995;15:7472–7484. doi: 10.1523/JNEUROSCI.15-11-07472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrera VP, Lisberger SG. The effect of a moving distractor on the initiation of smooth-pursuit eye movements. Vis Neurosci. 1997a;14:323–338. doi: 10.1017/s0952523800011457. [DOI] [PubMed] [Google Scholar]
- 16.Ferrera VP, Lisberger SG. Neuronal responses in visual area MT and MST during smooth pursuit target selection. J Neurophysiol. 1997b;78:1433–1446. doi: 10.1152/jn.1997.78.3.1433. [DOI] [PubMed] [Google Scholar]
- 17.Findlay JM. Global visual processing for saccadic eye movements. Vision Res. 1982;22:1033–1045. doi: 10.1016/0042-6989(82)90040-2. [DOI] [PubMed] [Google Scholar]
- 18.Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res. 1983;260:21–26. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- 19.Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature. 2000;404:390–394. doi: 10.1038/35006062. [DOI] [PubMed] [Google Scholar]
- 20.Green DM, Swets JA. Signal detection theory and psychophysics. Wiley; New York: 1966. [Google Scholar]
- 21.Groh JM, Born RT, Newsome WT. How is a sensory map read out? Effects of microstimulation in visual area MT on saccades and smooth pursuit eye movements. J Neurosci. 1997;17:4312–4330. doi: 10.1523/JNEUROSCI.17-11-04312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He PY, Kowler E. The role of location probability in the programming of saccades: implications for “center-of-gravity” tendencies. Vision Res. 1989;29:1165–1181. doi: 10.1016/0042-6989(89)90063-1. [DOI] [PubMed] [Google Scholar]
- 23.James W. The principles of psychology. Holt; New York: 1890. [Google Scholar]
- 24.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 25.Kawano K, Miles FA. Short-latency ocular following responses of monkey. II. Dependence on a prior saccadic eye movement. J Neurophysiol. 1986;56:1355–1380. doi: 10.1152/jn.1986.56.5.1355. [DOI] [PubMed] [Google Scholar]
- 26.Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res. 1991;11:79–107. doi: 10.1016/0168-0102(91)90048-4. [DOI] [PubMed] [Google Scholar]
- 27.Kowler E, Steen JVD, Tamminga EP, Collewijn H. Voluntary selection of the target for smooth eye movement in the presence of superimposed, full-field stationary and moving stimuli. Vision Res. 1984;24:1789–1798. doi: 10.1016/0042-6989(84)90010-5. [DOI] [PubMed] [Google Scholar]
- 28.Krauzlis RJ, Miles FA. Decreases in the latency of smooth pursuit and saccadic eye movements produced by the “gap paradigm” in the monkey. Vision Res. 1996a;36:1973–1985. doi: 10.1016/0042-6989(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 29.Krauzlis RJ, Miles FA. Release of fixation for pursuit and saccades in humans: evidence for shared inputs acting on different neural substrates. J Neurophysiol. 1996b;76:2822–2833. doi: 10.1152/jn.1996.76.5.2822. [DOI] [PubMed] [Google Scholar]
- 30.Krauzlis RJ, Stone LS. Tracking with the mind's eye. Trends Neurosci. 1999;22:544–550. doi: 10.1016/s0166-2236(99)01464-2. [DOI] [PubMed] [Google Scholar]
- 31.Krauzlis RJ, Zivotofsky AZ, Miles FA. Target selection for pursuit and saccadic eye movements in humans. J Cogn Neurosci. 1999;11:641–649. doi: 10.1162/089892999563706. [DOI] [PubMed] [Google Scholar]
- 32.Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol. 1998;79:1918–1930. doi: 10.1152/jn.1998.79.4.1918. [DOI] [PubMed] [Google Scholar]
- 33.Lisberger SG, Ferrera VP. Vector averaging for smooth pursuit eye movements initiated by two moving targets in monkeys. J Neurosci. 1997;17:7490–7502. doi: 10.1523/JNEUROSCI.17-19-07490.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- 36.Missal M, De Brouwer S, Lefèvre P, Olivier E. Activity of mesencephalic vertical burst neurons during saccades and pursuit. J Neurophysiol. 2000;83:2080–2092. doi: 10.1152/jn.2000.83.4.2080. [DOI] [PubMed] [Google Scholar]
- 37.Mooney CZ, Duval RD. Bootstrapping: a nonparametric approach to statistical inference. Sage; Newbury Park, CA: 1993. [Google Scholar]
- 38.Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ottes FP, Van Gisbergen JA, Eggermont JJ. Latency dependence of colour-based target vs nontarget discrimination by the saccadic system. Vision Res. 1985;25:849–862. doi: 10.1016/0042-6989(85)90193-2. [DOI] [PubMed] [Google Scholar]
- 40.Petit J, Klam F, Grantyn A, Berthoz A. Saccades and multisaccadic gaze shifts are gated by different pontine omnipause neurons in head-fixed cats. Exp Brain Res. 1999;125:287–301. doi: 10.1007/s002210050685. [DOI] [PubMed] [Google Scholar]
- 41.Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78:1574–1589. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- 42.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 43.Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol (Lond) 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Recanzone GH, Wurtz RH. Shift in smooth pursuit initiation and MT and MST neuronal activity under different stimulus conditions. J Neurophysiol. 1999;82:1710–1727. doi: 10.1152/jn.1999.82.4.1710. [DOI] [PubMed] [Google Scholar]
- 45.Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J Neurophysiol. 2000;83:777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- 46.Robinson DA. The mechanics of human smooth pursuit eye movement. J Physiol (Lond) 1965;180:569–591. doi: 10.1113/jphysiol.1965.sp007718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- 48.Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- 49.Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sparks DL, Mays LE. Signal transformations required for the generation of saccadic eye movements. Annu Rev Neurosci. 1990;13:309–336. doi: 10.1146/annurev.ne.13.030190.001521. [DOI] [PubMed] [Google Scholar]
- 51.Tomlinson RD, Bance M. Brain stem control of coordinated eye-head gaze shifts. In: Berthoz A, Graf W, Vidal PP, editors. The head-neck sensory motor system. Oxford UP; Oxford: 1992. pp. 356–361. [Google Scholar]
- 52.Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 53.Weber H, Latanov A, Fischer B. Context dependent amplitude modulations of express and regular saccades in man and monkey. Exp Brain Res. 1993;93:335–344. doi: 10.1007/BF00228403. [DOI] [PubMed] [Google Scholar]
- 54.Wurtz RH. Visual receptive fields of striate cortex neurons in awake monkeys. J Neurophysiol. 1969;32:727–742. doi: 10.1152/jn.1969.32.5.727. [DOI] [PubMed] [Google Scholar]