Abstract
Introduction
Selecting units of rare blood for transfusion to patients with complex immunisation is one of the most critical processes of a Transfusion Centre. In January 2005 the ‘Rare Blood Components Bank – Reference Centre of the Region of Lombardy’ w as established with the following goals: 1) identifying regional rare blood donors; 2) creating a regional registry of rare donors; 3) organising a regional bank of liquid and frozen rare blood units; 4) setting up a regional Immunohaematology Reference Laboratory (IRL) to type donors and resolve complex cases.
Methods
The key elements in establishing the Bank were periodic meetings organised by the directors and representatives of the regional Departments of Transfusion Medicine and Haematology (DTMH) and the institution of three working groups (informatics, regulations, finance).
Results
The regional IRL was set up, the relevant operating procedures were distributed region-wide, software features were defined and later validated upon activation, and the funds assigned were allocated to various cost items. The number and characteristics of the donors to be typed were identified and 14 regional DTMHs started to send samples. Overall, 20,714 donors were typed, for a total of 258,003 typings, and 2,880 rare donors were identified. Of these, 97% were rare donors because of combinations of antigens (2,139 negative for the S antigen and 659 negative for the s antigen) and 3% (n=82) because they were negative for high-frequency antigens. In the first 2 years of activity, the IRL carried out investigations of 140 complex cases referred from other Centres and distributed 2,024 units with rare phenotypes to 142 patients.
Conclusions
The main goal achieved in the first 24 months from the start of the project was to set up a regional network able to meet the transfusion needs of patients with complex immunisation.
Keywords: complex red cell immunisation, transfusion support, banks and registries of rare donors
Introduction
The presence of antibodies against red cell antigens is a problem occurring with different incidences, depending on the type of diagnostic method used, the underlying pathology and the ethnicity of the patients involved. The clinical effects of immunisation are heightened in complex cases, such as those involving antibodies against high-frequency antigens or combinations of antibodies against clinically significant common antigens, in that the specificities involved are difficult to identify. In these cases, when units of red blood cells must be transfused, it becomes essential to turn to immunohaematology reference laboratories (IRLs), highly specialised facilities which are able to solve the complex immunohaematological problems involved and to obtain compatible units of red blood cells of the required rare group1–3. However, despite the importance of this transfusion problem, until 2005 few rare donors had been identified in the Region of Lombardy, which has a population of over 9 million, and the Region did not have an IRL.
The ‘Rare Blood Components Bank – Regional Reference Centre’ was established in January 2005 and funded by the Region of Lombardy for 2005–2007 (Regional Government Resolution No. VII/20103). The aims of this project were to:
identify rare donors;
create a registry of rare donors;
set up a regional IRL for the study of complex cases of red blood cell immunisation detected in the Region;
organise a bank of rare blood units, stored fresh or frozen.
It was possible to establish the Bank following a financial and technical feasibility study, which forms integral part of the Regional Resolution cited above and was carried out by an ad-hoc working group set up by the Scientific-Technical Commission of the IV Regional Blood Plan. In order to evaluate the feasibility of the project, the working group considered the following aspects:
definition of the methodology, purposes and objectives;
identification of needs in the regional context;
ethnic composition of the population;
red cell antigens to be considered for the prevalent group of Caucasian donors and for the most numerous ethnic minorities;
functions of the IRL;
administration.
The Centre for Blood Transfusion and Immunohaematology of the Fondazione IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena of Milan was chosen as the location of the Bank. The most relevant features of this Centre are:
-
an IRL, accredited by the American Association of Blood Banks (AABB), which is able to:
- carry out immunohaematological investigations on complex cases of immunisation, with standardised techniques used internationally;
- regularly type periodic blood donors for red blood cell antigens of the common and high-frequency antigen systems;
- use serological and molecular biology techniques for the characterisation of red cell antigens;
- keep, through participation in international programmes, an inventory of rare typing sera and antisera and of cells with rare antigenic profiles, in accordance with international standards for IRLs;
- have access to international banks and registries in order to obtain blood units with rare antigenic profiles not available in the local inventory.
cryobiology facilities run in compliance with relevant legislation, endowed with sufficient cold spaces and reserve freezers, and connected to a centralised alarm system which ensures that the storage conditions of the units are kept under continuous local and remote control.
regular red blood cell (RBC) cryopreservation.
Methods
In order to achieve the goals of the project, periodic meetings have been held with the coordinators of the regional Departments of Transfusion Medicine and Haematology (DTMHs), during which three working groups (1-informatics, 2-regulations, 3-financial issues) were established, composed of technical and medical representatives from each DTMH, alongside the co-ordinators themselves. The working groups defined the stages of the project, the relevant guidelines, the design and functions of the software to be employed, and the use of financial resources.
Results
The main results achieved in the first 2 years of activity are described below.
Stages of the project
Figure 1 illustrates the stages of the project and the times in which they were achieved.
Figure 1.
Stages of the project
Guidelines
The ‘Regulations’ working group drafted, validated and distributed the Standard Operating Procedures and the forms to be used for donor selection, sample transportation, selection of blood units to freeze, informed consent, and criteria for access to the IRL.
Definition of rare status
Internationally accepted criteria4 were adopted to define rare donors (according to the profile considered, blood groups are considered rare if they occur with a frequency lower than 1:1,000 or 1:5,000 in random donors). The donors were divided into two main categories:
rare donors for combinations of antigens of the RH, KEL, JK, Fy and MNS systems (Table I);
donors negative for high-frequency antigens (Table II).
Table I.
Characteristics of the donors of rare blood types according to the combinations of antigens
| System
| |||||
|---|---|---|---|---|---|
| KEL | RH | JK | FY | MNS | |
| K−k+ | CCDee | Jk(a+b−) | Fy(a+b−) | S−s+ | |
| or | or | or | or | ||
| ccDEE | Jk(a−b+) | Fy(a−b+) | S+s− | ||
| or | |||||
| ccdee | |||||
| or | |||||
| ccDee | |||||
Group O and A
Table II.
Characteristics of the donors of rare blood types negative for high-frequency antigens
| System | Phenotype |
|---|---|
| RH | CCDEE, CCdee, ccdEE, CCdEE, deletions |
| KEL | Ko, K var, Kp(a−b−), Kp(b−), Js(b−), k− |
| JK | Jk(a−b−) |
| FY | Fy(a−b−) |
| LW | LW− |
| DO | Do(a−), Hy−, Gy(a−) |
| IN | In(b−) |
| MNS | U−, En(a−), S−s− |
| CO | Co(a−b−), Co(a−) |
| LU | Lu(a−b−), Lu(b−) |
| SC | Sc:−1,−2, Sc:−1 |
| GE | Ge:−2,−3, Ge:−2, 3 |
| CROM | Cr(a−), Es(a−), Tc(a−) |
| YT | Yt(a−) |
| DI | Di(b−), Wr(b−) |
| 901 Series | Vel−, Lan−, At(a−), Jr(a−), Ok(a−), Lan− |
| Collections | I−, i−, PP1Pk−, Pk, LKE−, Er(a−) |
| H | Oh |
| JMH | JMH− |
All ABO groups and Rh phenotypes
Donors included in the project
Of the 212,627 active donors in the Region of Lombardy at the start of the project, 50,000 donors of group A or O, with selected Rh phenotypes (CCDee, ccDEE, ccdee, ccDee), aged ≤ 55 years, who had made two or more donations, were considered for typing.
Computer programmes
The macrofunctions of the information system (Figure 2) were defined. The software was designed with the aim of enabling connections between the Bank and the 15 regional DTMH through the server of the Regional Centre for Coordination and Compensation (RCCC). The computer programmes were produced by INSIEL (Udine, Italy), a softwarehouse which supplies the EmoNet management software, currently in use in most of the Region’s transfusion facilities. The programmes produced incorporated the following features:
Figure 2.
Macrofunctions of the information system
identification of donors to type: the programme allows the DTMH to view the status of donors to be typed (‘candidate for typing’, ‘rare donor – typing to be confirmed’) and print the labels for samples to be sent to the Bank;
typing: the programme activates the work flow, sending the donors’ data from the DTMHs to the Bank through the regional server of the RCCC; the typing results are sent back from the Bank to the RCCC server;
identification of rare donors: the programme identifies each rare donor with an algorithm and transfers this information to the RCCC server.
As of 31 December 2006, the following functions had not been activated:
possibility for each DTMH to attribute typing results to each individual donor and to manage typing data;
a regional inventory of rare blood units; in the future, there will be a regional inventory of rare RBC units: this will be accessible to all the DTMHs, which will be able to select the rare units available in liquid phase or frozen;
requests for investigations of complex cases of immunisation: each DTMH will be able to ask the Bank for investigations on patients with complex immunisation; the results of the investigations will be transferred from the Bank to the DTMHs;
production of periodic activity reports, covering in particular such items as number of donors typed, typings carried out, units of rare blood collected and used, and cases of complex immunisation.
Transport
A daily service of transport for samples to be typed was established between the Bank and those DTMH lacking direct connections with the Bank.
Identification of costs
The funds assigned for the realisation of the project were allocated to the following main cost categories: reagents (37%), personnel (23%), general overhead (15%), immunohaematological and molecular biology tests for immunised patients and rare donors (8%), laboratory materials and consumables (6%), the IT system (4.5%), transport (3%), instruments and equipment (2.5%), depreciation and services (1%).
Setting up the reference laboratory
The IRL was activated in January 2005, as its staff of biologists and technicians was formed. In the first 2 years of activity, the IRL received 220 requests for information from outside Centres. In 140 instances, investigations were carried out, revealing the presence of combinations of antibodies against common antigens in about 44% of cases (Table III) and of antibodies against high-frequency antigens in 12% of cases. In six cases, no red cell antibodies were found.
Table III.
Antibodies identified in cases of complex red cell immunisation sent to the IRL for investigation
| Type | Years 2005–2006 |
|---|---|
| Alloantibodies against high-frequency antigens | 16 |
| Family studies in subjects with alloantibodies against high-frequency antigens | 5 |
| Mixtures of alloantibodies against high-frequency and common antigens | 5 |
| Mixtures of alloantibodies against common antigens | 62 |
| Single alloantibody against low-frequency or common antigens | 13 |
| Single autoantibody | 13 |
| Variants of red cell antigens | 20 |
Typing activities
A completely automated, high turnover system (Galileo, Immucor, Norcross, GA, USA), based on solid-phase technology (Capture-R Select, Immucor), was selected, validated and acquired for the typing of 14 red blood cell antigens (Fya, Fyb, Jka, Jkb, S, s, Coa, Jsb, Ge, Lub, Kpb, PP1P k, U, Vel) in the cohort of 50,000 donors included in the project. The instrument was also used to confirm the antigen profile in rare donors, including K/k antigens.
A combination of the following was used:
commercial polyclonal antisera (anti-Fya, anti-Fyb, anti-Jka, anti-Jkb, anti-S, anti-s, anti-Coa, anti-Jsb, anti-Lub, anti-Kpb, Immucor) for the typing of all samples;
samples of plasma from immunised donors (anti-Ge2, anti-PP1Pk, anti-U, anti-Vel), diluted 1:5 in physiological saline and stored at +4 °C until used, for the typing of all samples;
commercial polyclonal antisera different from those described in point a) (anti-K, anti-k, anti-Fya, anti-Fyb, anti-Jka, anti-Jkb, anti-S, anti-s, Immucor), for the confirmation of the antigen profile of rare donors on a second sample;
samples of undiluted plasma obtained from immunised donors different from those mentioned in point b) (anti-Ge2, anti-PP1Pk, anti-U, anti-Vel, anti-Coa, anti-Jsb, anti-Lub, anti-Kpb) for the confirmation on a second sample of the typing of donors found to be rare for high-frequency antigens.
The blood samples used for typing were anticoagulated with EDTA, and processed within 3–6 days of collection in batches of 50–100. The typing instrument dispenses 12 samples per plate, using 7 typing reagents and 1 negative control per sample. In the case of indeterminate/doubtful/invalid results, the tests have been repeated by the automated instrument. Other reagents and the test-tube method have been used to determine the antigen involved only in case of inadequate results from a second test performed by the automated instrument. A method of genomic typing, based on a PCR-SSP technique, was used for confirmation of donors found to be rare for high-frequency antigens, when commercial kits based on this technology were available (KKD-Type - BAGene, Lich, Germany; KKD-SSP, MNSs-SSP, Rare Antigen-SSP- Inno-Train Diagnostic GmbH, Kronberg/ Taunus, Germany).
From June 2005, the donors’ blood samples were sent at weekly intervals, according to a schedule agreed upon with the DTMHs. As of 31 December 2006, 14 of 15 regional DTMHs were active in sending samples. The DTMH of the Province of Milan-Northeast and the San Matteo Polyclinic Hospital in the DTMH of the Province of Pavia did not send samples, since they were not connected to the regional EmoNet software. In the Pavia DTMH, however, peripheral transfusion structures (Vigevano, Voghera), which are connected to the regional IT network, are active. The site of the DTMH of the Province of Bergamo was not activated because of organisational problems, although samples have regularly been sent from some peripheral structures (Alzano Lombardo, Gazzaniga, Seriate, Treviglio).
Overall, 20,714 periodic donors were typed, of whom 20,614 were Caucasian (99.5%) and 100 non-Caucasian (0.5%); samples from 13,296 donors (65%) were sent in 2006. Four DTMHs which have been active since 2005 (Cremona, Lodi, Mantua, Central Milan) sent samples from over 90% of donors eligible for typing (Figure 3). The total number of typings performed was 258,003, of which 74% (191,894) were carried out in 2006. One percent of all the typings gave indeterminate/doubtful/invalid results at the first test carried out with the automated instrument. Commercial polyclonal antisera were involved in 310 cases (0.12%). Human plasma was involved in 2,322 (0.88%) of the first tests that were inconclusive, with two antisera, anti-Vel and anti-PP1Pk, leading to a high number of repetitions (1,579 for anti-Vel, 325 for anti- PP1Pk). In 418 samples the definition of the Vel and PP1Pk antigens was only possible after recourse to a manually executed test-tube method. At the second test on a second sample, the rare donor status was confirmed for all donors negative for high-frequency antigens and not confirmed for 4 (0.14%) of the donors initially found to be rare for combinations of antigens. Molecular typing was necessary in 28 cases of donors negative for high-frequency antigens.
Figure 3.
Donors typed, divided according to DTMH (June 2005 – 31 December, 2006)
Rare group donors identified
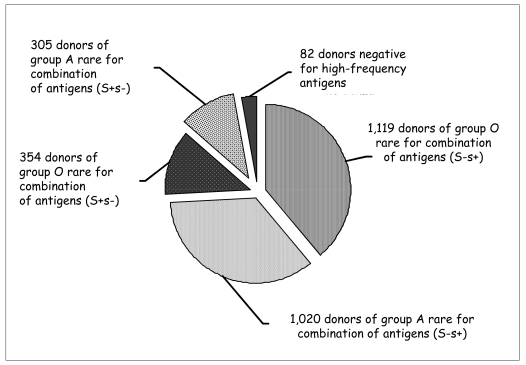
Overall, 2,880 donors of rare blood groups were identified, of whom 1,888 (66% of the total) were found in 2006; 2,798 donors (97%) had rare blood groups for combinations of antigens of the RH, KEL, JK, FY and MNS systems (Figure 4). Of these, 2,139 were negative for the S antigen, 659 negative for the s antigen and 82 negative for high-frequency antigens (Table IV). Among 100 non-Caucasian donors, 19 rare donors for high-frequency antigens were identified.
Figure 4.
Distribution of the types of rare blood donors identified
Table IV.
Distribution of the donors of rare blood types negative for high-frequency antigens
| Antigens | Total |
|---|---|
| Lu(a+b−) | 11 |
| Lu(a−b−) | 13 |
| Co(a−) | 12 |
| Co(a−b−) | 1 |
| Vel− | 23 |
| Fy(a−b−)* | 16 |
| Fy(a−b−) U−** | 1 |
| Fy null Fyb+w** | 2 |
| Ge:−2 | 2 |
| K+k− | 1 |
including one Caucasian donor
non-Caucasian donors
Use of rare blood units
In the 2 years considered, 153 rare RBC units were frozen, of which 43 were sent from regional DTMHs other than the one where the Bank is located. Fifty units of rare blood were deglycerolised for transfusion of patients with complex red cell immunisation; of these, 22 units were requested by regional DTMHs other than the one where the Bank is located. Because some features of the regional IT network were not implemented, information on the use of the rare blood units for transfusion purposes is available only for patients treated at the facility, which hosts the Bank. In the 2 years considered, the IRL used 2,024 units of rare blood, including the deglycerolised units, which were assigned to 142 patients with chronic blood disorders (thalassaemia major, thalassaemia intermedia, sickle cell disease). In the same period, the transfusion Centre including the IRL issued about 57,000 units of RBC.
Recruiting donors of rare blood groups
Posters and leaflets (Figure 5) were designed and displayed in all regional blood transfusion centres with the aim of illustrating the purpose of the project to the public. Furthermore, in collaboration with AVIS of the Region of Lombardy and with the Association ‘Friends of the Polyclinic – Blood Donors’, a project was drafted for the promotion of blood donation among ethnic minorities present in the Region.
Figure 5.
Information leaflet distributed to the Regional DTMHs
Discussion
Finding compatible units of blood components for subjects with complex red cell immunisation is one of the most important challenges facing immunohaematology. Data published in the literature indicate that about 25% of such patients receive unsatisfactory transfusion support5 and that for some of them it can be impossible to find suitable units6–8. Various strategies are adopted internationally to tackle this important problem. The first involves setting up a regional or national IRL9, whose staff is numerically sufficient and suitably skilled to carry out multiple and uncommon investigations, which has a large inventory of diagnostic material and is able to perform family studies in order to identify potential blood donors. The second strategy is to set up registries and banks of rare donors10, in order to ensure suitable transfusion support for the most critical cases. This latter service is usually guaranteed by the IRL, which undertakes the regular typing of a large number of donors from the area for which it has competence.
These needs have been identified internationally by various countries11–22. Starting in the 1950s and 1960s, programmes to recruit rare donors, coordinated by regional or national laboratories, were set up and fostered in Europe (France, The Netherlands, Germany, United Kingdom), in Asia (Korea, Malaysia, Japan, China), in Oceania (Australia, New Zealand), in the United States, in Africa (South-Africa) and in the Middle East (Israel). In 1965, the World Health Organisation also recognised the importance of an international register23–25 of blood donors rare for high-frequency antigens, managed by an accredited laboratory, in which 4,000 donors are currently enrolled. These donors are notified by 26 member States, which have active rare donor recruitment programmes.
Although these international facilities can be of some help in Italy for the transfusion treatment of patients with very rare antigen profiles, using them can lead to a delay in transfusion, which is unacceptable in most urgent cases, and to a reduction in the number of units that can be transfused; moreover, the units desired may not always be available5,6,8,22. Recourse to international Centres is also complicated by a number of problems related to transport, regulations on the import of human blood, relevant health requirements and the expiry dates of the units supplied26.
It is therefore important to organise, at a local level, an appropriate programme to identify donors and manage rare blood units. Some significant Italian experiences should be mentioned in this context. These include the Bank of Rare Phenotypes and Antibodies27,28, set up by a specific working group of the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI). The programme (coordinated by Dr. Giorgio Assali of the Immunotransfusion Service of San Gerardo Hospital, Monza) applied a specific protocol, but was limited to only some centres in Lombardy, which had established reciprocal exchanges of human sera for typing (for some high-frequency specificities), and had planned the freezing of rare blood units in a centre in Milan (the Lambrate site of Niguarda Hospital, co-ordinated by Dr. Elena Fonti). The Lombardy SIMTI group also collaborated with the National Red Cross Blood Transfusion Centre in Rome to set up a similar project in the central and southern area of the country. Another experience in Lombardy, which is worth mentioning, is our Centre’s regular typing of its donors performed between 1980 to 200429,30. Nevertheless, these initiatives had the important limitation of involving only low numbers of donors and Centres, and they were not useful for the transfusion support of all subjects with complex immunisation, coming from a vast area. This problem was recognised in our country by Law n. 107 of 1990, ‘Regulation of transfusion activities concerning human blood and its components and the production of plasma derivates’, which stated that every Region should have a bank of frozen blood components, collected from donors of rare or uncommon blood groups, in connection with the National Institute of Health. However, accredited IRLs and a regional network aimed at finding rare units were not present in every Region, and financial resources as well as cryopreservation programmes were lacking: these factors prevented this legislation from being implemented at a national level.
In order to solve this difficult problem, in 2005 the Department of Health of the Region of Lombardy financed – a move which has not yet been followed by others in Italy – the institution of the ‘Rare Blood Components Bank – Reference Centre of the Region of Lombardy’, co-ordinated by an IRL complying with the international criteria for this specific function. All the regional transfusion structures, all the donor Centres co-ordinated by these structures and all the voluntary associations in the region were involved in the project. The most important goal achieved in the first 24 months of activity of the Regional Bank was the setting up, by common agreement, of a regional network aimed at ensuring safe transfusions also for patients with complex immunisation. In an area with about nine million residents, 2,884 donors of rare blood groups were identified. The results achieved by this collaborative effort are significant, even in comparison with other, more established experiences such as the American Rare Donor Program (ARDP) in Philadelphia6,21.
Conclusions
The experience described in this report refers to selected data from a 3-year project of great transfusion relevance. The main goal achieved in the first 24 months since the start of the ‘Rare Blood Components Bank – Reference Centre of the Region of Lombardy’ was the creation of a regional network with the purpose of identifying rare donors in order to enable transfusion of subjects with complex red cell immunisation.
The organisational model established by the Region of Lombardy could provide valid indications for other regions that lack such a system.
Acknowledgements
The authors would like to thank the Health Department of the Region of Lombardy for having financed this project and the Associations of Blood Donors active in the region for having motivated their members to help realize this important program.
References
- 1.Daniels G. Human Blood Groups. Oxford: Blackwell Science; 2002. [Google Scholar]
- 2.Daniels G, Poole J, de Silva M, et al. The clinical significance of blood group antibodies. Transfus Med. 2002;12:287–95. doi: 10.1046/j.1365-3148.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 3.Reid ME, Lomas-Francis C. The Blood Group Antigen Facts Book. San Diego: Academic Press; 1997. [Google Scholar]
- 4.Technical Manual. 15. American Association of Blood Banks Press; Bethesda (USA): 2005. [Google Scholar]
- 5.Seltsam A, Wagner FF, Salama A, et al. Antibodies to high-frequency antigens may decrease the quality of transfusion support: an observational study. Transfusion. 2003;43:1563–6. doi: 10.1046/j.1537-2995.2003.00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Flickinger C, Petrone T, Church A. Review: American Rare Donor Program. Immunohematology. 2004;20:239–43. [PubMed] [Google Scholar]
- 7.Church A, Nance S ARDP Program Staff, ARDP Program Advisory Committee. Unfilled transfusion needs for phenotyped rare donor products. Transfusion. 2002;40(Suppl):145S. [Google Scholar]
- 8.Nance S, Church A. Unfilled transfusion needs for phenotyped rare donor products. Vox Sang. 2002;11 (Suppl 2):029. [Google Scholar]
- 9.Woodfield G, Poole J, Nance ST, et al. A review of the ISBT rare blood donors program. Immunohematology. 2004;20:244–8. [PubMed] [Google Scholar]
- 10.Poole J. The screening, identification and use of rare blood. Ch J Blood Transfus. 2001;14(Suppl):73. [Google Scholar]
- 11.Moullec J. The National Index of Rare Type Donors. Transfusion (Paris) 1966;9:163–6. [PubMed] [Google Scholar]
- 12.Woodfield G. The ISBT Working Party on rare blood, mission and achievements and its relationship to a rare blood programme in Asian countries. Ch J Blood Transfus. 2001;14 (Suppl S211):60–3. [Google Scholar]
- 13.Woodfield DG. Rare blood donors. A review of the present situation. Plenary and State of the Art Book. VIII European Congress; Istanbul. 2003. pp. 267–9. [Google Scholar]
- 14.Rouger P, Ansart-Pirenne H, Le Pennec PY. Annual report 2004 – French reference centre for rare blood groups and immunohaematology. Tranfus Clin Biol. 2005;12:345–52. doi: 10.1016/j.tracli.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Dazhuang Liu. Development of the work on rare blood types in China. Ch J Blood Transfus. 2001;14 (Suppl S212):64–71. [Google Scholar]
- 16.Tani Y. Rare blood typing and reference laboratory in Japan. Proceedings. The Third Red Cross and Red Crescent Symposium on Blood Programs in the Asian Region; Bangkok. 2001; pp. 202–7. [Google Scholar]
- 17.Novaretti MCZ, Woodfield DG. Rare blood usage in American countries. Vox Sang. 2002;83(Suppl 2):26. P-078. [Google Scholar]
- 18.Smith-Whitley K, Friedman DF, Lucas ML, et al. A program to direct blood donated by African Americans to children with sickle cell disease. Transfusion. 1998;38(Suppl):340. [abstract] [Google Scholar]
- 19.Kavitsky D, Zimmaro C, Maurer J, et al. Summary of the activity of a large national database of rare donors. Transfusion. 1997;37(Suppl):32S. [abstract] [Google Scholar]
- 20.Smart E. South African Rare Donor Panel. ISBT Science Series. 2006;1:210–2. [Google Scholar]
- 21.Mallory D, Malamut D, Sandler SG. A decade of rare donor services in the United States. Report of the American Red Cross Rare Donor Registry (1981–90) Vox Sang. 1992;63:186–91. doi: 10.1111/j.1423-0410.1992.tb05098.x. [DOI] [PubMed] [Google Scholar]
- 22.Nance S. The American Rare Donor Program – a collaborative program, of the American Red Cross and the AABB. ISBT Science Series. 2006;1:220–1. [Google Scholar]
- 23.Mourant AE. The establishment of an international panel of blood donors of rare types. Vox Sang. 1965;10:129–32. doi: 10.1111/j.1423-0410.1965.tb04330.x. [DOI] [PubMed] [Google Scholar]
- 24.Anstee D, Levene C, Mallory D, et al. Rare Blood. An ISBT Working party report on rare blood donors. Vox Sang. 1999;77:58–62. doi: 10.1159/000031075. [DOI] [PubMed] [Google Scholar]
- 25.Poole J. The International Rare Donor Panel. ISBT Science Series. 2006;1:209. [Google Scholar]
- 26.Novaretti M, Woodfield G. An international survey of rare blood activities. Transfusion Today. 2001 Sept.:6–7. [Google Scholar]
- 27.Assali G. Gruppo di Lavoro SIMTI. Il Servizio Trasfusionale. Bollettino di Informazione sulle attività trasfusionali italiane. 1997 Year XXVI, Number 2 [abstract] [Google Scholar]
- 28.Ghessi A, Raffaldoni E, Catalani C, Assali G Gruppo di Studio SIMTI. Costituzione Banca Fenotipi e Anticorpi Rari. Convegno Interregionale per Operatori Sanitari del Servizio Trasfusionale. ‘Simposio di Immunoematologia Eritrocitaria’; Genova. 13–15 November 1997; [abstract] [Google Scholar]
- 29.Morelati F, Villa A, Revelli N, et al. La banca dei gruppi rari. Convegno Interregionale per Operatori Sanitari del Servizio Trasfusionale ‘Simposio di Immunoematologia Eritrocitaria’; Genova. 13–15 November 1997; [abstract] [Google Scholar]
- 30.Morelati F, Revelli N, Musella A, et al. Automated red cell phenotyping. Transfusion. 2001;41(Suppl):SP262. [abstract] [Google Scholar]