Abstract
Background
Severe FX deficiency is a rare disorder with a variable bleeding tendency but spontaneous life threatening haemorrhage can occur. Treatment for invasive procedures and spontaneous bleeding is with prothrombin complex concentrates (PCC). When used in large or repetitive doses these are associated with a thrombotic tendency. FX:C levels of 0.15 – 0.30 IU/ mL are thought to be haemostatic during surgery . There is only limited information on the outcome and management of pregnancy in severe FX deficiency. Caesarean section is suggested as delivery mode to reduce the risk of intracranial/abdominal neonatal haemorrhage, but successful vaginal deliveries are also described. The calibrated automated thrombin generation assay (CAT) is a global coagulation test that measures the time course of thrombin generation. It has been reported to correlate with prothrombotic states and the severity of bleeding in rare coagulation disorders. The variability in phenotype, the uncertainty of the minimal haemostatic FX:C concentration and the association of PCC’s with thrombosis make thrombin generation of interest in the management of FX deficient patients.
Patient
We describe the use of CAT as a possible means to monitor treatment with PCC (Beriplex® ) in a patient with severe FX deficiency (FX:C < 0.01 IU/mL) during successful vaginal delivery and epidural anaesthesia.
Results
Thrombin generation was normal at FX:C 0.80 IU/mL but only borderline normal at FX:C 0.25 IU/mL. Repetitive doses over 3 days increased thrombin generation to the upper limit of normal at FX:C 0.25 IU/mL consistent with a prothrombotic tendency after multiple doses. The increase in thrombin generation was not related to prothrombin levels.
Conclusion
The data suggest that CAT may be used to monitor treatment with PCC in FX deficiency. Higher levels than previously thought may be needed to normalize thrombin generation. Further studies into the correlation with bleeding or thrombosis are needed before the approach can be accepted in clinical practice.
Keywords: FX deficiency, thrombin generation, endogenous thrombin potential, prothrombin complex concentrate, pregnancy
Introduction
Severe FX deficiency is a rare disorder with an estimated prevalence of 1:106, but is commoner in areas with a high proportion of consanguineous marriages1. The bleeding tendency is variable, but can be life threatening and includes umbilical stump bleeding in neonates, mucosal bleeding (in particular epistaxis), haemarthrosis, central nervous system and post operative bleeding2. Treatment depends on the severity and site of bleeding and includes antifibrinolytics, (virally inactivated) fresh frozen plasma and prothrombin complex concentrates (PCC)2. PCC’s contain FII, FVII, FIX and FX. They are obtained from thousands of pooled human plasma donations and heat treated to minimize the risk of viral transmission3. The United Kingdom Haemophilia Centre Doctors Organisation (UKHCDO) recommends the use of PCC for the treatment of severe spontaneous bleeding and to cover invasive procedures, but caution is needed in situations with high risk of DIC2. The use of PCC is also associated with both arterial and venous thrombotic events, particularly after large or repetitive doses4.
FX:C 0.15 – 0.30 IU/mL is suggested to be haemostatic during surgery2. Little information is available on the management of pregnancy and delivery in severe FX deficiency with only 14 pregnancies in 9 patients described to date (reviewed by Romagnolo et al5). Described complications in these cases include recurrent miscarriage, uterine bleeding, premature labour and post partum haemorrhage. Although the Authors suggest caesarean section to reduce the risk of intracranial/abdominal neonatal haemorrhage, 4 uncomplicated vaginal deliveries have also been described6–8. Spinal haematomas can occur spontaneously in patients with coagulopathies and as a complication of epidural anaesthesia9,10. The report by Rezig et al8, however, suggests that FX:C levels greater than 0.50 IU/mL may be sufficient to permit epidural anaesthesia.
The calibrated automated thrombin generation assay (CAT) is a global coagulation test, that measures the time course of thrombin generation, peak thrombin generation and the endogenous thrombin potential [total amount of thrombin that can be generated in plasma over the time course (ETP11)]. It reflects the action of all coagulation factors leading to thrombin generation and may have a better correlation with the phenotype of hypo and hyper coagulable states than traditional coagulation tests.
Thrombin generation has been reported to correlate with the severity of bleeding in rare coagulation disorders12 and haemophilia13–15 and is thought to reflect hypercoagulable states15,16.
Given the variability in phenotype, uncertainty about the minimal haemostatic FX:C concentration and the association of PCC’s with thrombosis, therefore make CAT measurements of interest in the management of patients with severe FX: deficiency. We describe the use of CAT to monitor treatment with PCC (Beriplex® ) in a patient with severe FX deficiency during epidural anaesthesia and vaginal delivery.
Methods
Blood sampling
The study was approved by the local ethics committee and written consent obtained before venepuncture.
Blood (2.7 mL) was taken using a 21 gauge needle employing minimal suction into Sarstedt S Monovette blood collection tubes (Nuembrecht, Germany) containing 0.3 mL 0.106 M sodium citrate and 0.05 mL corn trypsin inhibitor (1.1 mg/mL).
The latter is necessary to inhibit contact factor activation, when low tissue factor (TF) concentrations are used to trigger the assay17,18. Platelet poor plasma was obtained by double centrifugation at 2,000g for 10 minutes at room temperature and stored at − 80 ºC until testing.
Thrombin generation measurements
Thrombin generation was measured as previously described11. Briefly, 20 μL of a mixture of TF (1 pM final concentration, Innovin® , Dade Behring, Marburg, Germany.) and 4 μM phospholipids (DOPS, DOPE, DOPC in proportion of 20/20/60, Avanti Polar Lipids, Alabama, USA) and 80 μL PPP were manually pipetted in triplicate into 96 well round bottom microtiter plates (Immulon 2HB, U bottom plate, Thermo Lab systems, USA).
Twenty μL of calibrator with 80 μL PPP was pipetted in duplicate into the plate. Each sample used its own calibrator. The plate was then inserted into a Fluoroskan Ascent (Thermolab system, Helsinki, Finland), with an excitation filter at 390 nM and an emission filter at 460 nM. Twenty microliter 2.5 mM fluorogenic substrate (Z-Gly-Gly-Arg-AMC, Bachem, Switzerland) in 2.5% DMSO (Sigma Aldrich, Poole, UK) with 0.1 M CaCl2 prepared freshly was pipetted automatically into all wells, starting the reaction. The fluorescence signal was measured at 20 second intervals over 90 minutes. Thrombin generation was calculated using Thrombinoscope® software v 3.0.0.26 (Synapse b.v., Maastricht, The Netherlands). In all measurements we also used three control plasmas, two normal plasmas and one plasma with low thrombin generation prepared from a pool of anticoagulated samples with a pooled INR of the control of 2.1.
The ETP of two of the three control samples needed to be in a predefined range of the mean ± 2 SD for the results of an experiment to be accepted.
The effect of treatment
The in vitro effect of Beriplex® was assessed by spiking the plasma of a 31 year old lady with FX:C<0.01 IU/mL and life threatening spontaneous intra-abdominal bleeding in the past with 0.0 U/mL (baseline sample), 0.05 U/mL, 0.2 U/mL and 0.6 U/mL Beriplex® .
Thrombin generation was also measured during vaginal delivery and epidural anaesthesia covered with Beriplex® .
We aimed for FX:C > 0.50 IU/mL during delivery until removal of the epidural catheter. Measurements for CAT, FII, FVII, FIX and FX were done at the following time points: Day –1 pre and 20 minutes post treatment with 500 units Beriplex® , day 0 (delivery with epidural) pre and 20 minutes post 1,500 units Beriplex® . A further 500 units was given during delivery, on day 1 and on day 2. No samples for CAT were taken on these occasions. A final sample for CAT was taken on day 3. All measurements were done in parallel.
Coagulation factor assays
FIX was measured by one stage APTT based clotting assay, triggered with SynthaSil® and FII, FVII and FX by one stage PT based clotting assay, triggered with Innovin® .
Normal ranges
Normal ranges were established on 18 healthy women (26 – 54 years, median 41 years) without a history of thrombosis or bleeding and on no medication, including over the counter preparations and the oral contraceptive pill.
Results
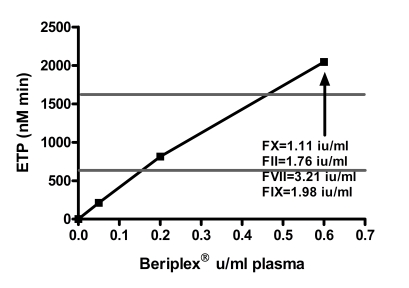
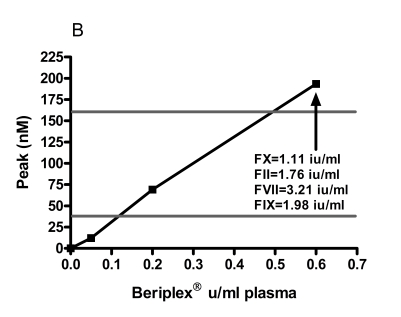
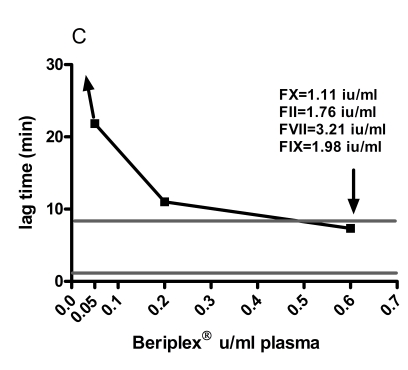
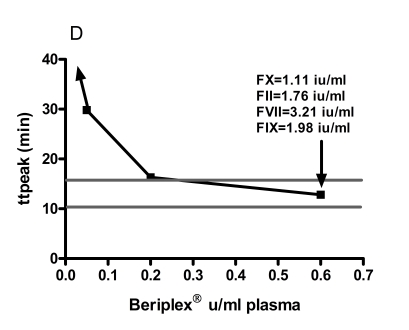
The normal range (mean ± 2 SD) for ETP was 634 – 1,621 nMmin −1, peak thrombin 37.6 – 162.5 nM, lag time 1.2 – 8.5 min, ttpeak 10.4 – 15.8 min. The baseline levels of FII, FVII and FIX and FX of the patient were 0.89 IU/mL, 1.93 IU/mL, 0.82 IU/ mL and <0.01 IU/mL respectively. Spiking with 0.05 IU/mL and 0.6 IU/mL corresponded to measured FX:C levels in the samples of 0.10 and 1.11 IU/mL respectively. The increments of FII, FVII and FIX at spiking with 0.6 IU/mL Beriplex® were 0.83 IU/mL, 1.13 IU/mL and 1.16 IU/mL respectively. Spiking showed a linear relation between the Beriplex® concentration and both ETP and peak. Both reached the normal range at spiking with 0.2 U/mL (FX : C 0.40 IU/mL) and values above the normal range at 0.6 U/mL (FX:C 1.11 IU/mL) plasma concentration. There is an inverse hyperbolic relation with the lag time and ttpeak. The relation between the Beriplex concentration and all thrombogram parameters is shown in figure 1.
Figure 1.
The response of A) ETP, B) peak thrombin generation, C) lag time and D) time to peak to in vitro spiking with increasing concentrations of Beriplex® . The horizontal grey lines represent the normal range for women
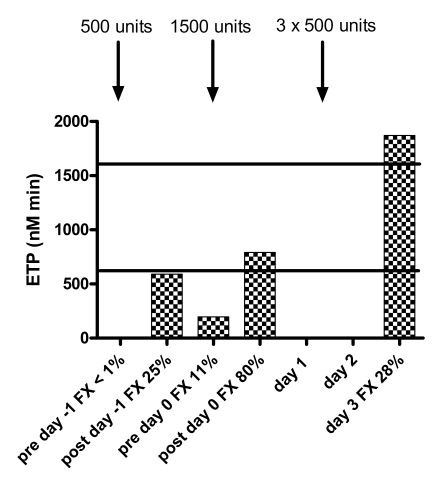
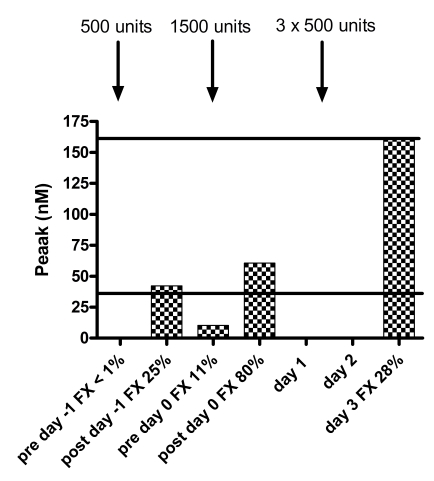
There was an obvious response of all thrombogram parameters to treatment with Beriplex® . Baseline thrombin generation (day -1 pre treatment, FX:C <0.01 IU/mL) was not detectable. After treatment on day -1 (FX:C 0.25 IU/mL), the ETP and peak thrombin generation were borderline normal (ETP 591 nMmin−1, peak 42.3 nM) and prior to treatment on day 0 (FX:C 0.11 IU/mL), both were below the normal range (ETP 195 nMmin−1, peak 10.3 nM) .
After treatment on day 0 (FX:C 0.80 IU/mL), both were within the normal range (ETP 790 nMmin−1, peak 60.6 nM). On day 3 (FX:C 0.28), both were at the upper level of normal (ETP 1870 nMmin−1, peak 162.1 nM). The responses of all thrombogram parameters are shown in figure 2. There was no particular relation with the levels of FII, FVII or FIX to explain the difference in thrombin generation on day -1 after treatment and day 3, despite similar FX levels. The measurements of FII, FVII, FIX and FX are given in table I.
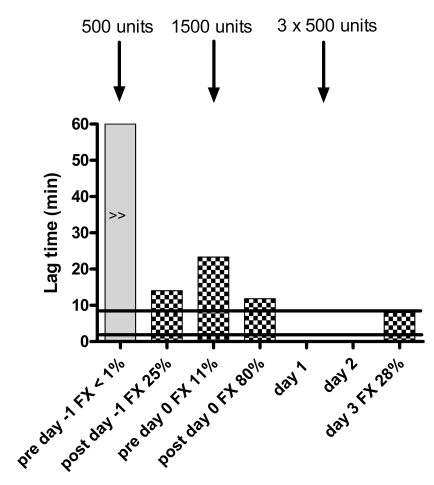
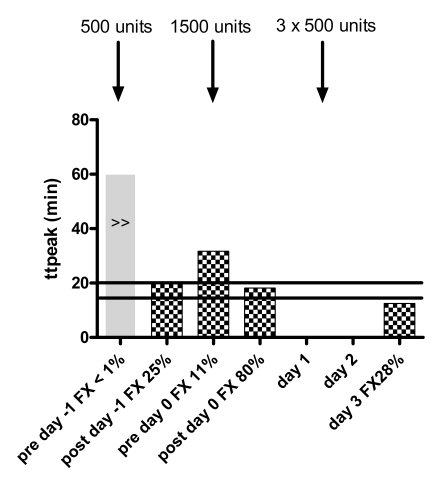
Figure 2.
Response of thrombogram parameters to treatment with Beriplex® . The solid black lines indicate the normal ranges for women. Note absent thrombin generation prior to treatment, normal ETP and peak after treatment to FX=0.80 IU/mL and increased thrombin generation on day 3 with comparable FX levels to day -1. No samples for thrombin generation were taken on day 1 and day 2. No lag time or ttpeak were reached
Table I.
Factor II, VII, IX and X levels at the same time points as thrombin generation measurements
| FII IU/mL | FVII IU/mL | FIX IU/mL | FX IU/mL | |
|---|---|---|---|---|
| Day -1 pre | 0.89 | 1.93 | 0.82 | <0.01 |
| Day -1 post | 1.35 | 2.31 | 1.13 | 0.25 |
| Day 0 pre | 1.09 | 1.75 | 0.86 | 0.11 |
| Day 0 post | 1.53 | 2.05 | 1.14 | 0.80 |
| Day 3 | 1.23 | 1.86 | 1.17 | 0.28 |
Discussion
Our data suggest that CAT may be used to monitor treatment with PCC in FX deficiency. The in vitro spiking experiments showed normalization of both the ETP and peak thrombin generation at a Beriplex® concentration of 0.2 U/mL, equivalent to approximately FX:C 0.40 IU/mL whereas both were clearly below normal at a concentration of 0.05 U/mL (measured FX:C 0.10 IU/mL). Thrombin generation was at the upper limit of normal at a concentration of 0.6 U/mL (measured FX:C 1.11 IU/ mL). This is similar to the response of the ETP and peak thrombin generation after treatment. Both were just below normal on day -1 at FX:C 0.25 IU/mL, levels thought to be haemostatic2, whereas both fully normalized at FX:C 0.80 IU/mL. The latter supports the report by Rezig et al suggesting that FX:C levels of greater than 0.50 IU/mL are needed to safely perform epidural anaesthesia in these patients19. Although a correlation between the severity of the bleeding phenotype and thrombin generation has been reported12–15, studies directly investigating the relation between thrombin generation and bleeding are not available and difficult to perform. The clinical implication of our finding that higher levels of FX than suggested to be necessary for haemostasis were necessary for normalization of thrombin generation in this patient is therefore uncertain. We also found that thrombin generation after multiple doses was significantly higher than after a single dose at a similar FX:C level. This is in keeping with the notion that repeated doses over a short time interval are a risk factor for thrombosis4. There is increasing evidence that accumulation of prothrombin may be a mechanism for the thrombogenicity in PCC20,21 and is important in the mode of action of activated prothrombin complex concentrates22. In our patient, however, prothrombin does not appear to be the main reason for increased thrombin generation, but it rather reflects the interplay between all coagulation factors leading to thrombin generation. Other factors that strongly influence thrombin generation at 1pM TF, and may be important in this respect, include antithrombin23 and tissue factor pathway inhibitor through the protein C independent action of protein S24. Another reason for increased thrombin generation on day three may be related to post partum changes in coagulation. To our knowledge, there is no information in the literature about normal thrombin generation in the peri partum period and it seems unlikely to cause an increase in thrombin generation to this degree. Overall, however, our results indicate that thrombin generation may be used to monitor treatment with PCC and higher levels of FX:C than generally thought may be needed to normalize thrombin generation. Further studies into the relationship between thrombin generation and bleeding are needed. The patient described had an uneventful vaginal delivery and no complications related to the epidural catheter.
References
- 1.Mannucci PM, Duga S, Peyvandi F. Recessively inherited coagulation disorders. Blood. 2004;104:1243–52. doi: 10.1182/blood-2004-02-0595. [DOI] [PubMed] [Google Scholar]
- 2.Bolton-Maggs PH, Perry DJ, Chalmers EA, et al. The rare coagulation disorders—review with guidelines for management from the United Kingdom Haemophilia Centre Doctors’ Organisation. Haemophilia. 2004;10:593–628. doi: 10.1111/j.1365-2516.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 3.UKHCDO. Guidelines on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. Haemophilia. 2003;9:1–23. doi: 10.1046/j.1365-2516.2003.00711.x. [DOI] [PubMed] [Google Scholar]
- 4.Kohler M. Thrombogenicity of prothrombin complex concentrates. [Review] Thrombosis Research. 1999;95:S13–S17. doi: 10.1016/s0049-3848(99)00079-1. [DOI] [PubMed] [Google Scholar]
- 5.Romagnolo C, Burati S, Ciaffoni S, et al. Severe factor X deficiency in pregnancy: case report and review of the literature. Haemophilia. 2004;10:665–8. doi: 10.1111/j.1365-2516.2004.01012.x. [DOI] [PubMed] [Google Scholar]
- 6.Bofill JA, Young RA, Perry KG., Jr Successful pregnancy in a woman with severe factor X deficiency. Obstet Gynecol. 1996;88:723. doi: 10.1016/0029-7844(96)00289-x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar M, Mehta P. Congenital coagulopathies and pregnancy: report of four pregnancies in a factor X-deficient woman. Am J Hematol. 1994;46:241–4. doi: 10.1002/ajh.2830460315. [DOI] [PubMed] [Google Scholar]
- 8.Rezig K, Diar N, Benabidallah D, Audibert J. Factor X deficiency and pregnancy. Ann Fr Anesth Reanim. 2002;21:521–4. doi: 10.1016/s0750-7658(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 9.Hausmann ON, Kirsch EC, Tolnay M, Gratzl O. Intramedullary spinal cord tumours: a clinical outcome and radiological follow-up study. Swiss Med Wkly. 2001 Oct 6;131:582–7. [PubMed] [Google Scholar]
- 10.Heppner PA, Monteith SJ, Law AJ, et al. Spontaneous spinal hematomas and low-molecular-weight heparin. Report of four cases and review of the literature. [Review] J Neurosurg Spine. 2004;1:232–6. doi: 10.3171/spi.2004.1.2.0232. [DOI] [PubMed] [Google Scholar]
- 11.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 12.Al Dieri R, Peyvandi F, Santagostino E, et al. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost. 2002;88:576–82. [PubMed] [Google Scholar]
- 13.Beltran-Miranda CP, Khan A, Jaloma-Cruz AR, Laffan MA. Thrombin generation and phenotypic correlation in haemophilia A. Haemophilia. 2005;11:326–34. doi: 10.1111/j.1365-2516.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 14.Dargaud Y, Beguin S, Lienhart A, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93 :475–80. doi: 10.1160/TH04-10-0706. [DOI] [PubMed] [Google Scholar]
- 15.Hemker HC, Giesen P, AlDieri R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–53. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 16.Hron G, Kollars M, Binder BR, et al. Identification of Patients at Low Risk for Recurrent Venous Thromboembolism by Measuring Thrombin Generation. JAMA. 2006 Jul 26;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 17.Luddington R, Baglin T. Clinical measurement of thrombin generation by calibrated automated thrombography requires contact factor inhibition. J Thromb Haemost. 2004;2:1954–9. doi: 10.1111/j.1538-7836.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 18.van Veen JJ, Gatt A, Cooper PC, et al. Corn trypsin inhibitor in fluorogenic thrombin generation measurements is only necessary at low tissue factor concentrations and influences the relationship between FVIII:C and thrombogram parameters. Blood Coagul Fibrinolysis. 2007 doi: 10.1097/MBC.0b013e3282f4bb47. in press. [DOI] [PubMed] [Google Scholar]
- 19.Rezig K, Diar N, Benabidallah D, Audibert J. [Factor X deficiency and pregnancy] Ann Fr Anesth Reanim. 2002;21:521–4. doi: 10.1016/s0750-7658(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 20.Grundmann C, Plesker R, Kusch M, et al. Prothrombin overload causes thromboembolic complications in prothrombin complex concentrates:In vitro and in vivo evidence. Thromb Haemost. 2005;94:1338–9. [PubMed] [Google Scholar]
- 21.Dusel CH, Grundmann C, Eich S, et al. Identification of prothrombin as a major thrombogenic agent in prothrombin complex concentrates. Blood Coagul Fibrinolysis. 2004;15:405–11. doi: 10.1097/01.mbc.0000114437.81125.2b. [DOI] [PubMed] [Google Scholar]
- 22.Gallistl S, Cvirn G, Leschnik B, et al. Respective roles of factors II, VII, IX, and X in the procoagulant activity of FEIBA. Blood Coagulation & Fibrinolysis. 2002;13 :653–5. doi: 10.1097/00001721-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Butenas S, van’t Veer C, Mann KG. “Normal” thrombin generation. Blood. 1999 Oct 1;94:2169–78. [PubMed] [Google Scholar]
- 24.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. PNAS. 2006 Feb 28;103:3106–11. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]