Abstract
Previous reports have indicated that in vitro biliary clearance (Clbiliary) determined in sandwich-cultured hepatocytes correlates well with in vivo Clbiliary for limited sets of compounds. This study was designed to estimate the in vitro Clbiliary in sandwich-cultured rat hepatocytes (SCRH) of angiotensin II receptor blockers and HMG-CoA reductase inhibitors that undergo limited metabolism, to compare the estimated Clbiliary values with published in vivo Clbiliary data in rats, and to characterize the mechanism(s) of basolateral uptake and canalicular excretion of these drugs in rats. Average biliary excretion index (BEI) and in vitro Clbiliary of olmesartan, valsartan, pravastatin, rosuvastatin, and pitavastatin were 15%, 19%, 43%, 45%, and 20%, respectively, and 1.7, 3.2, 4.4, 46.1, and 34.6 ml/min/kg, respectively. Clbiliary predicted from SCRH, accounting for plasma unbound fraction, correlated with reported in vivo Clbiliary for these drugs. The rank order of Clbiliary values predicted from SCRH was consistent with in vivo Clbiliary values. Bromosulfophthalein inhibited the uptake of all drugs. BEI and Clbiliary values of olmesartan, valsartan, pravastatin, and rosuvastatin, known multidrug resistance-associated protein (Mrp)2 substrates, were reduced in SCRH from Mrp2-deficient (TR−) compared to wild-type (WT) rats. Although Mrp2 plays a minor role in pitavastatin biliary excretion, pitavastatin BEI and Clbiliary were reduced in TR− compared to WT SCRH; Bcrp expression in SCRH from TR− rats was decreased. In conclusion, in vitro Clbiliary determined in SCRH can be used to estimate and compare in vivo Clbiliary of compounds in rats, and to characterize transport proteins responsible for their hepatic uptake and excretion.
Keywords: Metabolism, Transport, Pharmacogenomics
Introduction
Biliary excretion is a predominant route of elimination for bile acids and many xenobiotics (Trauner and Boyer, 2003; Shitara et al., 2006). ATP-dependent canalicular transport systems are responsible for the biliary excretion of xenobiotics (Chandra and Brouwer, 2004). One approach that drug development scientists have employed recently to decrease the lipophilicity of a drug candidate is to exchange the hydrophobic moiety for a more hydrophilic moiety, such as a carboxylic acid. While this modification may enhance some drug-like properties of the molecule (e.g., increased solubility, decreased metabolism), it increases the possibility that the molecule will be recognized by hepatic transport systems. Accordingly, knowledge regarding the extent of biliary excretion of compounds in the early stages of drug development may be as important as absorption and metabolic properties when selecting drug candidates. From the point of view of drug-drug interactions and disease state alterations, elucidation of the biliary excretion properties of a drug candidate also is a critical issue.
Freshly-isolated hepatocytes are widely accepted as a reliable model for characterizing drug uptake across the basolateral membrane (Hirano et al., 2004; Lam et al., 2006). However, hepatocytes lose cellular polarity rapidly after isolation (Groothuis et al., 1981; Talamini et al., 1997), and canalicular transport proteins are internalized or confined to junctions between adjacent cells (Bow et al., 2008). Therefore, suspended hepatocytes are not an appropriate system to study canalicular excretion of drugs. Sandwich-cultured rat hepatocytes (SCRH) rapidly regain polarity and maintain expression levels of uptake and efflux transport proteins for several days (Hoffmaster et al., 2004; Zhang et al., 2005). In vitro biliary clearance (Clbiliary) determined in sandwich-cultured rat and human hepatocytes correlates well with in vivo Clbiliary (Liu et al., 1999a; Ghibellini et al., 2007), suggesting that this system is useful for predicting the in vivo biliary excretion of drug candidates. However, there are few reports to systematically elucidate the prediction of biliary clearance, and assess the involvement of uptake or canalicular transport proteins in SCRH using compounds which have similar chemical structures. Such data would be useful in the preliminary evaluation of the hepatobiliary disposition of candidate compounds, predicting the rank order of in vivo Clbiliary, and assessing the potential for drug-drug interactions in hepatobiliary transport.
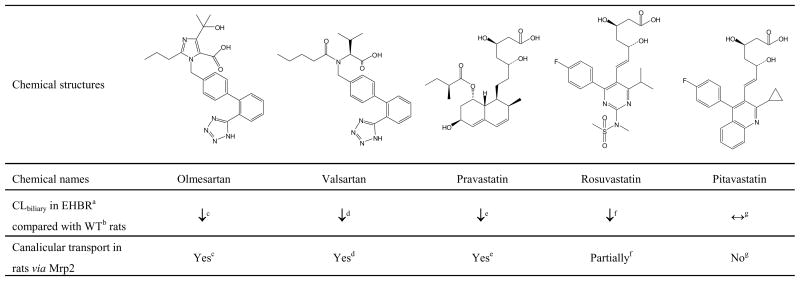
Angiotensin II receptor blockers (ARBs) and HMG-CoA reductase inhibitors (statins) are used widely in the treatment of cardiovascular disease (Brousil and Burke, 2003; Basile and Chrysant, 2006; Shitara and Sugiyama, 2006). Olmesartan and valsartan (ARBs), and pravastatin, rosuvastatin, and pitavastatin (statins), are all organic anions which have a carboxylic acid moiety and are known to be excreted extensively into bile as unchanged compound in rats (Nakagomi-Hagihara et al., 2006; Yamashiro et al., 2006; Yamazaki et al., 1997; Kitamura et al., 2005; Hirano et al., 2005; Fig. 1). Data obtained from multidrug resistance-associated protein (Mrp) 2-deficient Eisai hyperbilirubinemic rats (EHBR) suggested that Mrp2 is primarily involved in the biliary excretion of olmesartan, valsartan and pravastatin, partially involved in the biliary excretion of rosuvastatin, but plays a minor role in the biliary excretion of pitavastatin in rats (Fig. 1).
Figure 1.
Chemical structures of ARBs (olmesartan, valsartan) and statins (pravastatin, rosuvastatin, pitavastatin), and information regarding biliary excretion in rats. aEisai hyperbilirubinemic rats, bwild-type, cNakagomi-Hagihara et al., 2006, dYamashiro et al., 2006, eYamazaki et al., 1997, fKitamura et al., 2005, gHirano et al., 2005.
The purpose of this study was to determine in SCRH the in vitro Clbiliary of these ARBs and statins that undergo limited metabolism, to compare the predicted in vivo Clbiliary values based on the in vitro data with previously published in vivo Clbiliary data in rats, and to evaluate the involvement of basolateral uptake and canalicular efflux transporters in the hepatobiliary disposition of these drugs in rats.
Materials and Methods
Chemicals
Olmesartan was kindly provided by Daiichi-Sankyo Co., Ltd (Tokyo, Japan). Valsartan, pravastatin, rosuvastatin, pitavastatin, d6-olmesartan, d3-valsartan and d6-rosuvastatin were obtained from Toronto Research Chemicals Inc. (Ontario, Canada). Bromosulphophthalein (BSP) was obtained from Sigma-Aldrich, Inc (St. Louis, MO). [3H]Taurocholate (5 Ci/mmol; purity >97%) and [3H]estradiol-17β-D-glucuronide (E2–17βG; 46.9 Ci/mmol; purity >97%) were purchased from PerkinElmer Inc. (Waltham, MA). Collagenase (type I, class I) was purchased from Worthington Biochemicals (Freehold, NJ). Dulbecco’s modified Eagle’s medium (DMEM), MEM non-essential amino acids and insulin were purchased from Invitrogen (Carlsbad, CA). Insulin/transferrin/selenium culture supplement (ITS™), Matrigel™ and BioCoat™ plates were purchased from BD Biosciences Discovery Labware (Bedford, MA). Penicillin-streptomycin solution, fetal bovine serum (FBS), taurocholic acid, dexamethasone and Triton X-100 were purchased from Sigma-Aldrich, Inc. All other chemicals and reagents were of analytical grade and readily available from commercial sources.
Animals
Male Wistar (wild-type; WT) rats (177–318 g) from Charles River Laboratories, Inc. (Raleigh, NC) or male Mrp2-deficient (TR−) rats bred at the University of North Carolina (206–346 g; breeding stock obtained from Dr. Mary Vore, University of Kentucky, Lexington, KY) were used for hepatocyte isolation. Animals had free access to water and food prior to surgery. All animal procedures complied with the guidelines of the Institutional Animal Care and Use Committee (University of North Carolina, Chapel Hill, NC).
Hepatocyte Isolation and Culture
Hepatocytes were isolated from WT or TR− male rats by a modification of the two-step collagenase digestion method as described previously (Liu et al., 1999b). Hepatocyte viability was >85% as determined by trypan blue exclusion. Hepatocytes were seeded at a density of 1.75 million cells per well on 6-well BioCoat™ plates in 1.5 ml Dulbecco’s modified Eagle’s medium (DMEM) containing 5% FBS, 10 mM insulin, 1 μM dexamethasone, 2 mM L-glutamine, 1% MEM non-essential amino acids, 100 units penicillin G sodium and 100 µg streptomycin sulfate. Cells were incubated at 37°C in a humidified incubator with 95% O2/5% CO2 and allowed to attach for 2 hr, at which time the media was aspirated to remove unattached cells, and fresh media was added. Twenty-four hours later, cells were overlaid with BD Matrigel™ basement membrane matrix at a concentration of 0.25 mg/ml in 2 ml ice-cold DMEM containing 1% ITS™, 0.1 μM dexamethasone, 2 mM L-glutamine, 1% MEM non-essential amino acids, 100 units penicillin G sodium and 100 ng streptomycin sulfate. Hepatocytes were cultured for 4 days; media was changed daily.
Accumulation Studies
On day 4, hepatocytes were rinsed twice and then pre-incubated for 10 min at 37°C with 2 ml of warmed Hank’s balanced salt solution (HBSS) containing Ca2+ (standard; cells + bile) or Ca2+-free HBSS (cells), in order to maintain or disrupt the tight junctions sealing bile canalicular networks, respectively. Subsequently, hepatocytes were incubated with test compound (0.5–30 μM for ARB or statin; 1 μM for [3H]taurocholate or [3H]E2-17βG) in standard HBSS for 5–20 min (ARB or statin) or 10 min ([3H]taurocholate or [3H]E2–17βG) at 37°C. For uptake inhibition studies, BSP was added simultaneously with test compound to the hepatocytes. After incubation, the dosing solution was aspirated from the cells and uptake was stopped by washing the cells 3 times with ice-cold standard HBSS. For radiolabeled compounds, cells were lysed with 1 ml of 0.5% Triton X-100 in phosphate-buffered saline. For ARBs and statins, cells were lysed with 1 ml of 70% (v/v) methanol, and sonicated for 20 sec with a sonic dismembrator (model 100, Fisher Scientific, Pittsburgh, PA) and stored at <−70°C until analysis. The samples were analyzed for drug concentrations by liquid scintillation counting or by liquid chromatography with tandem mass spectrometry. Substrate accumulation was corrected for nonspecific binding by using Matrigel™-precoated dishes without cells. The total protein concentration in cell lysates was quantified by the BCA protein assay (Pierce Biotechnology, Inc., Rockford, IL) using bovine serum albumin as the reference standard, and accumulation was normalized to protein concentration. Due to incompatibility of the protein assay with methanol, the average protein concentration for standard HBSS or Ca2+-free HBSS incubations in the same liver preparation was used to normalize accumulation.
Analysis of ARBs and Statins
The cell lysate samples were centrifuged at 12,000 ×g for 2 min at 4°C and the supernatant was diluted 1:6 with water and methanol containing internal standard (d6-olmesartan for olmesartan; d3-valsartan for valsartan; d6-rosuvastatin for pravastatin, rosuvastatin and pitavastatin). A Shimadzu solvent delivery system (Columbia, MD) and a Leap HTC Pal thermostated autosampler (Carrboro, NC) connected to an Applied Biosystems API 4000 triple quadruple mass spectrometer with a TurboSpray ion source (Applied Biosystems, Foster City, CA) were used for analysis. Tuning, operation, integration and data analysis were performed in positive mode using multiple reaction monitoring (Analyst software v.1.4.1, Applied Biosystems). Analysis required 10 μL of sample and a solvent flow of 0.75 mL/min. Reverse phase chromatography (aqueous phase: water with 0.1% formic acid, v/v and organic phase: methanol with 0.1% formic acid, v/v) was used to elute the various compounds from an Aquasil C18, 50×2.1 mm column, with a 5 μm particle size (ThermoElectron, San Jose, CA). Initial gradient conditions (20% organic) were held for 0.75 min. From 0.75 min to 1.39 min, the mobile phase composition increased linearly to 40% organic and the eluent was directed to the mass spectrometer. At 3.3 min, the organic composition was increased to 90%. The flow was held at 90% organic until 4 min. At 4 min, the column was equilibrated with 20% organic. The total run time, including equilibration, was 5 min per injection. Eight point calibration curves (0.5–1000 nM for olmesartan; 2–1000 nM for pravastatin, rosuvastatin, and valsartan; 2–2000 nM for pitavastatin) were constructed based on peak area ratios of analyte and appropriate internal standard using the following transitions: olmesartan (447.5→207.2), valsartan (436.4→235.2), pravastatin (447.1→327.4), rosuvastatin (482.2→258.2), pitavastatin (422.1→290.3), d6-olmesartan (453.5→207.2), d3-valsartan (439.4→235.2), and d6-rosuvastatin (488.2→264.2). All points on the curves back-calculated to within ±15% of the nominal value.
Data Calculation
The accumulation (pmol/mg protein), biliary excretion index (BEI; %), and in vitro intrinsic Clbiliary (ml/min/kg) were calculated in hepatocytes using B-CLEAR® technology (Qualyst, Inc., Raleigh, NC) based on the following equations (Liu et al., 1999a).
| (1) |
| (2) |
where AUCmedium was determined as the product of the incubation time and the medium concentration. The concentration of drug in the medium was defined as the initial substrate concentration in the incubation medium, since the medium concentration at the end of incubation did not differ by more than 10% from the medium concentration at the beginning of incubation. In the absence of exogenous protein in the incubation medium, unbound intrinsic Clbiliary (intrinsic Cl’biliary) is assumed to be equivalent to in vitro intrinsic Clbiliary [Equation (2)]. The in vitro intrinsic Clbiliary (ml/min/mg protein) was scaled to kilogram of body weight assuming the following: 200 mg protein/g of rat liver tissue and 40 g of rat liver tissue/kg of body weight (Seglen, 1976). The predicted in vivo Clbiliary values were estimated according to the equations below based on the well-stirred model of hepatic disposition assuming that red blood cell partitioning of test compounds was minimal.
| (3) |
where Qp represents the hepatic plasma flow rate (40 ml/min/kg).
In Equation (3), plasma unbound fraction (fu,p) was assumed to be unity. Taking into consideration the unbound fraction,
| (4) |
The observed in vivo Clbiliary values were obtained from references.
Immunoblot
Freshly isolated rat hepatocytes on day 0 and SCRH on day 4 of culture were washed once with HBSS, and then lysed with 400 μl of lysis buffer [1 mM EDTA, 1% sodium dodecyl sulfate (SDS), pH 8, with Complete™ protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany)]. Lysates were stored at −80°C until immunoblot analysis. The whole cell lysates were thawed and protein concentrations were determined using the BCA protein assay. Protein samples (30 μg/well) were resolved on NuPAGE 4–20% Bis-Tris gel (Invitrogen), and electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Blots were blocked with Tris-buffered saline with 0.1% Tween 20 (TBS-T) containing 5% nonfat dry milk for 1 hr at room temperature. Subsequently, the membrane was incubated with appropriate primary antibodies for 2 hr at room temperature or overnight at 4°C and then rinsed three times at 10-min intervals with TBS-T. Mrp2, breast cancer resistance protein (Bcrp) and β-actin were detected using monoclonal antibodies M2III-6 (1:1000 dilution, Alexis Biochemicals, San Diego, CA), BXP-53 (1:1000 dilution, Alexis Biochemicals) and C4 (1:5000 dilution, Chemicon, San Fransisco, CA), respectively. After incubation with HRP-conjugated secondary antibody (Amersham Biosciences, Uppsala, Sweden), immunoreactive protein bands were detected by chemiluminescent substrate SuperSignal West Dura (Pierce Biotechnology) using a Bio-Rad VersaDoc imaging system (Bio-Rad Laboratories, Hercules, CA).
Results
Cumulative Uptake in SCRH
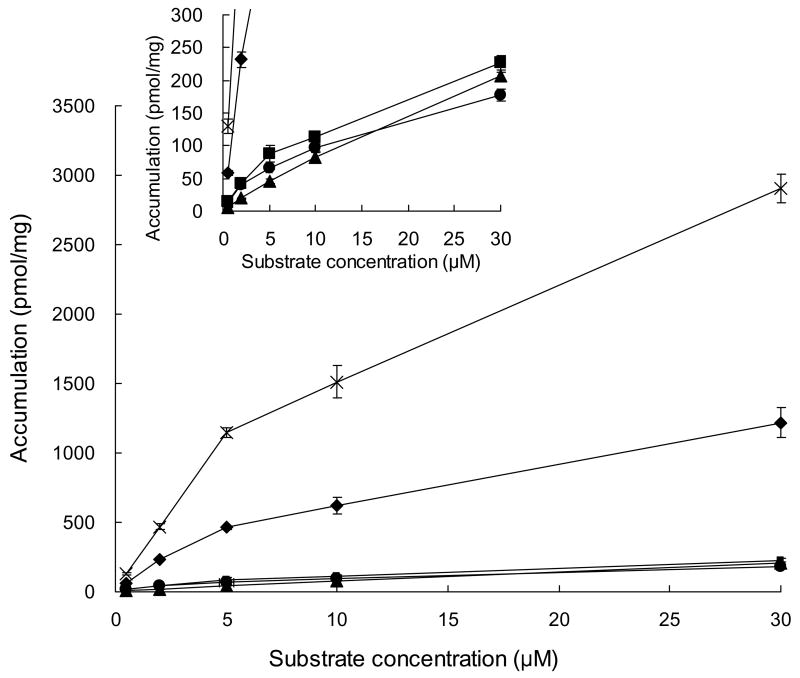
Substrate accumulation in SCRH was concentration-dependent and appeared to be in the linear range of uptake at concentrations between 2 and 5 μM for all drugs incubated for 10 min (Fig. 2). Accumulation was also time-dependent; BEI, equivalent to the percentage of retained substrate in the canalicular networks, was measurable for all drugs at each incubation time (Fig. 3). For a given drug, the BEI determined following 10- and 20-min incubations was comparable. Rosuvastatin and pravastatin have high BEI values. Both rosuvastatin and pitavastatin exhibited strikingly higher accumulation in SCRH compared to the other drugs, suggesting higher uptake activity (Fig. 3). The BEI of taurocholate ranged from 82.1% to 90.4% in these experiments, demonstrating functional excretion of substrates into the canalicular network in the SCRH system.
Figure 2.
Substrate concentration dependency of accumulation (cells + bile) for olmesartan (●), valsartan (■), pravastatin (▲), rosuvastatin (◆) and pitavastatin (×) in sandwich-cultured rat hepatocytes. Incubation time: 10 min. Data represent mean ± SD of three replicates from one liver. Inset: plot expanded scale of Y-axis
Figure 3.
Accumulation [cells + bile (solid bars) and cells (white bars)] and biliary excretion index of olmesartan (A), valsartan (B), pravastatin (C), rosuvastatin (D) and pitavastatin (E) in sandwich-cultured rat hepatocytes. Dosing concentration: 5 μM. Data represent mean + SEM (n = 3 livers)
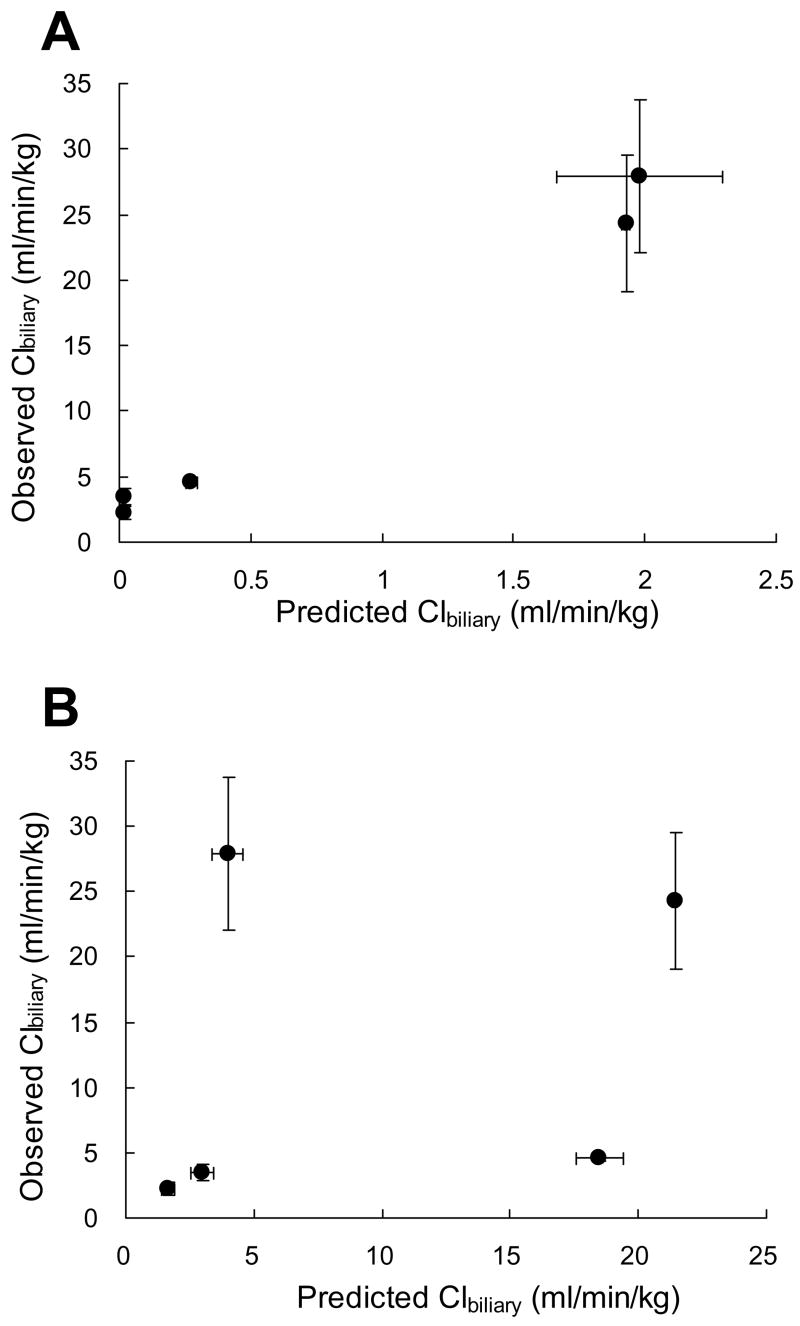
In Vitro-In Vivo Correlation of Clbiliary of ARBs and Statins in Rats
The in vitro intrinsic Clbiliary values were higher for both rosuvastatin and pitavastatin compared to the other drugs (Table 1). The relationship between predicted Clbiliary values from SCRH using Equation (4) and observed in vivo Clbiliary values for these drugs was excellent, although the compounds selected clustered into one of two categories, either low or high Clbiliary (Fig. 4). Even though the predicted Clbiliary values were 10–100-fold lower than the in vivo Clbiliary values, the rank order of the predicted Clbiliary values was consistent with the in vivo Clbiliary values for these drugs. Both the predicted [based on Equation (4)] and the in vivo Clbiliary values of pravastatin were the highest, followed by rosuvastatin, pitavastatin, valsartan and olmesartan (Fig. 4, Table 1). As shown in Fig. 4, there was no discernable relationship between predicted Clbiliary based on Equation (3) and observed in vivo Clbiliary values for these drugs.
TABLE 1.
In vitro intrinsic Clbiliary and predicted Clbiliary calculated based on Equations (3) and (4) from data generated in sandwich-cultured rat hepatocytes, compared with observed in vivo Clbiliary for selected angiotensin II receptor blockers and HMG-CoA reductase inhibitors. Data represent mean ± SEM of three individual experiments.
| Predicted Clbiliary (ml/min/kg) | |||||
|---|---|---|---|---|---|
| Compound | In vitro intrinsic Clbiliary (ml/min/kg)a | Plasma unbound fraction (fu,p) | from Equation (3) | from Equation (4) | Observed in vivo Clbiliary(ml/min/kg) |
| Olmesartan | 1.72 ±0.25 | 0.010b | 1.65 ±0.23 | 0.0172 ±0.0025 | 2.22 ±0.47b |
| Valsartan | 3.24 ±0.51 | 0.006c | 2.99 ±0.45 | 0.0194 ±0.0031 | 3.5 ±0.6c |
| Pravastatin | 4.43 ±0.75 | 0.472c | 3.97 ±0.60 | 1.98 ±0.32 | 27.9 ±5.8c |
| Rosuvastatin | 46.1 ±0.4 | 0.044c | 21.4 ±0.1 | 1.93 ±0.02 | 24.3 ±5.2c |
| Pitavastatin | 34.6 ±3.1 | 0.008d | 18.5 ±0.9 | 0.275 ±0.024 | 4.55 ±0.13e |
In vitro intrinsic Clbiliary values were calculated according to Equation (2) based on a 10-min incubation at 5 μM substrate concentration.
Olmesartan regulatory documentation,
Pitavastatin regulatory documentation,
Figure 4.
In vitro - in vivo correlation of Clbiliary of ARBs and statins in rats. Clbiliary predicted from in vitro data (mean ± SEM, n = 3 livers) was calculated from data generated in sandwich-cultured rat hepatocytes based on Equation (4) (A) or based on Equation (3) (B). When not visible, error bars are within the size of the symbol.
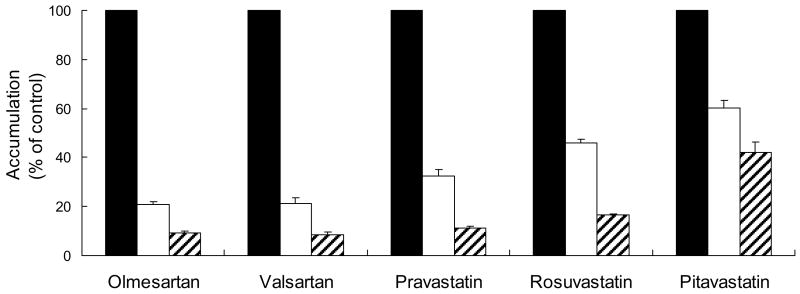
Effect of BSP on Accumulation of ARBs and Statins in SCRH
BSP (30 and 100 μM) inhibited the uptake of each drug tested (5 μM) in day 4 sandwich-cultured hepatocytes in a concentration-dependent manner (Fig. 5).
Figure 5.
Effect of BSP on accumulation (cell + bile) of ARBs and statins in day 4 sandwich-cultured rat hepatocytes. Open, solid and hatched bars represent 10-min incubation with vehicle control, 30 μM BSP, or 100 μM BSP, respectively. Dosing concentration: 5 μM. Data represent mean + SEM (n = 3 livers).
BEI of ARBs and Statins in SCRH from WT and TR− Rats
BEI values and in vitro intrinsic Clbiliary values (Table 2) of E2–17βG, an Mrp2 substrate (Morikawa et al., 2000), olmesartan, valsartan, pravastatin, and rosuvastatin in TR− SCRH were reduced compared to WT SCRH, consistent with decreased biliary excretion observed in vivo in EHBR compared to WT rats, as mentioned and referenced in Fig. 1. Surprisingly, the BEI value and in vitro intrinsic Clbiliary of pitavastatin also were reduced in TR− SCRH compared to WT SCRH, although Mrp2 plays a minor role in pitavastatin biliary excretion in rats (Hirano et al., 2005). There were marginal differences in accumulation (cell + bile) between WT and TR− SCRH for all of these compounds (data not shown), suggesting no differences in uptake and hepatocyte concentrations.
TABLE 2.
Biliary excretion index (BEI) and in vitro intrinsic Clbiliary of ARBs and statins in sandwich-cultured hepatocytes from WT and TR− rats. Data represent mean ± SEM (n = 3–4 livers)
| Compound | BEI (%) | In vitro intrinsic Clbiliary (ml/min/kg)a | ||
|---|---|---|---|---|
| WT | TR− | WT | TR− | |
| E2–17βG | 18.4 ±3.9 | 0.378 ±0.378 | 17.8 ±0.4 | 0.363 ±0.363 |
| Olmesartan | 14.7 ±0.5 | 7.05 ±0.93 | 1.72 ±0.25 | 0.931 ±0.167 |
| Valsartan | 18.9 ±2.6 | 1.63 ±1.33 | 3.24 ±0.51 | 0.406 ±0.341 |
| Pravastatin | 43.4 ±2.3 | 10.7 ±3.4 | 4.43 ±0.75 | 1.13 ±0.33 |
| Rosuvastatin | 44.9 ±6.1 | 20.6 ±3.9 | 46.1 ±0.4 | 21.2 ±6.0 |
| Pitavastatin | 19.9 ±1.4 | 6.54 ±3.38 | 34.6 ±3.1 | 10.4 ±5.4 |
In vitro intrinsic Clbiliary values were calculated according to Equation (2) based on a 10-min incubation at concentrations of 1 μM for E2–17βG and 5 μM for all other compounds.
Expression Levels of Mrp2 and Bcrp in Hepatocytes from WT and TR− Male Rats
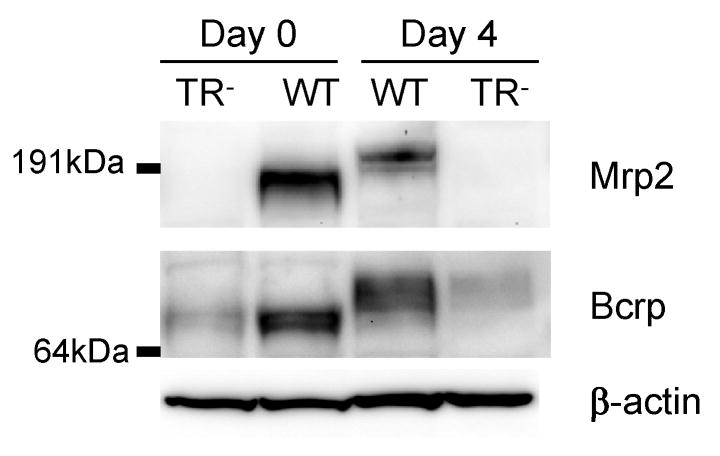
Protein levels of Mrp2 and Bcrp in freshly isolated hepatocytes on day 0 and SCRH on day 4 of culture were determined by immunoblot analysis and compared between WT and TR− male rats (Fig. 6). The absence of Mrp2 protein in hepatocytes from TR− rats in both day 0 freshly isolated hepatocytes and day 4 SCRH was confirmed. Interestingly, Bcrp protein levels in hepatocytes from TR− rats were much lower than in hepatocytes from WT rats in freshly isolated hepatocytes and day 4 SCRH. The molecular weight of both Mrp2 and Bcrp protein in the hepatocytes from WT rats on day 4 of culture was greater than their counterpart in freshly isolated hepatocytes.
Figure 6.
Comparison of Mrp2 and Bcrp expression in WT and TR− rat hepatocytes. Cell lysates from day 0 freshly isolated hepatocytes and day 4 sandwich-cultured rat hepatocytes were blotted for Mrp2 and Bcrp. Representative results of 2 experiments are shown (30 μg protein/well). After striping, the same blot was probed with β-actin antibody to serve as a loading control.
Discussion
The present study was undertaken to examine the relationship between in vitro and in vivo biliary excretion of ARBs and statins and to investigate the utility of SCRH as an in vitro model to identify transport proteins involved in the basolateral uptake and canalicular excretion of these drugs in rats.
As shown in Table 1, the predicted Clbiliary values from SCRH based on Equation (4) correlated well with the in vivo Clbiliary values for these drugs compared to predicted Clbiliary values based on Equation (3). Among statins, the predicted Clbiliary values assuming fu,p was unity [Equation (3)] did not reflect the rank order of the observed in vivo Clbiliary values. However, the predicted Clbiliary values for pravastatin, rosuvastatin and pitavastatin, accounting for plasma unbound fraction [Equation (4)], were consistent with the rank order observed for the in vivo Clbiliary values. The rank order of predicted Clbiliary values for ARBs was in good agreement with in vivo results, even though both ARBs have low in vivo Clbiliary values. Based on the assumptions of the well-stirred model of hepatic disposition, only unbound concentrations are able to traverse the hepatic basolateral membrane. Thus, accounting for the unbound fraction as shown in Equation (4) would be necessary to predict in vivo values. Fukuda et al. (2008) also reported that accounting for plasma protein binding was necessary to obtain a good in vivo prediction based on SCRH data using valsartan, pravastatin, rosuvastatin, and two other antibiotics. In contrast, Liu et al. (1999a) reported that predicted Clbiliary values determined in SCRH assuming fu,p was unity correlated well with reported in vivo Clbiliary using structurally different compounds (inulin, salicylate, methotrexate, [D-pen2,5]enkephalin, and taurocholate). According to the present data, when in vivo Clbiliary values are predicted based on SCRH data between structurally similar compounds, the unbound fraction should be included as shown in Equation (4).
The Clbiliary values predicted based on Equation (4) were 10–100-fold lower than the observed in vivo Clbiliary values for all compounds tested. These findings may be due to less extensive canalicular network formation in culture compared to liver tissue in vivo. Consistent underestimation of in vivo Clbiliary values also may be due to several factors including, but not limited to, decreased activity of transport proteins in culture or leakage from the biliary compartment over time in SCRH as discussed previously (Liu et al., 1999c; Hoffmaster et al., 2005).
Multiple organic anion transporting polypeptide (Oatp) isoforms are involved in the hepatic uptake of pravastatin (Yamazaki et al., 1993; Tokui et al., 1999), rosuvastatin (Nezasa et al., 2003) and pitavastatin (Fujino et al., 2005) in rats. Transport proteins responsible for the hepatic uptake of olmesartan and valsartan in rats were unknown, but these ARBs are substrates for multiple OATP isoforms in humans (Nakagomi-Hagihara et al., 2006; Yamashiro et al., 2006). Nezasa et al. (2003) reported that Km values for the hepatic uptake of pravastatin and rosuvastatin were 16.5 μM and 9.17 μM in rat hepatocytes, respectively; Km values for pitavastatin uptake in oocytes expressing rat Oatp1 and Oatp4 were 7.9 μM and 4.8 μM, respectively (Fujino et al., 2005). In the present study, uptake in SCRH for all drugs tested appeared to be in the linear range at concentrations between 2 and 5 μM, which was reasonable based on the reported Km values for pravastatin, rosuvastatin and pitavastatin. Thus, 5 μM was selected as the substrate concentration for the present study. Rosuvastatin exhibited approximately 10-fold higher accumulation in SCRH compared to pravastatin. This finding is consistent with the intrinsic clearance for the hepatic uptake of rosuvastatin, which was reported to be approximately 10-fold higher than pravastatin in rats (Nezasa et al., 2003), even though accumulation in SCRH was not determined as the initial uptake velocity.
BSP is a known inhibitor of Oatps, organic anion transporters (Oats) and Na+-taurocholate co-transporting polypeptide (Ntcp) (Tokui et al., 1999; Sekine et al., 1998; Hagenbuch et al., 1991). Inhibition of ARB and statin uptake in SCRH by BSP in a concentration-dependent manner (Fig. 5) suggests that one or more of these transport proteins is/are involved in the carrier-mediated uptake of these compounds from sinusoidal blood into hepatocytes. Inhibition of the hepatic uptake of pitavastatin by BSP was relatively less sensitive compared to the other compounds tested. This finding may be related to the higher contribution of passive diffusion to pitavastatin uptake, due to the hydrophobic structural features of pitavastatin compared to pravastatin and rosuvastatin (Shitara and Sugiyama, 2006).
Experiments in TR− SCRH demonstrated that Mrp2 was involved in the biliary excretion of olmesartan, valsartan, pravastatin, and rosuvastatin in rats, consistent with previously published in vivo data (see Fig. 1). Interestingly, the TR− SCRH data (Table 2) also suggested that pitavastatin was an Mrp2 substrate. However, as previously reported, the in vivo biliary excretion of pitavastatin was similar in EHBR and WT rats, suggesting that pitavastatin was not an Mrp2 substrate. Pitavastatin is hypothesized to be a Bcrp substrate (Hirano et al., 2005). This apparent discrepancy may be due to a novel finding based on immunoblots showing that Bcrp expression levels were much lower in SCRH from TR− rats used in this study compared to WT rats. Expression levels of Bcrp in TR− rats based on earlier studies (Hoffmaster et al., 2005; Johnson et al., 2006) were similar or decreased compared to WT rats. The decreased BEI for pitavastatin in TR− SCRH may be due to reduced Bcrp protein levels in these Mrp2-deficient TR− rats. Accordingly, reduced Bcrp levels in TR− SCRH could affect the evaluation of the other compounds tested. For instance, rosuvastatin has been shown to be a substrate of BCRP in humans (Huang et al., 2006). However, Mrp2 is known to be primarily involved in the biliary excretion of olmesartan, valsartan, and pravastatin in vivo, so the reduction of BEI values in TR− SCRH for these compounds should be due, in large part, to the absence of Mrp2. These data confirm that reduced transport protein expression in SCRH leads to decreased BEI, further demonstrating the utility of the SCRH system to evaluate transport protein function and the involvement of specific transport proteins in substrate excretion.
Immunoblot analysis demonstrated an increase in molecular mass of Mrp2 on day 4 compared to day 0, which has been attributed previously in SCRH to glycosylation of Mrp2 during the culture time (Zhang et al., 2005). Interestingly, immunoblot analysis also revealed an increase in Bcrp molecular mass at day 4 in culture compared to day 0.
Turncliff et al. (2006) demonstrated the utility of the SCRH model to evaluate the hepatobiliary disposition of generated metabolites. Although biliary excretion of pitavastatin is the main elimination pathway in rats, pitavastatin also is metabolized to pitavastatin lactone by UDP-glucuronosyl transferase as a minor component, and excreted into bile in rats (Kojima et al., 1999; Fujino et al., 2003). Simultaneous determination of the amount of pitavastatin and the lactone form in SCRH revealed pitavastatin lactone in both the cell and bile compartments, consistent with biliary excretion of pitavastatin lactone in vivo (data not shown). Studies currently are underway to examine the metabolic disposition of several drugs in SCRH.
In conclusion, in vitro Clbiliary determined in SCRH can be used to estimate and compare in vivo Clbiliary, and to ascertain the involvement of transport proteins in the basolateral uptake and canalicular excretion of ARBs and statins, that undergo limited metabolism. Knowledge regarding the contribution of hepatic uptake and efflux transporters to the pharmacokinetics of a drug is essential to predict potential alterations in systemic and hepatobiliary disposition when the expression or function of hepatic transport proteins is altered by disease states, genetic variability and/or transporter-mediated drug-drug interactions.
Acknowledgments
This research was supported by NIH GM41935. Koji Abe was supported by a scholarship from Daiichi-Sankyo.
Nonstandard abbreviations
- ARB
angiotensin II receptor blocker
- Bcrp
breast cancer resistance protein
- BEI
biliary excretion index
- BSP
bromosulfophthalein
- Clbiliary
biliary clearance
- E2–17βG
estradiol-17β-D-glucuronide
- EHBR
Eisai hyperbilirubinemic rats
- Mrp2
multidrug resistance-associated protein 2
- Oatp
organic anion transporting polypeptide
- SCRH
sandwich-cultured rat hepatocytes
- Statin
HMG-CoA reductase inhibitor
- TR−
Mrp2-deficient Wistar rat
- WT
wild-type rat
References
- Basile JN, Chrysant S. The importance of early antihypertensive efficacy: the role of angiotensin II receptor blocker therapy. J Hum Hypertens. 2006;20:169–175. doi: 10.1038/sj.jhh.1001972. [DOI] [PubMed] [Google Scholar]
- Bow DA, Perry JL, Miller DS, Pritchard JB, Brouwer KLR. Localization of P-gp (Abcb1) and Mrp2 (Abcc2) in Freshly Isolated Rat Hepatocytes. Drug Metab Dispos. 2008;36:198–202. doi: 10.1124/dmd.107.018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousil JA, Burke JM. Olmesartan medoxomil: an angiotensin II-receptor blocker. Clin Ther. 2003;25:1041–1055. doi: 10.1016/s0149-2918(03)80066-8. [DOI] [PubMed] [Google Scholar]
- Chandra P, Brouwer KLR. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res. 2004;21:719–735. doi: 10.1023/b:pham.0000026420.79421.8f. [DOI] [PubMed] [Google Scholar]
- Fujino H, Saito T, Ogawa S, Kojima J. Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. J Pharm Pharmacol. 2005;57:1305–1311. doi: 10.1211/jpp.57.10.0009. [DOI] [PubMed] [Google Scholar]
- Fujino H, Yamada I, Shimada S, Yoneda M, Kojima J. Metabolic fate of pitavastatin, a new inhibitor of HMG-CoA reductase: human UDP-glucuronosyltransferase enzymes involved in lactonization. Xenobiotica. 2003;33:27–41. doi: 10.1080/0049825021000017957. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Ohashi R, Tsuda-Tsukimoto M, Tamai I. Effect of plasma protein binding on in vitro-in vivo correlation of biliary excretion of drugs evaluated by sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2008 doi: 10.1124/dmd.107.019026. in press. [DOI] [PubMed] [Google Scholar]
- Ghibellini G, Vasist LS, Leslie EM, Heizer WD, Kowalsky RJ, Calvo BF, Brouwer KLR. In vitro-in vivo correlation of hepatobiliary drug clearance in humans. Clin Pharmacol Ther. 2007;81:406–413. doi: 10.1038/sj.clpt.6100059. [DOI] [PubMed] [Google Scholar]
- Groothuis GM, Hulstaert CE, Kalicharan D, Hardonk MJ. Plasma membrane specialization and intracellular polarity of freshly isolated rat hepatocytes. Eur J Cell Biol. 1981;26:43–51. [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B, Foguet M, Lübbert H, Meier PJ. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991;88:10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005;68:800–807. doi: 10.1124/mol.105.014019. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Turncliff RZ, LeCluyse EL, Kim RB, Meier PJ, Brouwer KLR. P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model opioid peptide. Pharm Res. 2004;21:1294–1302. doi: 10.1023/b:pham.0000033018.97745.0d. [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KLR. Multiple transport systems mediate the hepatic uptake and biliary excretion of the metabolically stable opioid peptide [D-penicillamine2,5]enkephalin. Drug Metab Dispos. 2005;33:287–293. doi: 10.1124/dmd.104.001420. [DOI] [PubMed] [Google Scholar]
- Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34:738–742. doi: 10.1124/dmd.105.007534. [DOI] [PubMed] [Google Scholar]
- Johnson BM, Zhang P, Schuetz JD, Brouwer KLR. Characterization of transport protein expression in multidrug resistance-associated protein (Mrp) 2-deficient rats. Drug Metab Dispos. 2006;34:556–562. doi: 10.1124/dmd.105.005793. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Maeda K, Sugiyama Y. Involvement of transporters in the hepatic transport of rosuvastatin. Drug Metab Rev. 2005;37(suppl 2):59–60. [Google Scholar]
- Kojima J, Fujino H, Abe H, Yosimura M, Kanda H, Kimata H. Identification of metabolites of NK-104, an HMG-CoA reductase inhibitor, in rat, rabbit and dog bile. Biol Pharm Bull. 1999;22:142–150. doi: 10.1248/bpb.22.142. [DOI] [PubMed] [Google Scholar]
- Lam JL, Okochi H, Huang Y, Benet LZ. In vitro and in vivo correlation of hepatic transporter effects on erythromycin metabolism: characterizing the importance of transporter-enzyme interplay. Drug Metab Dispos. 2006;34:1336–1344. doi: 10.1124/dmd.106.009258. [DOI] [PubMed] [Google Scholar]
- Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KLR. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos. 1999a;27:637–644. [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KLR. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999b;277:G12–21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KLR. Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther. 1999c;289:1592–1599. [PubMed] [Google Scholar]
- Morikawa A, Goto Y, Suzuki H, Hirohashi T, Sugiyama Y. Biliary excretion of 17beta-estradiol 17beta-D-glucuronide is predominantly mediated by cMOAT/MRP2. Pharm Res. 2000;17:546–552. doi: 10.1023/a:1026412915168. [DOI] [PubMed] [Google Scholar]
- Nakagomi-Hagihara R, Nakai D, Kawai K, Yoshigae Y, Tokui T, Abe T, Ikeda T. OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab Dispos. 2006;34:862–869. doi: 10.1124/dmd.105.008888. [DOI] [PubMed] [Google Scholar]
- Nezasa K, Higaki K, Takeuchi M, Nakano M, Koike M. Uptake of rosuvastatin by isolated rat hepatocytes: comparison with pravastatin. Xenobiotica. 2003;33:379–388. doi: 10.1080/0049825031000066259 . [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett. 1998;429:179–182. doi: 10.1016/s0014-5793(98)00585-7. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425–446. doi: 10.1016/j.ejps.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Talamini MA, Kappus B, Hubbard A. Repolarization of hepatocytes in culture. Hepatology. 1997;25:167–172. doi: 10.1002/hep.510250131. [DOI] [PubMed] [Google Scholar]
- Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, oatp2. Pharm Res. 1999;16:904–908. doi: 10.1023/a:1018838405987. [DOI] [PubMed] [Google Scholar]
- Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34:1247–1254. doi: 10.1124/dmd.105.008938. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Suzuki H, Hanano M, Tokui T, Komai T, Sugiyama Y. Na(+)-independent multispecific anion transporter mediates active transport of pravastatin into rat liver. Am J Physiol. 1993;264:G36–44. doi: 10.1152/ajpgi.1993.264.1.G36. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Akiyama S, Niinuma K, Nishigaki R, Sugiyama Y. Biliary excretion of pravastatin in rats: contribution of the excretion pathway mediated by canalicular multispecific organic anion transporter. Drug Metab Dispos. 1997;25:1123–1129. [PubMed] [Google Scholar]
- Zhang P, Tian X, Chandra P, Brouwer KLR. Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Mol Pharmacol. 2005;67:1334–1341. doi: 10.1124/mol.104.004481. [DOI] [PubMed] [Google Scholar]