Because of the high failure rate after renal homotransplantation, there has been an air of pessimism concerning the possibility of long term function of the grafted kidney. The immunologic processes subserving rejection are generally thought to be so powerful and persevering that consistent success cannot be expected with the use of any of the currently available methods of antirejection therapy.
Recent personal experience in caring for patients with renal homografts has resulted in alterations in many of our preconceived notions concerning the management of such patients. It has led to the beliefs that the rejection process can almost never be entirely prevented, but that its effects can be reversed with a high degree of regularity and completeness. Furthermore, the subsequent behavior of patients who have been brought through a successfully treated rejection crisis suggests the early development of some degree of host-graft adaptation, since the phenomenon of vigorous secondary rejection has been encountered only once.
METHODS
The material consists of our first 10 patients who received renal homografts from living donors. All recipients were males. The operations were performed between 24 November 1962 and 15 May 1963. The patients have been followed up to 1 July 1963. Excluded from consideration are 2 patients in whom cadaveric kidneys were used as well as a homotransplantation between identical twins, which has been described previously (16). The 10 patients have in common that the homografts were previously normal organs and that injury during transfer was minimized by the provision of cooling during the average ischemic period of 39 minutes. There was one donation from mother to son, and there were 5 from siblings, including 2 pairs of fraternal twins. In the other 4 patients, there was no genetic relationship (Table I). In half the patients, the donor was of a different blood type than the recipient and in 4, of a different sex, factors which did not materially influence the results.
TABLE 1.
DATA REGARDING 10 PATIENTS WHO RECEIVED RENAL HOMOGRAFTS FROM LIVING DONORS
| Rejection crisis | ||||
|---|---|---|---|---|
| Patient No. | Date of transplant | Donor | Onset | Duration |
| 1 | 11-24-62 | Mother | 14th day | 20 days |
| 2 | 1-30-63 | Sister | 25th day | 31 days |
| 3 | 2- 9-63 | Frat. twin | None | |
| 4 | 2-25-63 | Wife | Unable to assess | |
| 5 | 3-26-63 | Unrelated | Early death | |
| 6 | 4-17-63 | Brother | 18th day | 19 days |
| 7 | 5- 3-63 | Wife | 18th day | 27 days |
| 8 | 5- 8-63 | Brother | 4th day | 35 days |
| 9 | 5- 10-63 | Frat. twin | 5th day
34th day |
10 days
Receding |
| 10 | 5- 15-63 | Unrelated | 4th | Receding |
Transplantation was performed with the technique of Murray and Harrison (12), and the kidney was placed in the contralateral iliac fossa. One to 19 days before operation, daily administration of azathioprine (imuran) in doses of 3 to 15 milligrams per kilogram of body weight was started in all but Patient 1. Patient 1 was initially treated with total body irradiation (Fig. 1), and the administration of azathioprine was begun in the postoperative period. An attempt was made to provide the maximum daily dose which would not produce leukopenia. Except when its discontinuance became mandatory because of the development of prolonged agranulocytosis, azathioprine was given each day for the entire subsequent course. At the time of rejection crisis, the dose was temporarily increased in most cases (Figs. 2 and 3). If complete reversal of rejection could not be achieved with the addition of the secondary drug therapy to be described, the dose of azathioprine was used to induce temporary leukopenia deliberately. This extreme measure was necessary in only 2 patients—Patients 2 and 7.
Fig. 1.
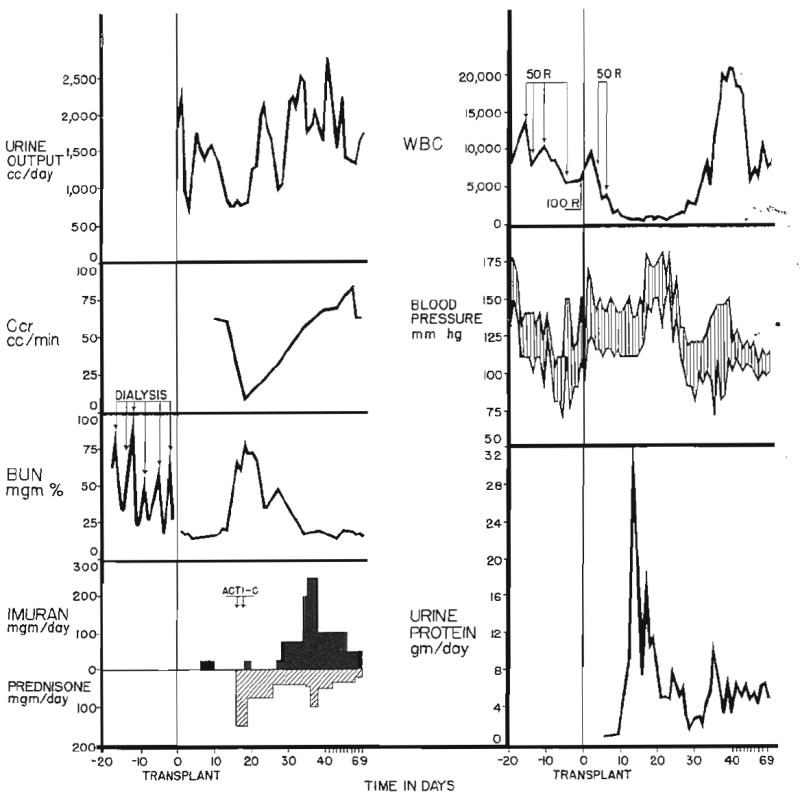
Rejection crisis in Patient 1. Note transient oliguria, depression of creatinine clearance, and elevation in blood urea nitrogen, blood pressure, and urinary protein excretion. The changes were all reversible. The patient previously had undergone bilateral nephrectomy, splenectomy, and thymectomy. R, Dose total body irradiation; Acti-C, actinomycin C, each arrow equals 200 micrograms of actinomycin C administered intravenously. Imuran is synonymous with azathioprine.
Fig. 2.
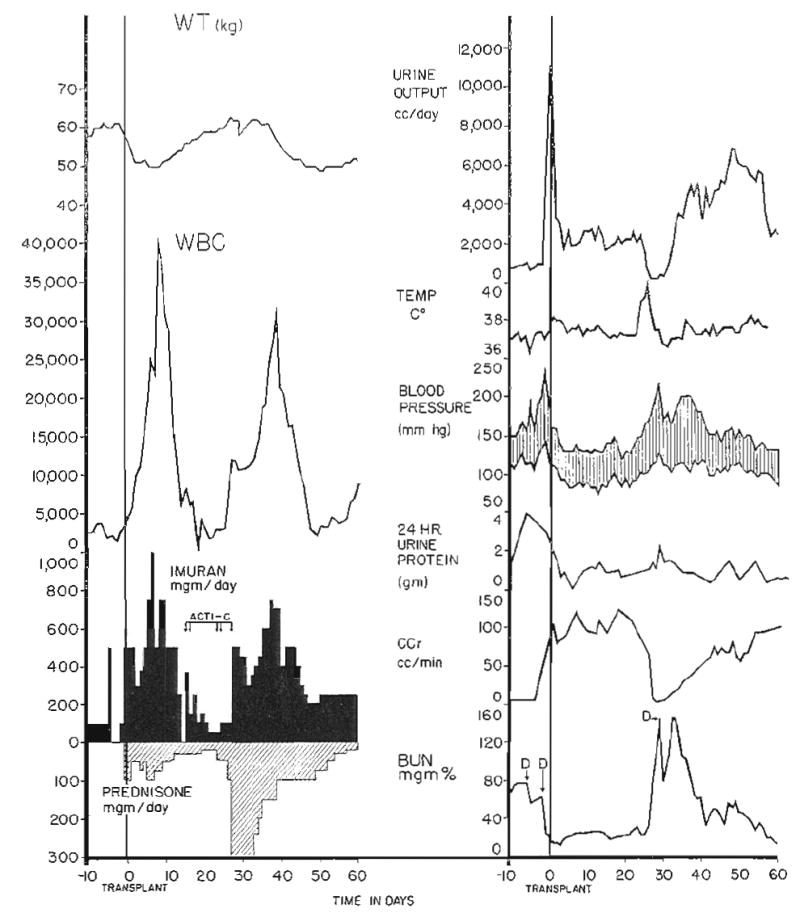
Rejection crisis in Patient 2. Note temporary deterioration of renal function, coincident with fever and weight gain starting on the twenty-fifth day after transplantation. The patient was essentially anuric for 3 days. Acti-C, Actinomycin C, each arrow equals 200 micrograms; D, diaysis. Imuran is synonymous with azathioprine.
Fig. 3.
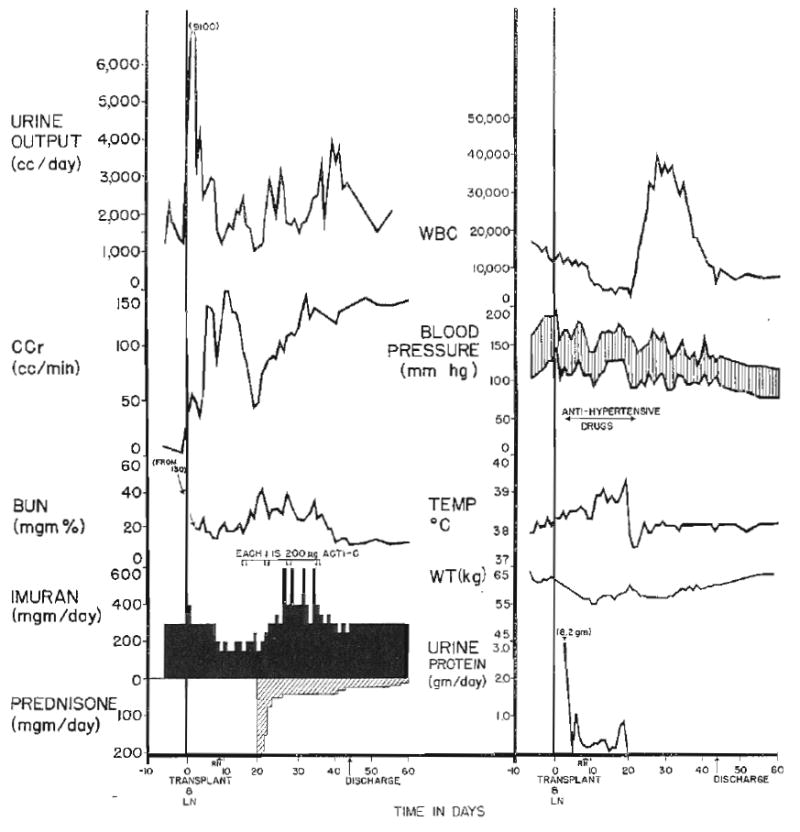
Rejection crisis in Patient 6. Deterioration of renal function began 19 days after transplantation. All stigmas of rejection are present except for acute hypertension and weight gain, which were successfully prevented by medical treatment. Acti-C, Actinomycin C; LN, left nephrectomy at time of transplantation; RN, right nephrectomy. Imuran is synonymous with azathioprine.
Prednisone was used for secondary antirejection therapy in all but Patient 3. At the time of active rejection, doses of 150 to 400 milligrams per day were employed (Figs. 1 to 3), either starting at this level, or with a sharp increase in dose as in Patients 2 and 7, who were already receiving maintenance steroid therapy (Fig. 2). As soon as clear evidence was available that reversal of rejection had occurred, the dose of prednisone was reduced. Antacid therapy was vigorously applied to prevent the development of steroid ulcers. Patient 4 differed from the others in that he was given large doses—100 milligrams per day—of prednisone from the time of transplantation (Fig. 4), rather than for the specific treatment of a rejection crisis.
Fig. 4.
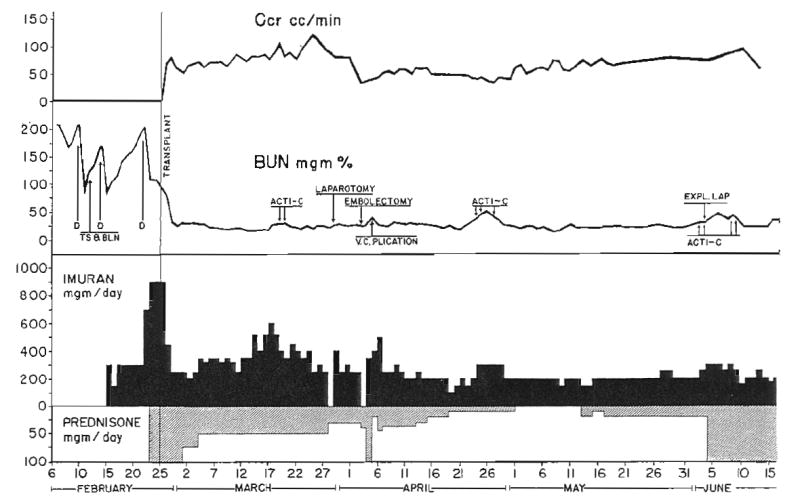
Course of Patient 4, the only patient who received large continuous prophylactic doses of prednisone from the time of operation. Despite provision of kidney by a genetically nonrelated donor, a definite rejection crisis did not occur. D, Dialysis; TS & BLN, thymectomy, splenectomy, and bilateral nephrectomy; Acti-C, actinomycin C, each arrow equals 200 micrograms of actinomycin C administered intravenously. Patient ultimately died of unrecognized intra-abdominal abscess.
During a rejection crisis, actinomycin C was given intravenously in a dose of 400 micrograms at intervals of 4 to 7 days.
In addition to the pharmacologic treatment of rejection, the host lymphoid mass was reduced by surgical measures either prior to or at the time of transplantation. All 10 patients underwent splenectomy and all but Patients 7, 8, and 10 also underwent thymectomy.
RESULTS
Eight of the 10 recipients are alive on 1 July 1963, from 218 to 46 days after transplantation. A ninth patient, Patient 4, died 113 days after operation from an unrecognized intra-abdominal abscess. The tenth patient, Patient 5, had cardiac arrest during transplantation which was successfully treated, but he died 10 days later of mediastinal cellulitis and septicemia.
Diagnosis of rejection
The diagnosis of rejection crisis is relatively easy provided that the homograft has functioned well from the beginning. In all but Patient 5 there was an immediate diuresis and prompt resolution of the pre-existing azotemia. A variable period of normal renal function then ensued, lasting from 4 to 25 days, which was ultimately interrupted by an episode of acute renal failure. This characteristic sequence, rather than any single symptom or finding, was the most easily recognized feature of a rejection crisis. The use of damaged donor organs, in which acute tubular necrosis may complicate the picture, leads to confusion in planning therapy. We (17) have observed this in the employment of cadaveric kidneys.
Several consequences of functional deterioration of the homograft were regularly observed (Figs. 1 to 3). The patients became oliguric or anuric. Severe hypertension developed. Urinary sodium excretion abruptly declined to as low as 8 milliequivalents per liter. Fluid retention and weight gain followed. The blood urea nitrogen rose and the creatinine clearance fell, often to less than 10 milliliters per minute. The urine protein excretion was increased. There was variability in the rapidity with which the rejection syndrome developed. In some cases, several days were required. In others, the crisis was violent in onset, occurring within a few hours. In 1 patient, Patient 2, an intravenous pyelogram obtained just before a severe rejection attempt revealed prompt and concentrated dye excretion (Fig. 5). Within the next 24 hours, a severe rejection crisis had fully developed (Fig. 2).
Fig. 5.
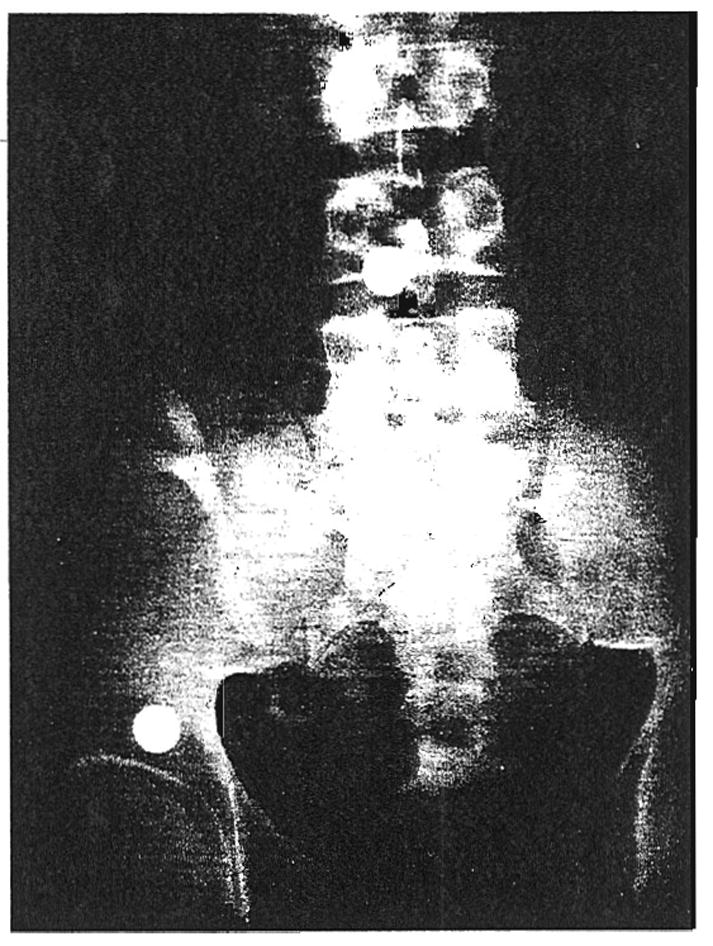
Four minute intravenous pyelogram or Patient 2 on 23 February 1963, 24 days after renal transplantation to the right iliac fossa. Note prompt and concentrated dye excretion. Within 24 hours after this roentgenogram was taken, the patient experienced a severe rejection crisis and became essentially anuric. Recovery was ultimately complete.
There was also a marked febrile systemic response at the time of a rejection attempt. Temperatures often rose to 40 degrees C or higher, with accompanying tachycardia. Malaise and mental depression were observed, and 2 patients made suicidal gestures at this time. Leukocyte counts were often strikingly elevated to as high as 45,000.
Incidence of rejection crisis
Nine of the 10 patients survived the immediate postoperative course. Of these, 7 experienced a severe and unequivocal rejection crisis. An eighth patient, who received a homograft from his wife, never had a definite episode of rejection (Fig. 4). This patient was the only one in the series who received massive steroid therapy prophylactically, 100 milligrams of prednisone per day. Histologic studies of his homograft at autopsy, 113 days after transplantation, showed excellent preservation of architecture (Fig. 6). A few scattered foci of mononuclear cells were present. It is noteworthy that renal function was adequate until death resulted from sepsis. Patients 2 and 7 received smaller prophylactic doses of prednisone—30 milligrams per day. In the latter patients, there was no demonstrable deterrent effect against rejection.
Fig. 6.

Autopsy specimen of renal homograft in Patient 4. Renal function had been adequate until death from sepsis 113 days after transplantation. Note good preservation of renal architecture. Only a few scattered mononuclear cells were present. X 39.
The other patient in the series who did not undergo an episode of rejection, Patient 3, received a kidney from his fraternal twin. There was a high degree of blood typing compatibility between the twins, extending to 21 of 23 minor subgroups. The other fraternal twin in the series, Patient 9, passed through an early severe rejection crisis and is currently recovering from a vigorous secondary rejection attempt.
Incidence of recurrent rejection crisis
A clear-cut secondary recurrence of renal failure was seen only in Patient 9. A presumed rejection crisis began on the fifth day and was readily reversed (Table I). On the thirty-fourth day after transplantation, an almost identical episode occurred, with fever and deterioration of renal function. Reversal of rejection was considerably more difficult on the second than on the first occasion, and the completeness of remission in this patient is still in doubt.
The predictability of rejection reversal
Each of the 8 patients whose homograft was threatened by rejection was successfully treated, in terms of improvement of renal function. The promptness of a demonstrable response to therapy was variable. Although the return of renal function did not occur immediately, return to a normothermic state was invariably noted within a few hours after the administration of prednisone was started.
There was value in studying several parameters of renal function during recovery from a rejection crisis. Blood ureanitrogen was often slow to return to completely normal values, the decline stopping at 30 to 40 milligrams per cent. Usually this was explicable by excessive protein break-down produced by the massive steroid therapy, since creatinine clearance and total urea excretion were normal at this time. The blood urea nitrogen returned to normal under these circumstances as the doses of steroids were reduced.
The degree of permanent functional damage to the kidney was either minimal or undetectable in the cases available for the longest follow-up. The most serious sequela was seen in Patient 1, consisting of the excretion of 2 to 4 grams of protein per day. Even in this patient, however, the blood urea nitrogen and creatinine clearances are normal. An intravenous pyelogram obtained after reversal of the rejection revealed prompt concentration of the dye (Fig. 7).
Fig. 7.
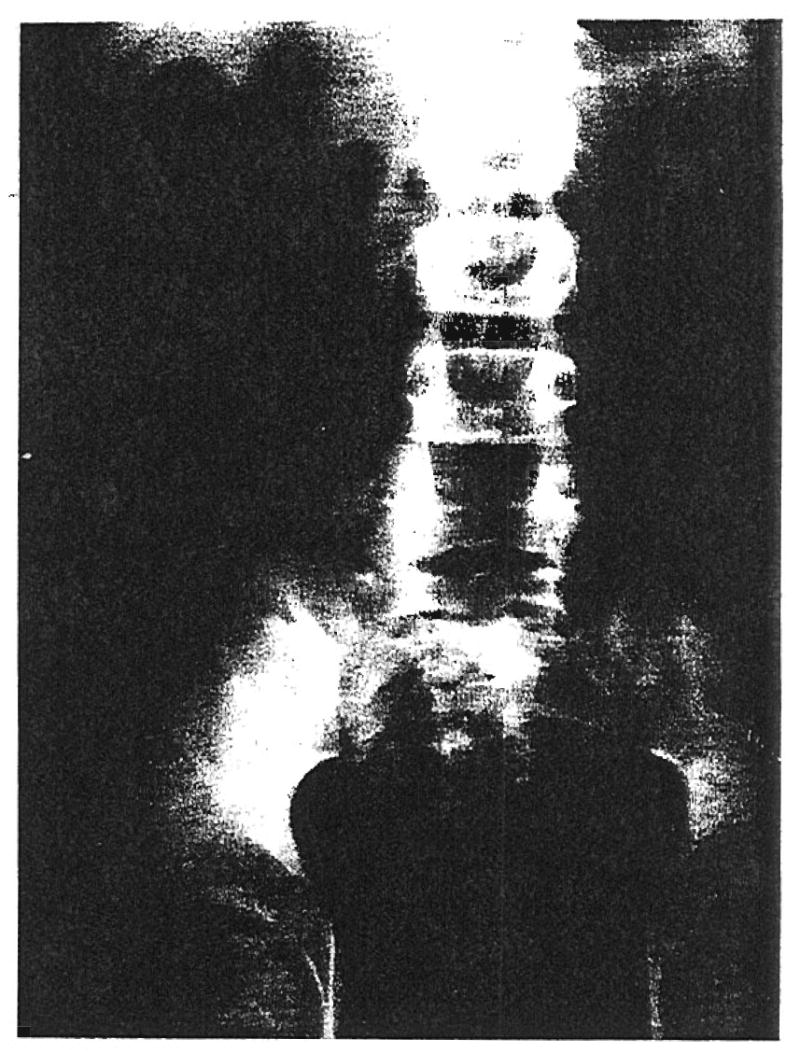
Three minute intravenous pyelogram of Patient 1 on 28 January 1963, 9½ weeks after transplantation and 4 weeks after complete recovery from a rejection crisis. Note prompt and concentrated dye excretion in the kidney which is located in the right iliac fossa.
DISCUSSION
As commonly used, the term rejection carries an implication of finality. The observations herein reported indicate that this phenomenon is not necessarily such a decisive event. It has been demonstrated that the features of rejection can be reversed in most cases in humans, even when the degree of acute renal functional impairment is profound. The extent of permanent histologic damage produced by such an immunologic crisis is not known in the surviving patients, inasmuch as renal biopsies have understandably not been performed. However, the functional capacity of these salvaged homografts has eventually returned to essentially normal standards in most cases.
The fact that rejection is not an all or none phenomenon was first suggested by the retransplantation experiments in dogs of Balankura and his associates. It was found that renal homografts which were undergoing active rejection resumed function and their normal histologic appearance was restored, if they were retransplanted back to the original donor. Subsequent isolated observations by Calne (2), Murray (11, 13, 14), Goodwin (4), Hume (7), and their associates have suggested the possibility of reversing a homograft reaction, in either dogs or man.
It is hoped that increased knowledge about the reversibility of rejection will allow wider application of the use of homografts than has been possible in the past. In addition, the prior knowledge that a rejection crisis is almost a certainty and that it can usually be managed by relatively conservative means should serve as a deterrent to the excessive early use of measures which may cause fatal bone marrow depression. The data collected by Goodwin and Martin (5) on all known cases of renal homotransplantation suggest that the natural tendency to apply marrow-depressant therapy to prevent the onset of rejection may have contributed heavily to unfavorable mortality statistics in the past.
Successful treatment of a rejection crisis is evidently dependent upon the synergistic action of a combination of agents which are individually ineffective. The most important drug in the regimen is azathioprine, an imidazole derivative of 6-mercaptopurine which was first used by Calne and Murray (3) to potentiate homograft survival. In most cases, azathioprine was given as the sole therapy until the beginning of rejection in doses which did not cause leukopenia. With the onset of homograft deterioration, it is mandatory that azathioprine be continued without a decrease and preferably with an increase in dose. This is possible only if previous therapy has not caused suppression of the leukocyte count. Prednisone and actinomycin C are then added.
If the combination of agents does not result in satisfactory reversal of clinical rejection, the doses of azathioprine are increased until the leukocyte count has fallen to low levels. This extreme measure has been necessary in 2 of the cases. When azathioprine is used as described, the maximum doses in such refractory cases are reserved for the time of greatest need, during the effort to reverse the events of rejection. If leukopenia results from this therapy, it is important to continue azathioprine at a reduced dose level despite the risk of producing fatal agranulocytosis rather than to discontinue it altogether. Experience at this and other clinics has demonstrated that the homograft rejection can proceed with great vigor in spite of the presence of leukopenia, if day to day antirejection therapy is not provided.
Of the secondary drugs, prednisone is probably the most important in altering the acute febrile illness which is precipitated by a rejection crisis. Temperature returns to normal within a few hours, and there is usually prompt evidence of improved renal function. The almost miraculous effect of prednisone at this critical time was first noted by Goodwin and his associates (4) in a recently reported clinical case, and confirmed by Marchioro and his colleagues (8), who used dogs in experiments designed specifically to test the effect of steroids in reversing rejection. The dose is probably important in obtaining the desired effect. In Patients 2 and 7 of the present series, 30 milligrams of prednisone per day were given before and after transplantation despite which rejection proceeded. An increase in dose to 200 milligrams per day as well as the addition of actinomycin C promptly alleviated the symptoms. In a third case doses of 100 milligrams per day were started before transplantation, and the rejection attempt was masked. It would, therefore, seem reasonable to believe that 30 milligrams per day is insufficient, and that doses of 100 milligrams per day or more are required. Stringent antacid therapy is a prerequisite with such intensive steroid therapy.
The value of actinomycin C in reversal of rejection was first demonstrated by Calne, Alexandre, and Murray (2), and confirmed in clinical use by Murray and his associates (14) and in the present series. The therapeutic mechanism of this drug, which has a selective cytocidal action on lymphocytes, has not yet been clarified. It is possible that it inhibits subtle chronic damage to the kidney by acting against parenchymal lymphocytic infiltrates already present at the time of the rejection crisis. It is noteworthy that both the secondary drugs, actinomycin C and prednisone, can be used without fear of confusing the status of the bone marrow injury since neither is a depressant drug.
The general medical care of the patient during the rejection crisis is of the utmost importance. The elevation of blood pressure which occurs at this time is treated with the usual antihypertensive drugs. With aggressive therapy, the hypertension can be prevented altogether in some patients (Fig. 3). Inadequate control of the blood pressure may result in irreversible parenchymal damage, as described by Parsons and his colleagues, and as recently observed by us (9) in a cadaveric homograft. It is usually necessary to restrict salt and fluids. In the present series, acute pulmonary edema developed in Patient 8 because of failure of early fluid limitation.
The clinical course of those patients who have survived a rejection crisis deserves special comment. A state of relative immunologic nonreactivity seems to have been produced, which has lasted for as long as 6 months. After successful management of the attempted rejection, the same drug regimen which did not prevent the initial attack on the homograft has been adequate for maintenance therapy. It is not known whether this is due to a change in the antigenic properties of the homograft or to an alteration in the specific antibody response to the stimulus of the grafted tissue. The apparent host-graft adaptation does, however, provide some hope for prolonged functional survival.
Two important questions remain unanswered from the present study. The first concerns the biologic significance of the rejection crisis as it applies to the long term survival of the homograft. If the graft repudiation can be reversed by the addition of prednisone and actinomycin C to pre-existing azathioprine therapy, it might seem reasonable to administer these agents prophylactically from the time of transplantation. This approach may eventually prove to be advisable. It is also conceivable, however, that the avoidance of a primary host-graft reaction by these means would prevent the adaptive process alluded to previously.
The other unanswered question concerns the possible role of thymectomy and splenectomy, first, in rendering the rejection crisis more controllable, and, second, in contributing to the subsequent state of host-graft nonreactivity. Miller and Gunderson and their colleagues have published the respective suggestions that the thymus and spleen govern to some extent the organization of immunologic response to antigenic stimuli under special experimental conditions. The importance, if any, of these adjuvant surgical measures in the achievement of a favorable outcome will eventually be clarified by comparison of the present results with other clinical series in which thymectomy or splenectomy have not been performed.
The application of the foregoing concepts to other than renal homografts may be of importance in the future. With the kidney, the functional status of the graft can be followed with precision following the specific parameters of urine volume, blood urea nitrogen, creatinine clearance, urine protein and urea, and blood pressure. The onset of rejection can be defined with great accuracy, and the indicated treatment instituted. The use of pulmonary, hepatic, spleen, cardiac, and endocrine homografts will each require the establishment of specific measurements to guide appropriate antirejection therapy. It would seem probable that the principles of reversal of rejection, as defined with the kidney, can eventually be applied to other organ homografts.
Although recognition that the rejection reaction is a highly reversible phenomenon constitutes an important step in the ultimate solution of the homograft problem, it would be erroneous to conclude that the present methods of therapy are anything but imperfect. The regimen used is not selective enough to permit acceptance of the homograft without seriously endangering the host at some time during the course, most commonly at the time of the rejection crisis. The rejection reaction can be controlled, but sometimes only at the expense of fatal sepsis, as in Patients 4 and 5.
SUMMARY
The rejection process in humans receiving renal homografts can seldom be entirely prevented. At varying periods after operation, acute deterioration of renal function signals the onset of graft repudiation, accompanied by fever and other evidence of a systemic response. The physiological derangements attending such a rejection crisis can be regularly reversed by the addition of actinomycin C and massive doses of prednisone to pre-existing therapy with azathioprine. In 1 of the 10 patients, a similar reversal was possible by the addition of actinomycin C and prednisone in a patient previously treated with total body irradiation. The degree of functional recovery has been essentially complete in those patients who could be followed up and observed carefully for the longest periods.
A state of host-graft nonreactivity has seemed to follow the successful treatment of such a rejection crisis. Resumption of the same therapy which failed to prevent the initial attack on the graft has been sufficient to prevent a secondary rejection attempt in all but 1 case.
Acknowledgments
Aided by Grants A-6283, A-6344, HE 00735-01, and AI-94152 from the U. S. Public Health Service.
References
- 1.Balankura O, Goodwin WE, Murray JE, Dammin GJ. Surgical Forum; Clinical Congress 1960. XI. Chicago: American College of Surgeons; 1960. Study of the homograft reaction by retransplantation of the canine kidney; p. 24. [PubMed] [Google Scholar]
- 2.Calne RY, Alexandre GPJ, Murray JE. A study of the effects of drugs in prolonging survival of homologous renal transplants in dogs. Ann N York Acad Sc. 1962;99:743. doi: 10.1111/j.1749-6632.1962.tb45358.x. [DOI] [PubMed] [Google Scholar]
- 3.Calne RY, Murray JE. Surgical Forum; Clinical Congress 1961. XII. Chicago: American College of Surgeons; 1961. Inhibition of the rejection of renal homografts in dogs by Burroughs Wellcome 57-322; p. 118. [PubMed] [Google Scholar]
- 4.Goodwin WE, Kaufman JJ, Mims MM, Turner RD, Glassock R, Goldman R, Maxwell MM. Human renal transplantation – I, clinical experiences with 6 cases of renal homotransplantation. J Urol Balt. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin WE, Martin DC. Transplantation of the kidneys. J Urol Balt. 1963 spring; in press. [PubMed] [Google Scholar]
- 6.Gunderson CM, Juras D, La Via MF, Wissler RW. Tissue and cellular changes associated with antibody formation in the rat spleen. J Am M Ass. 1962;180:1038. [PubMed] [Google Scholar]
- 7.Hume DM, Magee JM, Kauffman MJ, Jr, Rittenbury MS, Prout GA. Renal homotransplantations in man in modified recipients. Ann Surg. doi: 10.1097/00000658-196310000-00010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchioro TL, Axtell HK, La Via MF, Waddell WR, Starzl TE. The role of adrenocortical steroids in reversing established homograft rejection. Surgery. in press. [PMC free article] [PubMed] [Google Scholar]
- 9.Marchioro TL, Huntley RT, Waddell WR, Starzl TE. The use of extracorporeal perfusion for obtaining post mortem hornografts. Surgery. in press. [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JFAP. Immunologic significance of the thymus of the adult mouse. Nature, Lond. 1962;195:1318. [Google Scholar]
- 11.Murray JE, Balankura O, Greenberg JB, Dammin GJ. Reversibility of the kidney homograft reaction by retransplantation and drug therapy. Ann N York Acad Sc. 1962;99:768. doi: 10.1111/j.1749-6632.1962.tb45360.x. [DOI] [PubMed] [Google Scholar]
- 12.Murray JE, Harrison JH. Management of 50 patients with kidney transplants including 18 pairs of twins. Am J Surg. 1963;105:205. doi: 10.1016/0002-9610(63)90292-7. [DOI] [PubMed] [Google Scholar]
- 13.Murray JE, Merrill JP, Dammin GJ, Dealy JB, Alexandre GW, Harrison JH. Kidney transplantation in modified recipients. Ann Surg. 1962;156:337. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N England J M. 1963;268:1315. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 15.Parsons FM, Markland C, Raper FP, Fox M. Cadaveric renal transplantation. Brit M J. 1963;1:930. [Google Scholar]
- 16.Starzl TE, Brittain RS, Stonnington O, Coppinger W, Waddell WR. Renal transplantation in identical twins. Arch Surg. 1963;86:600. doi: 10.1001/archsurg.1963.01310100084013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Marchioro TL, Holmes JH, Brittain RS, Hermann G, Stonnington O, Knight ICS, Talmage DW, Waddell WR. A study of current clinical problems involved in renal homotransplantation. J Am M Ass. in press. [Google Scholar]


