The antigen-antibody reaction is widely used in laboratory diagnostics, including immunohaematology. It is a reversible chemical reaction:
| (1) |
The forces joining the antigen-antibody complex are not strong covalent bonds but weaker bonds, appropriately named “weak interactions”1.
Types of weak interactions
According to quantum mechanics, all chemical bonds are based on electrostatic forces. A list of types of weak bonds is shown in table I, together with their strength (energy). Van der Waals forces are the weakest, but they are able to attract all kinds of molecules. Hydrogen or ion-dipole bonds require oppositely charged atoms. Hydrogen bonds are very important in aqueous solutions because water easily forms strong hydrogen bonds. Actually, “hydrophobic” bonds are simply the result of the exclusion from water molecules of other molecules that cannot participate in good hydrogen bonds. These molecules are called “non-polar” because their atoms do not form electric dipoles. At 37 °C, water molecules have an average kinetic energies higher than the weakest bonds. Moreover, the kinetic energy is not distributed uniformly. Therefore, even the strongest single weak interaction has an ephemeral life of a fraction of a second at physiological temperatures. For a stable binding, several weak bonds must be present contemporaneously and this requires steric complementarity between the molecules1.
Table I.
Types of weak interactions. Their strength is equal to the change in free energy that takes place during binding. By comparison, the average kinetic energy of water molecules at 37 °C is 2.6 kJ/mol.
| Bond | Energy (kJ/mol) |
|---|---|
| Van der Waals | 4 (1–20) |
| Hydrogen | 20 (5–40) |
| "Hydrophobic" | <40 |
| Ion-dipole | 20 (10–50) |
Weak interactions involved in epitope-paratope binding
The specific binding between the antigenic determinant on the red cell (epitope) and the antigen-combining site on the immunoglobulin molecule (paratope) involves very small portions of the molecules 2, comprising just a few amino acids and a surface area between 0.4 and 8 nm2. Specific binding must overcome an overall repulsion between the two molecules. As presently understood, events at a molecular level occur as follows2: when the epitope and the paratope casually come to a distance of several nanometres, they are attracted by long-range forces, such as ionic and hydrophobic bonds. These attractive forces locally overcome the hydration energies of the two molecules, water molecules are expelled and the epitope and the paratope approach each other more closely. At this distance, van der Waals forces prevail, but ionic groups still play a role. At that point, the overall strength of the binding depends on the goodness of fit between the two surfaces (van der Waals forces are very short-range and decay with the seventh power of the interatomic distance3) and their total contact area.
The law of mass action
At the beginning, a chemical reaction proceeds predominantly in one direction, but the reverse rate progressively increases until the forward and reverse speeds are equal. At this point, the reaction is said to have reached its equilibrium.
According to the law of mass action* :
| (2) |
at equilibrium, the ratio between the concentrations of the product ([complex]) and the reactants ([antigen] and [antibody]) is constant. Keq is called the equilibrium constant and is equal to the ratio between the association (ka) and the dissociation (kd) rate constants.
In order to improve antibody detection, the ratio between bound and free antigen should be increased as much as possible. Rearranging (1):
we note that this can be obtained in two ways: increasing either the equilibrium constant or the antibody concentration4.
The greater the strength of the bond, the higher its equilibrium constant. This relationship is expressed by the following formula** :
| (3) |
where ln means the natural logarithm, Δ G is the change in free energy, R is the universal gas constant (= 8.314472 J·K−1·mol−1) and T is the absolute temperature (310 K at 37 °C).
Table II shows a list of values of equilibrium constants and the corresponding free energy changes.
Table II.
Equilibrium constants and their associated free energy changes (ΔG) at 37 °C. Chemical reactions only occur spontaneously when ΔG is negative
| Keq (L/mol) | Δ G (kJ/mol) |
|---|---|
| 105 | −40 |
| 106 | −46 |
| 107 | −52 |
| 108 | −58 |
| 109 | −64 |
| 1010 | −70 |
In order for a reaction to proceed spontaneously, the free energy change must be negative.
As shown by the following formula:
free energy change has two components: enthalpy (H), i.e. the energy contained in the chemical bonds of reactants and products, and entropy (S), i.e. the degree of randomness of the system (the symbol Δ indicates the change in the variable that occurs when the reactants are transformed into the products).
Hydrogen bonds are exothermic3 and the heat derives from the energy released with the formation of the chemical bonds of the product. Thus, hydrogen bonds predominantly form because of the enthalpic factor. In contrast, hydrophobic bonds are endothermic and probably driven by the entropic factor3. However, others disagree and believe that the entropic and enthalpic contributions to the hydrophobic interaction vary in relation to the individual organic compound2.
Equilibrium constants of red cell antibodies
The equilibrium constants of a few samples of red cell antibodies have been measured experimentally. They are listed in Table III.
Table III.
Equilibrium constants of red cell antibodies, as measured experimentally at normal ionic strength (I=0.16–0.17) 5–14. Values for anti-C, -E and –e were estimated on single samples8,13, anti-c on three samples5,8, and anti-D on more than 50 (some were anti-D immunoglobulin preparations) 9–12.
| Antibody | Keq |
|---|---|
| Anti-D | 2x107 – 3x10 9 |
| Anti-C | 0.5x107 |
| Anti-c | 1.9x107 – 5.6x10 7 |
| Anti-E | 4x108 |
| Anti-e | 2.5x108 |
| Anti-K | 6x109 – 4.5x10 10 |
Comparing Tables I, II and III, it is easy to conclude that two or more weak bonds must be involved in the formation of red cell antigen-antibody complexes.
Detailed thermodynamic data are available for anti-c5 and anti-D6 (one sample each): in both cases, most of the free energy change was due to an increase in entropy. Moreover, it was estimated that for anti-D there were three or four pairs of oppositely charged ionic groups involved in the reaction15.
Judging from Table III, anti-D seems to be more variable than the others, but this is, probably, just a reflection of the far greater number of samples studied.
On average, the equilibrium constants of anti-c and anti-K antibodies are 10-fold lower and 10-fold higher, respectively, than that of anti-D.
As an explanation for the high affinity of anti-K, it has been suggested that it has a particularly large binding site7.
Limitations of the measurement of the equilibrium constant
Polyclonal antibodies are in fact mixtures of antibodies with different affinities. A single value for the equilibrium constant is just an average and fails to convey the extent of heterogeneity.
For this purpose, an index of heterogeneity (a) may be calculated16, which is the slope of the curve obtained plotting against log[antibody free], where [antibody bound] is equal to [complex] and [antibody maximum] is the total number of antigen sites available to the antibodies. A value close to 1 indicates no heterogeneity. The few values concerning red cell antibodies, published in the past, ranged from 0.5 to 0.987,8,10,13, with an average around 0.77. However, the values reported more recently do not deviate significantly from unity14.
This may not be a matter of chance and highlights a significant source of error in the measurement of equilibrium constants: up to recently, it was necessary to label the primary antibody or the anti-IgG with radioiodine. Unfortunately, radioiodination interferes with the binding properties of the antibody17,18. When the primary antibody is radiolabelled, the equilibrium constant may be underestimated by 50–65%21.
Moreover, the extent of radioiodination is not uniform and this may conceivably result in an apparent heterogeneity of antibody affinity. However, the equilibrium constants of 22 anti-D samples, measured without radioiodination14, remained within the range shown in table III.
Another problem relates to the antibody valence. IgG molecules have two antigen binding sites. When both sites bind to the same red cell, the equilibrium constant increases by three orders of magnitude19. Anti-D, both IgG and IgM, apparently binds monovalently20,21. In contrast, IgG anti-A, -B, and –M bind by both sites 20. In these cases, the dissociation does not follow first-order kinetics, but two reactions occur at the same time: one involving the monovalently bound antibodies, and the other those bound by both sites22. Moreover, the proportion of the antibodies in the two states varies according to usually insignificant experimental conditions22, making the assumptions behind the law of mass action invalid.
A similar problem arises when the heterogeneity is on the part of the antigen: for example a monoclonal anti-Cw reacted with a small number of high affinity sites (Keq = 2–10x108) and a large number of low affinity sites (Keq = 1.2–5.5x107) 23.
Affinity maturation
Affinity maturation is the progressive increase in the equilibrium constant of antibodies produced during the immune response. Typically, early antibodies have equilibrium constants of around 105–106; those produced after a few months show 100-fold greater affinities24. The structural basis of affinity maturation seems to be a decrease in the conformational flexibility of the antigen binding site of the antibody25. The whole process can be described in this way: B-cell antigen receptors are able to recognise an enormous repertoire of different antigens because their binding sites cross-react with a variety of antigens and the activation process has a low threshold. Therefore, low affinity interactions are sufficient to induce a primary response25. Afterwards, antibody-producing B cells undergo rapid cell divisions in the germinal centres, during which the diversity of the binding sites is greatly amplified by casual mutations (somatic hypermutation). B cells producing high affinity antibodies are then preferentially selected for proliferation. In fact, early antibodies are coded by genes identical to those found in the germline, while mature antibodies are the product of somatically acquired point mutations24. Therefore, both affinity maturation and the seemingly impossible task of recognising a potentially infinite epitope repertoire with a finite paratope array, are fulfilled by the same mechanism, i.e. the modulation of the conformational flexibility of the antigen binding site25: the initial high flexibility permits cross-reactivity, but at the expense of low affinity, because of the unfavourable entropy changes (antigen binding entails a restriction in the conformational freedom). Conversely, the increased rigidity of the binding site of the mature antibody abolishes cross-reactivity but entails favourable entropic changes during antigen binding25, although enthalpic factors may also be involved26.
Re-stimulation of anti-D with D-positive red cells in immunised volunteers was accompanied by an increase in the equilibrium constant from 0.5x108 (range 0.3–1.3) to 2.1x108 (range 0.6–4.5)12. However, affinity maturation of red cell antibodies produced by non-deliberate immunisation has not been documented by reaction kinetics studies.
Factors affecting the antigen-antibody reaction
Many factors influence antigen-antibody reactions. They can be conveniently classified in two groups, according to whether they act on the equilibrium constant or not (Table IV).
Table IV.
Factors affecting the antigen-antibody reaction
|
Factors acting on the equilibrium constant
|
|
|
Other factors
|
|
Factors acting on the equilibrium constant
Temperature
Red cell antibodies are traditionally divided into “cold” and “warm” types, in relation to the thermal optimum of the antigen-antibody reaction. However, the thermal optimum probably depends on the chemical nature of both the epitope and paratope27 or, better, on the types of weak bonds involved. Hydrogen bonds are exothermic and are more stable at low temperature3. They are particularly important when the antigen is a carbohydrate3. Conversely, the strength of the hydrophobic bond increases with temperature3. Even in the case of warm antibodies, antigen-antibody reactions are expected to be stabilised at low temperature. However, the effect on the equilibrium constant is minimal or absent and is exceeded in practice by a decrease in the association rate: an example of anti-c showed equilibrium constants around 2.2x107 at 15–19 °C and 1.8x107 at 37–40 °C 5; no change in the equilibrium constant between 2 and 40 °C was found in an example of anti-D 6. On the other hand, at 4 °C, anti-D needs 20 times longer to reach equilibrium than at 37 °C4.
p H
The effect of pH on the equilibrium constant of anti-D is characterised by a symmetrical curve around a maximum lying between pH 6.5 and 8.4 15,28. At both sides of the maximum, the antigen-antibody reaction is strongly inhibited. For example, at pH 5.0 or 9.5, the equilibrium constant is 100-fold lower than at 6.5–7.0 15. In fact, this property has been exploited to elute antibodies from red cells29,30. Extreme pH values induce marked conformational changes in the antibody molecule that probably destroy the complementarity with the antigen28. Although no other red cell antibody has been evaluated with reaction kinetics studies, most of them should behave like anti-D, at least judging from serological results of acid elution31.
Ionic strength
Ionic strength (I) is defined as follows:
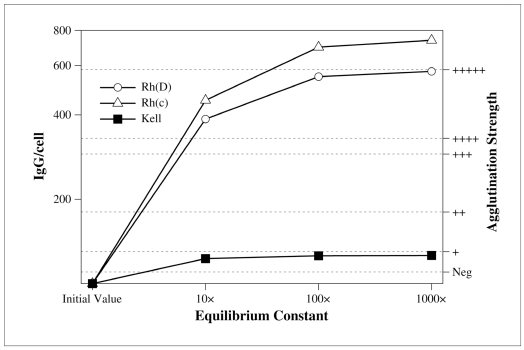
where [i] is the molar concentration of each ion in solution and vi is its valence. When there are only monovalent ions, I is equal to half of the sum of their molarity. The rate of decay of ionic interactions with distance strongly depends on I: e.g., at a distance of 0.8 nm in 0.15 mol NaCl (physiological saline), ionic bonds have the same strength as at 10 nm in 0.001 mol NaCl2. Ionic strength does not influence other weak interactions. Probably, ions bind to charged groups of epitopes or paratopes, obstructing their combination3. Early kinetic studies15,31 on ionic strength and red cell antibodies concerned anti-D and found a pronounced effect on the initial rate of association: reducing I from 0.17 to 0.03 increased the association rate constant 1000-fold15. It was calculated that a reaction that needed 1 hour to reach equilibrium at I=0.17, would be completed in 4 seconds at I=0.034. Plotting the log10 of the association rate constant against the square root of the ionic strength, a straight line was obtained from I=0.17 to I=0.03; then the curve reached a plateau15. The initial rate of dissociation was also influenced, but to a lesser degree: from 8x10−5 to 2.5x10−5 sec−1 15. Early serological studies4,32 extended the first observations and demonstrated that low ionic strength increased the titre of most antibodies tested, with the exception of anti-A and anti-B. Lewis antibodies were apparently enhanced in one study4 but not in the other32. Further experience revealed other notable exceptions: anti-K was not enhanced33 and a short incubation (10 min) could fail to detect weak examples34. The same was true for anti-k, -Kpa and – Kpb 35,36: therefore, all common Kell system antibodies behave similarly with respect to ionic strength. This is not surprising, because single base substitutions are responsible for the different Kell phenotypes37. Evidently, ionic bonds make no contribution to the specific binding of Kell system antibodies. Moreover, it is not surprising that antibodies against carbohydrate epitopes (ABH, Lewis) are not enhanced by low ionic strength, as the weak interactions involved are mainly hydrogen bonds3. Another characteristic of anti-K may be involved in its unusual behaviour at low ionic strength: its high equilibrium constant at normal ionic strength7. As already noted in one of the first studies on this subject, only low affinity antibodies (or the low affinity fraction of a polyclonal antibody) should be expected to be significantly enhanced by low ionic strength4. In fact, at equilibrium, low titre-high affinity antibodies already have most of the molecules bound to antigen. Therefore, even a marked increase in the equilibrium constant would produce a negligible increase in the number of antibody molecules per cell. This situation is illustrated in figure 1.
Figure 1.
Effect of increasing the equilibrium constant on three representative red cell antibodies. The y-axis on the left shows the number of IgG per red cell. The y-axis on the right is the agglutination strength corresponding to the number of IgG/cell (the correlation is based on a study by Merry et al.56 and must be intended as a gross approximation; it is shown for illustrative purposes only). Anti-D, -c, and – K are supposed to have an equilibrium constant of 2′108, 4.4′107, and 1.5′1010, respectively (typical values, see Table III) and a concentration below the minimum detectable in the antiglobulin test. If the equilibrium constant is increased 10 to 1,000 times, anti-D and anti-c are greatly enhanced but anti-K is not. The reason is that, in the initial conditions, anti-D and anti-c only have 17 and 13%, respectively, of the antibody molecules bound to the red cells, while anti-K already has 79%. Therefore, in the latter case, increasing the equilibrium constant 1,000-fold results in a mere 20% increment in antibody uptake, too small to be appreciated in the antiglobulin test.
False positive reactions at low ionic strength
False positive reactions were noted in the first serological studies on low ionic strength4,32, particularly at I≤0.04 (final concentration).
Further studies confirmed the first findings and showed that false reactions were mostly due to complement attachment38,39. The nature of this phenomenon had been independently investigated in a completely different field, albeit still related to transfusion medicine: red cell freezing40,41. When exposed to a very low ionic strength, gamma globulins aggregate and form a reversible complex with the lipoproteins of the red cell membrane. This causes the aggregation of the red cells, which rapidly sediment without centrifugation. Such a “reversible agglomeration” formed the basis of one of the first techniques for washing frozen glycerolised red cells42. Returning to immunohaematology, in the presence of active complement, aggregated immunoglobulins on the surface of red cells provoke the attachment of complement fractions. When the red cells are washed (at normal ionic strength) before adding the antiglobulin serum, immunoglobulins are removed but complement fractions are not and react with the anti-complement portion of broad-spectrum antiglobulin reagents. False positive reactions are not a problem with commercially available techniques, because the final ionic strength is usually around 0.09 and never falls under 0.06. However, when I is decreased to 0.03, so as to exploit the full effect on the association rate, anti-IgG must be substituted for a broad-spectrum antiglobulin43, or, as an alternative, complement must be inhibited using plasma instead of serum.
Enzyme treatment
Many proteolytic enzymes are known to enhance the antigen-antibody reaction, but the most used are papain, ficin and bromelin. Enzymatic pre-treatment almost doubles the amount of anti-D bound to D-positive red cells44. A detailed kinetic study showed that ficin increased the equilibrium constant of the D-anti-D reaction and that this effect was due to an increase in the association rate, while the dissociation rate was almost unaffected45. It has been suggested that enzymes expose D or D-like antigenic determinants44,46. Scatchard plots of equilibrium curves obtained in different studies gave contrasting results: in one study45, the maximum number of antibody molecules per cell (equivalent to the number of available antigens) was the same in enzyme-treated and control cells; in the other46, it was 46% more. In the last study, similar results were obtained with low ionic strength, but this finding is not easy to explain. Other antibodies known to be potentiated by enzymes are those directed against the other Rh antigens, Kidd, Colton, Dombrock, Lewis, P1, I, and i. Conversely, antigens of the Duffy and MNSs systems are destroyed or weakened, as are Xga, Ge, Ch, Rg and others20.
The effects of enzymes and ionic strength are not additive31. This suggests a common mechanism of action. However, a situation similar to that depicted in figure 1 is also possible: once the equilibrium constant is elevated, further increases would go unnoticed. Probably, enzymes act by removing obstructing molecules or charged chemical groups around the combining site45. Enzymes are also capable of inducing direct agglutination by incomplete antibodies. However, the effects on antibody binding and agglutination are independent, because normal D-positive cells do not agglutinate with anti-D, even though sensitised with a much greater amount of antibody than necessary for enzyme-treated cells45.
Polymers and other potentiators
Most chemical substances used for improving red cell antibody detection do not act on the first stage of agglutination, the antigen-antibody reaction, but on the second, agglutination proper. Typical examples are the polycations: polybrene47, protamine43, methylcellulose. Albumin and polyethylene glycol (PEG) are exceptions. Reagents containing 20–33% bovine albumin were used for many years in antiglobulin tests, before being supplanted by low ionic strength solutions. In retrospect, it appears probable that the effect of albumin reagents was due to their relatively low ionic strength, rather than to the properties of albumin.
PEG is the only potentiator also known to improve the detection of anti-K, although Kell antibodies are less enhanced than Rh and Kidd antibodies48. PEG must be used with an anti-IgG (or with plasma instead of serum) because, otherwise, false positive reactions due to the attachment of complement fractions would ensue49. There are no thermodynamic studies of its effects on the antigen-antibody reaction. PEG decreases the solubility of plasma proteins, a property due to the very unfavourable free energy of its interaction with proteins (steric exclusion)50. It is speculated that in this way, antigen and antibody behave as if they were more concentrated, increasing the probability of a contact51. In any case, the mechanism of action is probably not the same as that of low ionic strength. In fact, reagents have been described that combine PEG and low ionic strength52. Unfortunately, PEG at low ionic strength tends to precipitate plasma proteins in samples with hyperproteinemia or cryoglobulins. Apparently, this does not interfere with red cell sensitisation, but makes it difficult to re-suspend the cells if they are centrifuged before washing. PEG-enhanced tests are among the most sensitive manual antibody screening assays available today. Unfortunately, up to now, PEG has not been integrated in any automated test.
Other factors
Concentrations of antigen and antibody
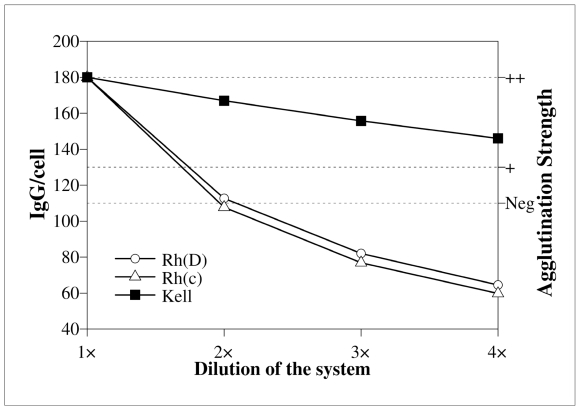
The left terms of the law of mass action (2) are concentrations. This means that a simple dilution or concentration of the system, without changing the absolute quantities, can markedly affect the number of IgG per cell. An example is shown in figure 2: three antibodies (anti-D, -c, and – K), with typical equilibrium constants, are supposed to have such a concentration in the sample as to give a ‘++’ in the antiglobulin test. If the system is diluted 1:2 or 1:4 by adding an appropriate medium, so as not to change temperature, pH, or ionic strength, the equilibrium constant does not change, but part of the antigen-antibody complex will dissociate. This happens because (2) has a product in the denominator and [antigen] or [antibody] or both must be increased to maintain the result of the fraction constant. As shown in figure 2, the dilution effect is much more pronounced for low affinity antibodies: both anti-D (supposed to have an equilibrium constant of 2x108) and anti-c (with an equilibrium constant of 4.4x107) would give a negative or doubtful reaction in a diluted system. On the other hand, anti-K (Keq=1.5x1010) would still be easily detectable.
Figure 2.
Effect of the dilution of the reaction system on antibody uptake. Y-axes are the same as in figure 1. Antibodies are also the same, but they are supposed to have an initial concentration corresponding to a ‘++’ in the antiglobulin test. If a suitable medium is added, which dilutes the system without changing temperature, pH, or ionic strength, anti-D and anti-c are markedly affected and the antibody uptake may be insufficient to be detected. A high affinity antibody, such as anti-K, is much less affected.
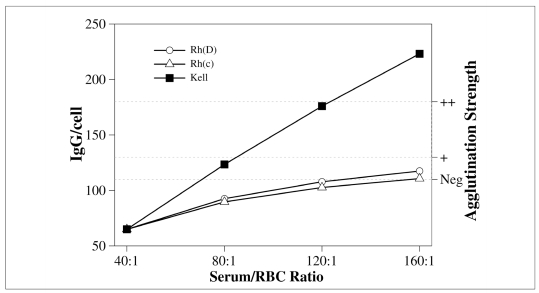
Serum/cell ratio
Similar considerations apply, when the serum/cell ratio is modified. This may be obtained in two ways: either by increasing the volume of serum/plasma or decreasing the volume or the concentration of the red cells. It is advisable to increase the serum/cell ratio in order to improve the sensitivity of the antiglobulin test4. However, only high affinity antibodies, such as anti-K, should be expected to be enhanced in this way, as shown in figure 3. These theoretical expectations about the behaviour of anti-D, -c, and -K have been confirmed in kinetic33 and serological studies53.
Figure 3.
Effect of an increased serum/cell ratio on antibody uptake. Y-axes and antibodies are the same as in previous figures. In the initial conditions, antibodies are supposed to have a concentration equal to half of the amount necessary to give a ‘+’ in the antiglobulin test. A serum/cell ratio of 40 corresponds to two volumes of serum/plasma for one volume of 5% red cells. Higher ratios are obtained by adding more serum/plasma. Anti-K is greatly enhanced. In contrast, the uptake of low affinity antibodies (anti-D and anti-c) is only modestly increased.
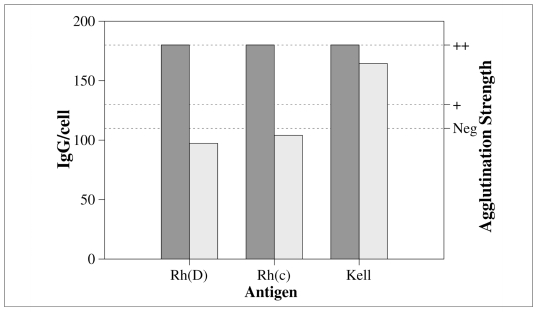
Antigen zygosity
Many red cell antigens are present in different numbers in homozygous and heterozygous cells54. The effects of antigen zygosity and red cell concentration are distinct: even when the concentration of the antigen in the reaction system is equal in both cases, and, for this reason, the concentration of the antigen-antibody complex too, the number of antibodies per cell changes and favours homozygous cells. An example is shown in figure 4. Once again, the behaviour of the antibodies is determined by their affinity: the two low affinity antibodies (anti-D, anti-c) are greatly influenced by zygosity, while the high affinity example (anti-K) is not.
Figure 4.
Effect of antigen zygosity on the number of antibodies per cell. Y-axes and antibodies are the same as in previous the figures. Dark and light bars represent homozygous and heterozygous red cells, respectively. Calculations are based on the following estimates for the number of antigen sites per cell: homozygous cells: D: 27,000, c: 78,000, K: 5,000; heterozygous cells: D: 12,000, c: 40,000, K: 3,00054. The antibodies (anti-D, -c, -K) are supposed to have such a concentration as to give a ‘++’ in the antiglobulin test with homozygous cells. In the case of low affinity antibodies (anti-D and antic), the use of heterozygous instead of homozygous cells causes a marked decrease in the number of IgG/cell. The high affinity antibody (anti-K) is much less affected.
Duration of incubation
For maximum sensitivity, the antigen-antibody reaction should be allowed to reach equilibrium. However, this may take 4 hours at normal ionic strength and at a temperature of 37 ° C20. Luckily, the kinetics is not linear and in the first hour the uptake is already 87% of the maximum (40% after 15 min)20. At low ionic strength, the reaction is faster but how much faster depends on how low the ionic strength is and on how sensitive the antibody is to its effect. For fear of false positive reactions, the ionic strength has usually been decreased only moderately (I≈0.09). In such conditions, the uptake of Rh and Kell antibodies increased at least up to 20 min (longer incubations were not reported)33. After 20 min at low ionic strength, Rh antibodies gave values 72% higher than after 45 min at normal ionic strength. After 10 min, they were already 40% higher. Anti-Fya and anti-Jka were also enhanced, but not so strongly. On the other hand, Kell antibodies gave slightly lower values (−5%) after 20 min than after 45 min at normal ionic strength, and after 10 min they were about 25% less33.
Another study employed almost the same experimental design, but only serological tests were performed and the reference method was a 60 min incubation at normal ionic strength55. After 5 min at low ionic strength (I=0.09), 24% of the antibodies gave weaker reactions than with the reference method and 2 out of 17 anti-K did not react at all. A case of anti-K, which caused a haemolytic transfusion reaction and had not been detected after 10 min at low ionic strength, has been cited above34. In a comparison between low (I=0.10, 20 min incubation) and very low (I=0.04, 5 min incubation) ionic strength, serological results showed that the very low ionic strength test gained sensitivity for Rh antibodies, notwithstanding the very short incubation36.
Duffy and Kidd antibodies gave almost equal results with both techniques, but Kell antibodies were clearly weaker in the very low ionic strength, 5 min incubation technique36. In a further experiment, eight examples of anti-K were incubated for 5, 20 or 60 min at normal (I=0.16), low (I=0.10) or very low (I=0.04) ionic strength.
There were no significant differences between the ionic strengths, but results were clearly better after 20 min than after 5 min. By prolonging the incubation up to 60 min, the score improved slightly but the difference was not statistically significant36.
In liquid phase tests, red cells sediment during incubation. The sedimentation rate depends on the viscosity of the medium, but after 20 min this phenomenon is usually clearly evident. The antigen is more concentrated and the antibody less in the sedimented layer. This decreases the number of antigen-antibody complexes per cell. Although no quantitative study has specifically addressed this point, unless the reaction mixture is mixed regularly, there should be no advantage from prolonging incubation beyond 20 min.
Overall sensitivity of a test method
The incubation phase is just one component: the washing step and the way the reaction is read are also important. During washing, the antigen-antibody complex partly dissociates, because free antibody is removed and the washing solution is usually at normal ionic strength and does not contain any potentiator. However, washing is usually performed at room temperature and this decreases the dissociation rate. Low affinity antibodies should be more affected by this problem. This probably explains why Rh antibodies are particularly enhanced by polycations, which bring about agglutination without washing being necessary36,43. The gel test, which avoids washing through the use of a gradient, should also be particularly advantageous for low affinity antibodies. In any case, the steps following incubation should be as fast as possible.
Conclusions: optimal conditions for red cell antibody detection
Affinity is the most important factor governing the behaviour of antibodies. It depends on the genetic system (Kell antibodies generally show a high affinity, Rh antibodies a low one) and on how mature the immune response is (affinity maturation). Low affinity antibodies are particularly sensitive to antigen and antibody concentrations and antigen zygosity. On the other hand, high affinity antibodies are sensitive to the serum/cell ratio. The second most important factor is the activity at low ionic strength: Rh antibodies are particularly enhanced, most other antibodies are less enhanced, but Kell antibodies are not enhanced at all.
A technique suitable for all clinically significant red cell antibodies should meet a series of requirements.
- Incubation at very low ionic strength (I=0.03–0.04) or in the presence of PEG.
- Duration of incubation not less than 20 min.
- The highest serum/cell ratio.
- The minimum amount of red cells.
- Homozygous red cells.
In practice, volume limitations force a compromise between low ionic strength and the amount of serum/plasma: to reach an ionic strength of 0.04, serum must be diluted at least 1:4 with a low ionic strength solution. Moreover, the minimum amount of red cells depends on the detection system: tube tests require more red cells than gel or solid phase tests.
Appendix A – Derivation of the formulae
An intuitive derivation of the law of mass action
During the course of the antigen-antibody reaction, the association rate (va) is proportional to the probability of contact between free epitope and paratope molecules. That is, in the ideal case where there is only one epitope per antigen molecule and one paratope per antibody molecule, and they are in solute form: va = ka × [ag]×[ab], where [ag] and [ab] are the concentrations of free antigen and antibody, respectively, and ka is a proportionality constant called the association rate constant.
Similarly, the dissociation rate (vd) is proportional to the concentration of the antigen-antibody complex ([complex]): vd = kd × [complex] , where kd is the dissociation rate constant. At equilibrium va = vd and ka × [ag]×[ab] = kd × [complex].
Rearranging, we obtain the law of mass action:
| (1) |
where Keq is the equilibrium constant.
Keq and the number of antibody molecules bound at equilibrium
The left terms of (1) are concentrations (mol/L). Let us suppose that we are performing a gel test, with 25 μL of serum/plasma and 50 μL of red cells at a 0.8% concentration. Serum contains anti-D with a Keq = 2′108 in the test conditions and red cells have 12,000 antigen sites per cell. Let us suppose, moreover, that the concentration of anti-D is sufficient to bind 100 antibody molecules per cell, at equilibrium. This amount would not be enough to give a positive reaction56. The final antigen concentration ([ag]tot) is 1.063′10−9mol/L.
In fact, there are parts of red cells in the final suspension, i.e. 5.333 mL per litre.
As there are 1010 red cells per mL, the antigen sites are 5.333′1010′12,000 = 6.4′1014 per litre.
Therefore the concentration is , where 6.022′1023 is Avogadro’s number.
The concentration of the antigen-antibody complex is and the free antibody concentration (4.202′10−11 mol/L) is obtained rearranging (1):
Thus, only 17% of the antibody molecules are bound at equilibrium.
The total antibody concentration ([ab]tot) is equal to the sum of the concentrations of the free antibody and the antigen-antibody complex:[ab]tot = [ab] + [complex]
If we repeat the calculations keeping constant [ag]tot and [ab]tot, and increasing Keq 10 times, equation (1) becomes:
Solving for [complex] we obtain a second degree equation:
that has two apparent solutions:
| (2) |
Only one of the solutions is correct, the one which is positive and less than [ag]tot and [ab]tot. The concentration of the antigen-antibody complex is now 3.423′10−11 mol/L, which corresponds to 387 antibody molecules per cell or 67% of all antibody molecules. This amount of antibody per cell would give a ‘++++’ reaction56.
Formula (2) can be used to calculate the concentration of the antigen-antibody complex, whenever [ag]tot, [ab]tot, and Keq are known.
Effect of the dilution of the reaction system35
Let us suppose that an antigen-antibody reaction has already reached its equilibrium. At that point, we dilute the system with an appropriate medium so as not to change the temperature, pH or ionic strength. The concentrations of both the numerator and the denominator of the left term of (1) will be affected in the same way, but the denominator is a product and therefore the result of the fraction will not be equal to the equilibrium constant, which is supposed to be unchanged. In other words, the system is no longer in equilibrium:
where , Vi is the initial volume and is the final volume of the system.
Part of the antigen-antibody complex will dissociate to increase both the free antigen and the free antibody concentrations:
Solving for the dissociated portion (d) we obtain a second degree equation:
that has the following two possible solutions:
Obviously, the system would reach the same equilibrium if the reactants were diluted since the beginning. The example is only meant to show that dilution necessarily entails a decrease in the ratio between bound and free antibody.
Appendix B – Programming a spreadsheet *
(The reader is referred to the first article57 of this series for a brief introduction to spreadsheets).
How to calculate the antibody concentrations at equilibrium, when the equilibrium constant and the number of antibody molecules bound per cell are known
Open a new sheet. Enter the text, values and formulae listed in Table V. Cell B12 contains the number of red cells per litre; B13 contains the total antigen concentration (mol/ L); B14, the antigen-antibody complex concentration (mol/ L); B15 and B16, the free antibody and the total antibody concentrations (mol/L), respectively; B17, the antibody molecules bound per cell, expressed as a percentage of the total antibody concentration.
Table V.
Instructions to calculate antibody concentration, when the equilibrium constant and the number of antibody molecules bound per cell are known.
| Cell | Text to be entered |
|---|---|
| A1 | Antibody concentration, when the equilibrium constant and the number of antibody molecules bound per cell are known |
| A3 | Volume of serum/plasma (μL) |
| A4 | Volume of RBC suspension (μL) |
| A5 | [RBC] (%) |
| A6 | Volume of diluent/potentiator (μL) |
| A7 | Antigen sites per cell |
| A8 | Equilibrium constant |
| A9 | Antibody molecules bound per cell |
| A10 | Avogadro’s number |
| A12 | Number of RBC per litre |
| A13 | Total antigen concentration (mol/L) |
| A14 | Antigen-antibody complex concentration (mol/L) |
| A15 | Free antibody concentration (mol/L) |
| A16 | Total antibody concentration (mol/L) |
| A17 | Bound antibody (%) |
|
| |
| Value to be entered | |
|
| |
| B3 | The volume of serum/plasma in the test system (μL), e.g. 25 |
| B4 | The volume of the RBC suspension (μL), e.g. 50 |
| B5 | The concentration of the RBC suspension (%), e.g. 0.8 |
| B6 | The volume of the diluent/potentiator (if not included in the RBC suspension) (μL), e.g. 0 |
| B7 | The number of antigen sites per cell, e.g. 12,000 |
| B8 | The equilibrium constant§, e.g. 2E8 |
| B9 | The number of antibody molecules bound per cell, e.g. 130 |
| B10 | Avogadro’s number§: 6.022E23 |
|
| |
| Formula to be entered | |
|
| |
| B12 | =B5/100*B4/(B3+B4+B6)*POWER(10,13) |
| B13 | =B7*B12/B10 |
| B14 | =B9*B12/B10 |
| B15 | =B14/((B13–B14)*B8) |
| B16 | =B14+B15 |
| B17 | =B14/B16*100 |
2E8 and 6.022E23 are short-hand ways to write 2x108 and 6.022x1023, respectively.
How to calculate the number of antibody molecules bound per cell at equilibrium, when the equilibrium constant and the total antibody concentration are known
Open a new sheet. Enter the text, values and formulae listed in Table VI. Cell B12 contains the number of red cells per litre; B13 contains the total antigen concentration (mol/ L); B16, the antigen-antibody complex concentration (mol/ L); B17, the free antibody concentration (mol/L); B18, the antibody molecules bound per cell, expressed as a percentage of the total antibody concentration; B19, the number of antibody molecules bound per cell.
Table VI.
Instructions to calculate the number of antibody molecules bound per cell, when the equilibrium constant and the total concentration of the antibody are known
| Cell | Text to be entered |
|---|---|
| A1 | Number of antibody molecules bound per cell, when the equilibrium constant and the total concentration of the antibody are known |
| A3 | Volume of serum/plasma (μL) |
| A4 | Volume of RBC suspension (μL) |
| A5 | [RBC] (%) |
| A6 | Volume of diluent/potentiator (μL) |
| A7 | Antigen sites per cell |
| A8 | Equilibrium constant |
| A9 | Total antibody concentration (mol/L) |
| A10 | Avogadro’s number |
| A12 | Number of RBC per litre |
| A13 | Total antigen concentration (mol/L) |
| A14 | First solution |
| A15 | Second solution |
| A16 | Antigen-antibody complex concentration (mol/L) |
| A17 | Free antibody concentration (mol/L) |
| A18 | Bound antibody (%) |
| A19 | Number of antibody molecules bound per cell |
|
| |
| Value to be entered | |
|
| |
| B3 | The volume of serum/plasma in the test system (μL), e.g. 25 |
| B4 | The volume of the RBC suspension (μL), e.g. 50 |
| B5 | The concentration of the RBC suspension (%), e.g. 0.8 |
| B6 | The volume of the diluent/potentiator (if not included in the RBC suspension) (μL), e.g. 0 |
| B7 | The number of antigen sites per cell, e.g. 12,000 |
| B8 | The equilibrium constant, e.g. 2E8 |
| B9 | The total antibody concentration (mol/L), e.g. 6.63E-11 |
| B10 | Avogadro’s number: 6.022E23 |
|
| |
| Formula to be entered | |
|
| |
| B12 | =B5/100*B4/(B3+B4+B6)* POWER (10,13) |
| B13 | =B7*B12/B10 |
| B14 | =(B13+B9+1/B8)/2+ POWER (POWER ((B13+B9+1/B8)/2,2)-B13*B9,0.5) |
| B15 | =(B13+B9+1/B8)/2- POWER (POWER ((B13+B9+1/B8)/2,2)-B13*B9,0.5) |
| B16 | =IF(OR(B14>B9,B14<0),B15,B14) |
| B17 | =B9-B16 |
| B18 | =B16/B9*100 |
| B19 | =B16*B10/B12 |
Footnotes
Concentrations are shown in square brackets.
Keq in formula (3) must be expressed in (mole fraction)−1 rather than in units of L/mol, as usually reported in the immunohaematological literature (and this article)2. The conversion formula is L/mol = 55.56(mole fraction) −1. In fact, a litre of water contains 55.56 moles of H2O.
Italian readers using the local (Italian) versions of the spreadsheets should follow the instructions in the Italian translation of this paper, which is available on line at http://www.transfusionmedicine.org/. Briefly, “ potenza” should be substituted for “ power” and “ ;” should be substituted for “ ,” .
References
- 1.Watson JD, Baker TA, Bell SP, et al. Molecular Biology of the Gene. San Francisco, CA: Benjamin Cummings; 2004. [Google Scholar]
- 2.van Oss CJ. Hydrophobic, hydrophilic and other interactions in epitope-paratope binding. Mol Immunol. 1995;32:199–211. doi: 10.1016/0161-5890(94)00124-j. [DOI] [PubMed] [Google Scholar]
- 3.Moore BPL. Antibody uptake: the first stage of the haemagglutination reaction. In: Bell CA, editor. A Seminar on Antigen-antibody Reactions Revisited. Arlington VA: AABB; 1982. pp. 47–66. [Google Scholar]
- 4.Hughes-Jones NC, Polley MJ, Telford R, et al. Optimal conditions for detecting blood group antibodies by the antiglobulin test. Vox Sang. 1964;9:385–95. doi: 10.1111/j.1423-0410.1964.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 5.Hughes-Jones NC, Gardner B, Telford R. The kinetics of the reaction between the blood-group antibody anti-c and erythrocytes. Biochem J. 1962;85:466–74. doi: 10.1042/bj0850466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes-Jones NC, Gardner B, Telford R. Studies on the reaction between the blood-group antibody anti-D and erythrocytes. Biochem J. 1963;88:435–40. doi: 10.1042/bj0880435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes-Jones NC, Gardner B. The Kell system studied with radioactively-labelled anti-K. Vox Sang. 1971;21:154–8. doi: 10.1111/j.1423-0410.1971.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 8.Hughes-Jones NC, Gardner B, Lincoln PJ. Observations of the number of available c, D, and E antigen sites on red cells. Vox Sang. 1971;21:210–6. doi: 10.1111/j.1423-0410.1971.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 9.Rochna E, Hughes-Jones NC. The use of purified 125I-labelled anti-γ globulin in the determination of the number of D antigen sites on red cells of different phenotypes. Vox Sang. 1965;10:675–86. doi: 10.1111/j.1423-0410.1965.tb05179.x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes-Jones NC. The estimation of the concentration and equilibrium constant of anti-D. Immunol. 1967;12:565–71. [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes-Jones NC, Gardner B. The equilibrium constants of anti-D immunoglobulin preparations made from pools of donor plasma. Immunol. 1970;18:347–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Holburn AM, Cleghorn TE, Hughes-Jones NC. Re-stimulation of anti-D in donors. Vox Sang. 1970;19:162–7. doi: 10.1111/j.1423-0410.1970.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 13.Skov F, Hughes-Jones NC. Observations on the number of available C antigen sites on red cells. Vox Sang. 1977;33:170–4. doi: 10.1111/j.1423-0410.1977.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 14.Debbia M, Brossard Y, Lambin P. Measurement of the affinity of anti-D in the serum of immunized mothers and in immunoglobulin preparations with unlabelled antibodies. Transfusion. 2005;45:975–83. doi: 10.1111/j.1537-2995.2005.04313.x. [DOI] [PubMed] [Google Scholar]
- 15.Hughes-Jones NC, Gardner B, Telford R. The effect of pH and ionic strength on the reaction between anti-D and erythrocytes. Immunol. 1964;7:72–81. [PMC free article] [PubMed] [Google Scholar]
- 16.Karush F. Immunologic specificity and molecular structure. Adv Immunol. 1962;2:1–40. [Google Scholar]
- 17.Matzku S, Kirchgessner H, Dippold WG, et al. Immunoreactivity of monoclonal anti-melanoma antibodies in relation to the amount of radioactive iodine substituted to the antibody molecule. Eur J Nucl Med. 1985;11:260–4. doi: 10.1007/BF00279081. [DOI] [PubMed] [Google Scholar]
- 18.Nikula TK, Bocchia M, Curcio MJ, et al. Impact of the high tyrosine fraction in complementarity determining regions: measured and predicted effects of radioiodination on IgG immunoreactivity. Mol Immunol. 1995;32:865–72. doi: 10.1016/0161-5890(95)00052-g. [DOI] [PubMed] [Google Scholar]
- 19.Hornick CL, Karush F. Antibody affinity. III. The role of multivalence. Immunochemistry. 1972;9:325–40. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- 20.Mollison PL, Engelfriet CP, Contreras M. Blood Transfusion in Clinical Medicine. 10. Oxford, Great Britain: Blackwell Science; 1997. [Google Scholar]
- 21.Debbia M, Lambin P. Measurement of anti-D intrinsic affinity with unlabelled antibodies. Transfusion. 2004;44:399–406. doi: 10.1111/j.1537-2995.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 22.Ong GL, Mattes MJ. Re-evaluation of the concept of functional affinity as applied to bivalent antibody binding to cell surface antigens. Mol Immunol. 1993;30:1455–62. doi: 10.1016/0161-5890(93)90107-m. [DOI] [PubMed] [Google Scholar]
- 23.Thorpe SJ, Boult CE, Thompson KM. Immunochemical characterization of the Rh Cw antigen using human monoclonal antibodies. Vox Sang. 1997;73:174–81. doi: 10.1046/j.1423-0410.1997.7330174.x. [DOI] [PubMed] [Google Scholar]
- 24.Foote J, Eisen HN. Kinetic and affinity limits on antibodies produced during immune response. Proc Natl Acad Sci USA. 1995;92:1254–6. doi: 10.1073/pnas.92.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manivel V, Sahoo NC, Salunke DM, Rao KVS. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity. 2000;13:611–20. doi: 10.1016/s1074-7613(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 26.Pauyo T, Hilinski GJ, Chiu PT, et al. Genetic and fluorescence studies of affinity maturation in related antibodies. Mol Immunol. 2006;43:812–21. doi: 10.1016/j.molimm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Hughes-Jones NC. Red cell antigens, antibodies and their interaction. Clin Haematol. 1975;4:29–43. [PubMed] [Google Scholar]
- 28.Barnes AE. The specificity of pH and ionic strength effects in the kinetics of the Rh(D)-anti-Rh(D) system. J Immunol. 1966;96:854–64. [PubMed] [Google Scholar]
- 29.Hughes-Jones NC, Gardner B, Telford R. Comparison of various methods of dissociation of anti-D, using 131I-labelled antibody. Vox Sang. 1963;8:531–6. doi: 10.1111/j.1423-0410.1963.tb04180.x. [DOI] [PubMed] [Google Scholar]
- 30.Howard PL. Principles of antibody elution. Transfusion. 1981;21:477–82. doi: 10.1046/j.1537-2995.1981.21582040807.x. [DOI] [PubMed] [Google Scholar]
- 31.Atchley WA, Bhagavan NV, Masouredis SP. Influence of ionic strength on the reaction between anti-D and D-positive red cells. J Immunol. 1964;93:701–12. [PubMed] [Google Scholar]
- 32.Elliot M, Bossom E, Dupuy ME, Masouredis SP. Effect of ionic strength on the serologic behaviour of red cell isoantibodies. Vox Sang. 1964;9:396–414. doi: 10.1111/j.1423-0410.1964.tb03308.x. [DOI] [PubMed] [Google Scholar]
- 33.Merry AH, Thomson EE, Lagar J, et al. Quantitation of antibody binding to erythrocytes in LISS. Vox Sang. 1984;47:125–32. doi: 10.1111/j.1423-0410.1984.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 34.Molthan L, Strohm PL. Haemolytic transfusion reaction due to anti-Kell undetectable in low ionic strength solutions. Am J Clin Path. 1981;75:629–31. doi: 10.1093/ajcp/75.4.629. [DOI] [PubMed] [Google Scholar]
- 35.Reverberi R, Moretti M, Campana F, et al. Soluzioni a bassa forza ionica: importanza della concentrazione dei ligandi nel test indiretto all’antiglobulina. La Trasf del Sangue. 1984;29:325–35. [Google Scholar]
- 36.Reverberi R, Ferrari L, Balugani S. Fattori che influenzano la sensibilità dei test per gli anticorpi anti-eritrocitari: confronto tra bassa e bassissima forza ionica e tra protamina ed anti-IgG. La Trasf del Sangue. 1996;41:10–6. [Google Scholar]
- 37.Lee S. Molecular basis of Kell blood group phenotypes. Vox Sang. 1997;73:1–11. doi: 10.1046/j.1423-0410.1997.7310001.x. [DOI] [PubMed] [Google Scholar]
- 38.Löw B, Messeter L. Antiglobulin test in low-ionic strength salt solution for rapid antibody screening and cross-matching. Vox Sang. 1974;26:53–61. doi: 10.1111/j.1423-0410.1974.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 39.Moore HC, Mollison PL. Use of a low-ionic-strength medium in manual tests for antibody detection. Transfusion. 1976;16:291–6. doi: 10.1046/j.1537-2995.1976.16476247048.x. [DOI] [PubMed] [Google Scholar]
- 40.Huggins CE. Reversible agglomeration used to remove dimethylsulfoxide from large volumes of frozen blood. Science. 1963;139:504–5. doi: 10.1126/science.139.3554.504. [DOI] [PubMed] [Google Scholar]
- 41.Van Oss CJ, Buenting S. Adsorbed euglobulins as the cause of agglomeration of erythrocytes in the Huggins blood-thawing method. Transfusion. 1967;7:77–8. (letter) [Google Scholar]
- 42.Huggins CE. Frozen blood. Europ Surg Res. 1969;1:3–12. doi: 10.1159/000127455. [DOI] [PubMed] [Google Scholar]
- 43.Rosenfield R, Shaikh SH, Innella F, et al. Augmentation of haemagglutination by low ionic conditions. Transfusion. 1979;19:499–510. doi: 10.1046/j.1537-2995.1979.19580059800.x. [DOI] [PubMed] [Google Scholar]
- 44.Masouredis SP, Dupuy ME, Elliot M. Reaction of I131 anti-Rh0(D) with enzyme treated red cells. Transfusion. 1962;2:363–74. doi: 10.1111/j.1537-2995.1962.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 45.Hughes-Jones NC, Gardner B, Telford R. The effect of ficin on the reaction between anti-D and red cells. Vox Sang. 1964;9:175–82. doi: 10.1111/j.1423-0410.1964.tb03677.x. [DOI] [PubMed] [Google Scholar]
- 46.Masouredis SP, Sudora EJ, Victoria EJ. Immunological and electron microscopic analysis of IgG anti-D saline haemagglutination of neuraminidase- and protease-modified red cells. J Lab Clin Med. 1977;90:929–48. [PubMed] [Google Scholar]
- 47.Lalezari P, Jiang AF. The manual polybrene test: a simple and rapid procedure for detection of red cell antibodies. Transfusion. 1980;20:206–11. doi: 10.1046/j.1537-2995.1980.20280169962.x. [DOI] [PubMed] [Google Scholar]
- 48.De Man AJM, Overbeeke MAM. Evaluation of the polyethylene glycol antiglobulin test for detection of red blood cell antibodies. Vox Sang. 1990;58:207–10. doi: 10.1111/j.1423-0410.1990.tb02092.x. [DOI] [PubMed] [Google Scholar]
- 49.Nance SJ, Garratty G. A new potentiator of red blood cell antigen-antibody reactions. Am J Clin Pathol. 1987;87:633–5. doi: 10.1093/ajcp/87.5.633. [DOI] [PubMed] [Google Scholar]
- 50.Arakawa T, Timasheff SN. Mechanism of poly(ethylene glycol) interaction with proteins. Biochemistry. 1985;24:6756–62. doi: 10.1021/bi00345a005. [DOI] [PubMed] [Google Scholar]
- 51.Wenz B, Apuzzo J. Polyethylene glycol improves the indirect antiglobulin test. Transfusion. 1989;29:218–20. doi: 10.1046/j.1537-2995.1989.29389162726.x. [DOI] [PubMed] [Google Scholar]
- 52.Verenini M, Reverberi R. Uso del PEG in immunoematologia: ottimizzazione della tecnica. La Trasf del Sangue. 1997;42:220–4. [Google Scholar]
- 53.Voak D, Downie M, Haigh T, et al. Improved antiglobulin tests to detect difficult antibodies: detection of anti-Kell by LISS. Med Lab Sci. 1982;39:363–70. [PubMed] [Google Scholar]
- 54.Daniels G. Human Blood Groups. Oxford, Great Britain: Blackwell Science; 1995. [Google Scholar]
- 55.Herron R, Smith DS. Use of low ionic strength salt solution in compatibility testing. J Clin Pathol. 1978;31:1116–7. doi: 10.1136/jcp.31.11.1116. [letter] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merry AH, Thomson EE, Rawlinson VI, et al. Quantitation of IgG on erythrocytes: correlation of number of IgG molecules per cell with the strength of the direct and indirect antiglobulin test. Vox Sang. 1984;47:73–81. doi: 10.1111/j.1423-0410.1984.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 57.Reverberi R, Reverberi L. Removal kinetics of exchange transfusion. Blood Transfusion. 2007;5:93–101. doi: 10.2450/2007.0018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]