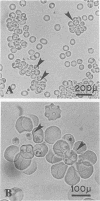
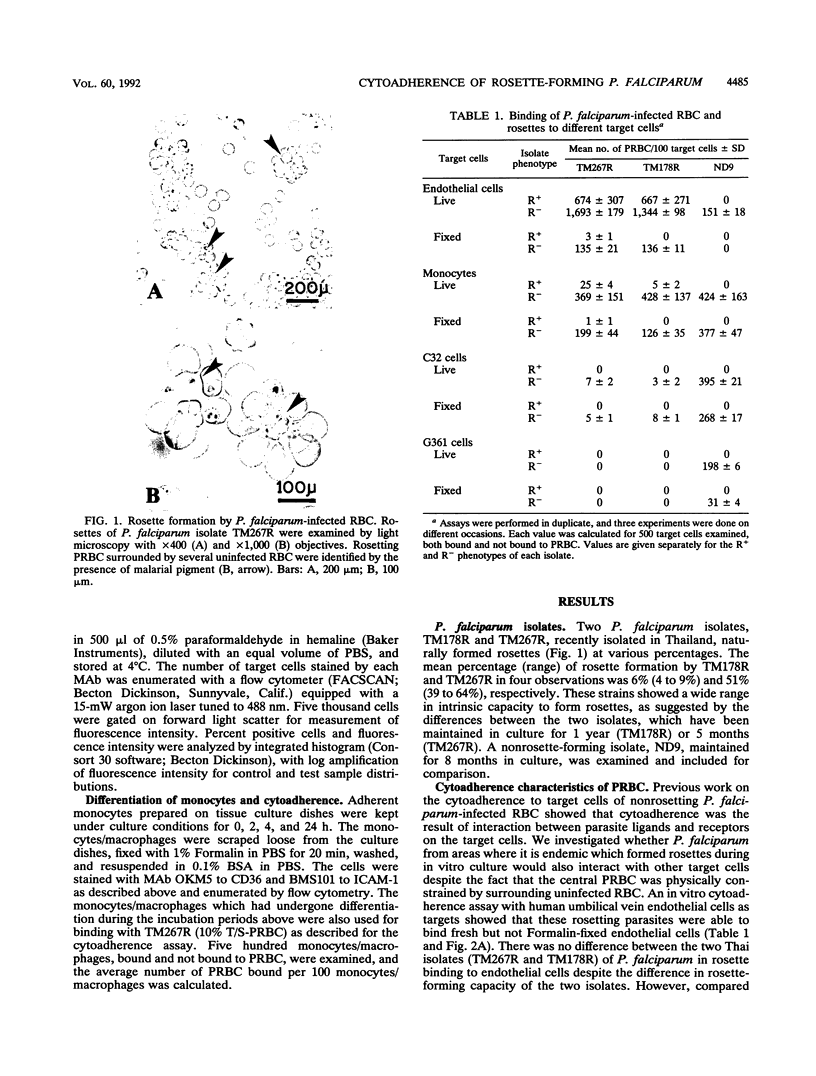
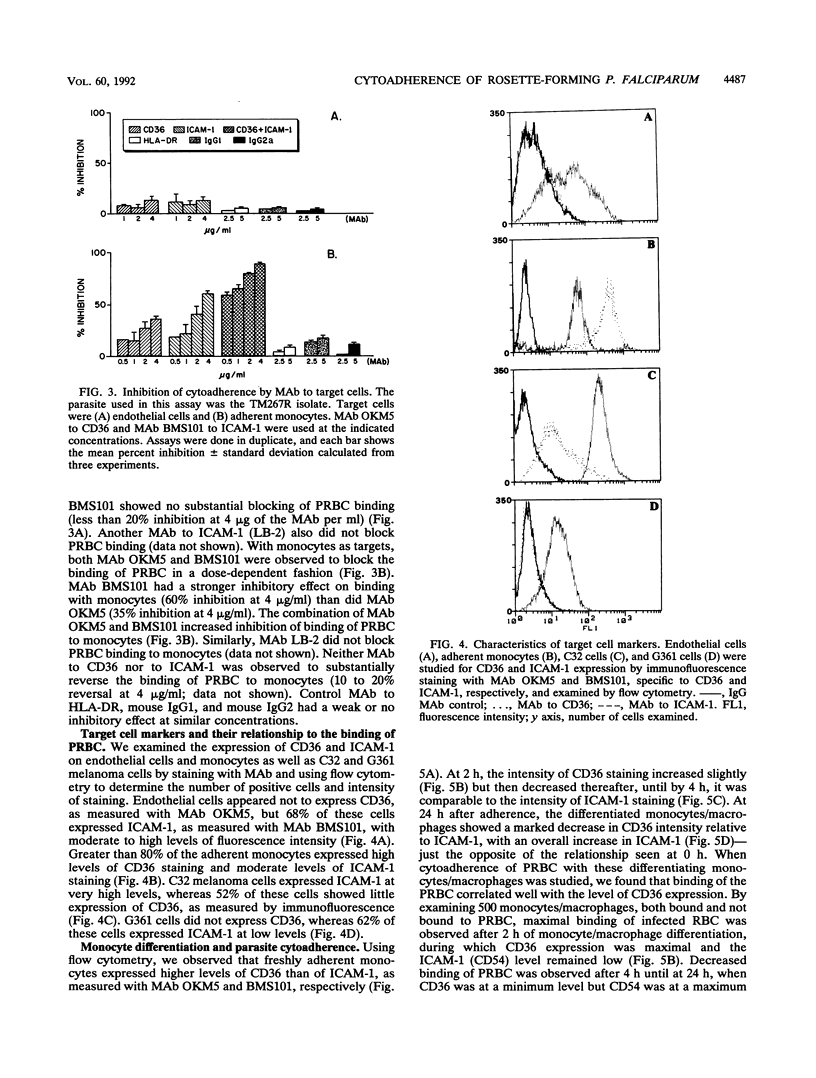
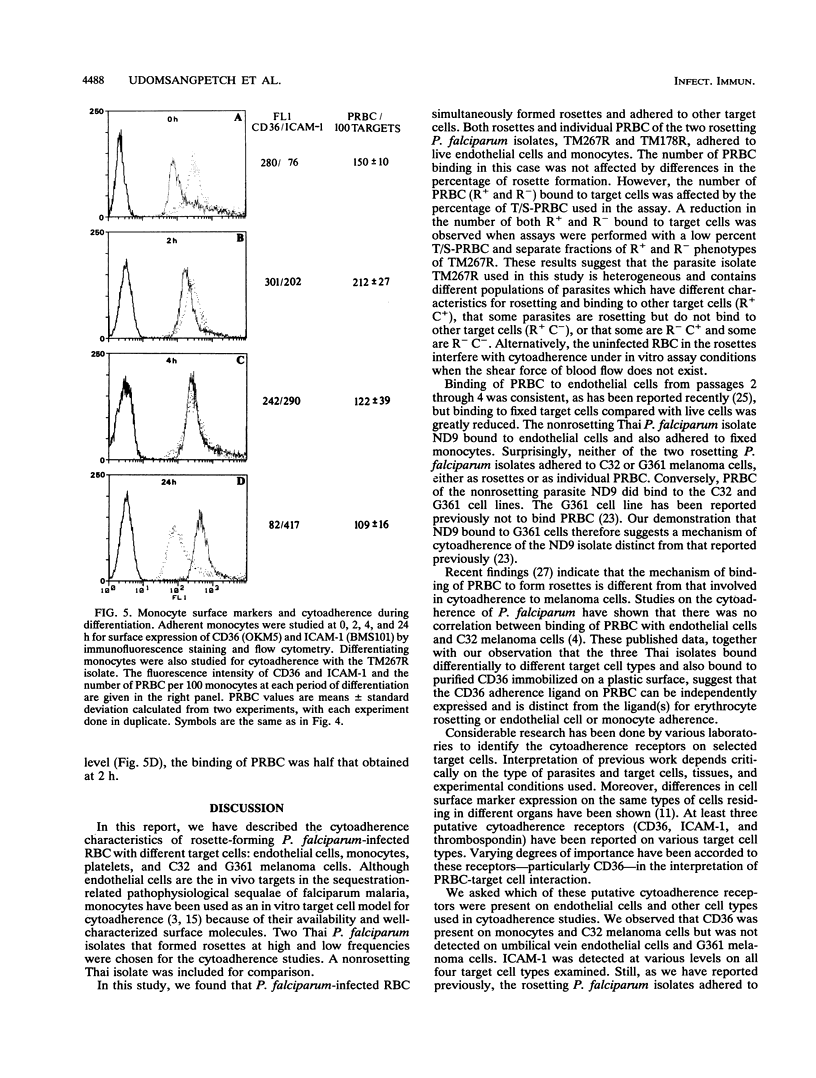
Abstract
Sequestration of Plasmodium falciparum-infected erythrocytes to the capillary endothelium can cause obstruction and localized tissue damage. Occlusion of vessels in falciparum malaria infection has been related to two properties of the parasite: adhesion to endothelial cells and rosette formation. Our study on P. falciparum isolates from Thailand producing variable numbers of rosettes suggests the involvement of rosettes in capillary blockage caused by direct adhesion of the rosette-forming infected erythrocytes to various target cells, e.g., live human umbilical vein endothelial cells, monocytes, and platelets. These rosettes did not bind Formalin-fixed target cells, nor did they bind to live or fixed C32 or G361 melanoma cells. Classification of the receptors involved in cytoadherence of endothelial cells and monocytes by specific antibody blocking and flow cytometry indicated that CD36 was involved in the adherence of monocytes but that other receptors besides CD36 may be involved in parasite adherence to endothelial cells. The cytoadherence of infected erythrocytes to monocytes was also associated with CD54 (ICAM-1). Further, differentiation of adherent monocytes resulted in an inversion of CD36 and CD54 levels on the cell surface which correlated with a decrease in surface binding of infected erythrocytes. This observation suggests that the state of cell activation and differentiation may also contribute to sequestration of parasites and to the pathogenesis of malaria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988 Jul;39(1):3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- Barnwell J. W., Asch A. S., Nachman R. L., Yamaya M., Aikawa M., Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest. 1989 Sep;84(3):765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell J. W., Ockenhouse C. F., Knowles D. M., 2nd Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum-infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J Immunol. 1985 Nov;135(5):3494–3497. [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Cranston H. A., Boylan C. W., Carroll G. L., Sutera S. P., Williamson J. R., Gluzman I. Y., Krogstad D. J. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science. 1984 Jan 27;223(4634):400–403. doi: 10.1126/science.6362007. [DOI] [PubMed] [Google Scholar]
- Handunnetti S. M., David P. H., Perera K. L., Mendis K. N. Uninfected erythrocytes form "rosettes" around Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989 Feb;40(2):115–118. doi: 10.4269/ajtmh.1989.40.115. [DOI] [PubMed] [Google Scholar]
- Ho M., Singh B., Looareesuwan S., Davis T. M., Bunnag D., White N. J. Clinical correlates of in vitro Plasmodium falciparum cytoadherence. Infect Immun. 1991 Mar;59(3):873–878. doi: 10.1128/iai.59.3.873-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J. Malarial proteins at the membrane of Plasmodium falciparum-infected erythrocytes and their involvement in cytoadherence to endothelial cells. Prog Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D. K., Roth E. F., Jr, Nagel R. L., Howard R. J., Handunnetti S. M. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood. 1991 Aug 1;78(3):812–819. [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Langreth S. G., Peterson E. Pathogenicity, stability, and immunogenicity of a knobless clone of Plasmodium falciparum in Colombian owl monkeys. Infect Immun. 1985 Mar;47(3):760–766. doi: 10.1128/iai.47.3.760-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Chulay J. D. Plasmodium falciparum sequestration: OKM5 antigen (CD36) mediates cytoadherence of parasitized erythrocytes to a myelomonocytic cell line. J Infect Dis. 1988 Mar;157(3):584–588. doi: 10.1093/infdis/157.3.584. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Magowan C., Chulay J. D. Activation of monocytes and platelets by monoclonal antibodies or malaria-infected erythrocytes binding to the CD36 surface receptor in vitro. J Clin Invest. 1989 Aug;84(2):468–475. doi: 10.1172/JCI114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tandon N. N., Magowan C., Jamieson G. A., Chulay J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989 Mar 17;243(4897):1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- Oo M. M., Aikawa M., Than T., Aye T. M., Myint P. T., Igarashi I., Schoene W. C. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol. 1987 Mar;46(2):223–231. doi: 10.1097/00005072-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Oquendo P., Hundt E., Lawler J., Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989 Jul 14;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Panton L. J., Leech J. H., Miller L. H., Howard R. J. Cytoadherence of Plasmodium falciparum-infected erythrocytes to human melanoma cell lines correlates with surface OKM5 antigen. Infect Immun. 1987 Nov;55(11):2754–2758. doi: 10.1128/iai.55.11.2754-2758.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Spitalnik S. L., Panton L. J., Howard R. J., Dixit V. M., Frazier W. A., Miller L. H., Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985 Nov 7;318(6041):64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- Rock E. P., Roth E. F., Jr, Rojas-Corona R. R., Sherwood J. A., Nagel R. L., Howard R. J., Kaul D. K. Thrombospondin mediates the cytoadherence of Plasmodium falciparum-infected red cells to vascular endothelium in shear flow conditions. Blood. 1988 Jan;71(1):71–75. [PubMed] [Google Scholar]
- Schmidt J. A., Udeinya I. J., Leech J. H., Hay R. J., Aikawa M., Barnwell J., Green I., Miller L. H. Plasmodium falciparum malaria. An amelanotic melanoma cell line bears receptors for the knob ligand on infected erythrocytes. J Clin Invest. 1982 Aug;70(2):379–386. doi: 10.1172/JCI110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood J. A., Roberts D. D., Spitalnik S. L., Marsh K., Harvey E. B., Miller L. H., Howard R. J. Studies of the receptors on melanoma cells for Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989 Feb;40(2):119–127. doi: 10.4269/ajtmh.1989.40.119. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Magowan C., Chulay J. D. Long-term cultured human vascular endothelial cells (EC-FP5) bind Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989 Oct;41(4):400–405. doi: 10.4269/ajtmh.1989.41.400. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Wåhlin B., Carlson J., Berzins K., Torii M., Aikawa M., Perlmann P., Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989 May 1;169(5):1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell D. A. Pathophysiology of severe falciparum malaria in man. Parasitology. 1987;94 (Suppl):S53–S76. doi: 10.1017/s0031182000085826. [DOI] [PubMed] [Google Scholar]
- White N. J., Warrell D. A., Looareesuwan S., Chanthavanich P., Phillips R. E., Pongpaew P. Pathophysiological and prognostic significance of cerebrospinal-fluid lactate in cerebral malaria. Lancet. 1985 Apr 6;1(8432):776–778. doi: 10.1016/s0140-6736(85)91445-x. [DOI] [PubMed] [Google Scholar]