Abstract
Background
Abdominal bloating and visible distention are common yet poorly understood symptoms. Epidemiological data distinguishing visible distention from bloating are not available. We aimed to evaluate the prevalence and potential risk factors for abdominal bloating and visible distention separately in a representative US population, and their association with other functional gastrointestinal disorders (FGIDs).
Methods
The validated Talley Bowel Disease Questionnaire was mailed to a cohort selected at random from the population of Olmsted County, Minnesota. The complete medical records of responders were abstracted; 2259 subjects (53% females; mean age 62 years) provided bloating and distention data.
Results
The age and sex-adjusted (US White 2000) overall prevalence per 100 for bloating was 19.0 [95% confidence interval (CI), 16.9 to 21.2] vs 8.9 (95% CI, 7.2 to 10.6) for visible distention. Significantly increased odds for bloating alone and separately for distention (vs neither) were detected in females, and in those with higher overall Somatic Symptom Checklist (SSC) scores and higher scores of each individual SSC item. Further, females [odds ratio (OR), 1.5; 95% CI, 1.0 to 2.1], higher SSC score (OR, 1.4; 95% CI, 1.1 to 1.8), constipation-predominant irritable bowel syndrome (OR, 2.3; 95% CI, 1.3 to 4.1), dyspepsia (OR, 1.9; 95% CI, 1.1 to 3.2), and gastro-intestinal symptom complex overlap (OR, 1.7; 95% CI, 1.1 to 2.7) significantly increased odds for distention over bloating alone.
Conclusions
Bloating and distention are common and have similar risk factors; somatisation probably plays a role.
Abdominal bloating and visible distention are common and bothersome symptoms, but remain poorly understood. The term “bloating” refers to the subjective sensation of abdominal inflation or swelling, while visible distention refers to an actual increase in abdominal girth.1 2 Previous studies have suggested that bloating with or without visible distention might affect up to 96% of patients with functional gastro-intestinal disorders (FGIDs),3 4 as well as 10-30% of the general population.5 More than 65% of patients presenting with bloating or distention rated the symptoms as moderate to severe in intensity, while 54% of them reported that the symptoms influenced their daily activities and 43% had to resort to the use of medication.1 6 However, little epidemiological data on bloating and separately on distention exist.
While often considered closely related, bloating is not always accompanied by visible distention; up to a quarter of irritable bowel syndrome (IBS) patients stated that their bloating was not associated with abdominal distention.7 8 Using objective measurement of abdominal inductance plethysmography, Houghton et al9 also reported that visible abdominal distention only occurred in 52% of patients with IBS reporting bloating. Whether this is true in the general population is unclear. Further, there are few data on the association of bloating or visible distention with specific FGIDs such as functional dyspepsia, or with symptomatic gastro-oesophageal reflux disease (GORD).
Most studies published on the mechanisms of bloating and distention have focused on patients with IBS who had consulted their medical practitioner.7-11 Some studies have reported that bloating and distention were more common in females10 and with constipation,9 11 12 and may worsen when other abdominal symptoms (pain/discomfort) increase.7 9 Flatulogenic foods such as wheat bran, fresh fruits or juices may precipitate bloating and/or distension.13 14 There is also some limited evidence that bloating is associated with obesity.15-17 No evidence exists to show that bloating is related to age18 or parity.17 The risk factors for bloating and distention as well as the factors that distinguish bloating from distention have not been well defined, however, especially in the general population.
Despite the potential clinical, social and economic importance,19 the epidemiology of bloating and visible distention has been largely ignored. We aimed to provide novel data on the prevalence and risk factors for bloating and distention separately in a representative population sample. We hypothesised that bloating and distention would be highly prevalent in the general population and commonly would coexist with other FGIDs. We also postulated that female gender and somatic distress would be independent predictors of bloating and distention.
METHODS
Study sampling frame
Olmsted County, Minnesota, is isolated from other urban centres. Its population comprises 124 277 persons (US Census 2000 data), of whom 89% are white; sociodemographically, the community is similar to the United States white population.20 Over 95% of County residents receive their medical care from one of the two group practices (Mayo Medical Center and Olmsted Medical Center). A common medical record system has been maintained for over 90 years. Recorded diagnoses and surgical procedures are indexed, including the diagnoses made for outpatients seen in office or clinic consultations, emergency room visits or nursing home care, as well as the diagnoses recorded for hospital inpatients, at autopsy examination or on death certificates. This system was further developed by the Rochester Epidemiology Project (REP). Annually, over 80% of the entire population is attended by one or both of these two practices, and 96% are seen at least once during any given 3-year period.20 The REP medical records linkage system therefore provides what is essentially an enumeration of the population from which random samples can be drawn.
Bowel Disease Questionnaire and Somatic Symptom Checklist
The original Talley Bowel Disease Questionnaire (BDQ) was designed in 198821 as a self-report instrument to measure symptoms experienced over the prior year and to collect medical history data.22 Extensive reliability and validity testing has been conducted; the BDQ has a median kappa chance corrected measure of agreement for symptom items of 0.8 (range, 0.5-1.0).22 The questionnaire has been modified several times to meet the needs of new studies. The modified Talley BDQ includes 46 gastro-intestinal (GI) symptoms and 17 non-GI items in the Somatic Symptom Checklist (SSC),23 a measure of somatisation. Somatisation refers to the presence of physical symptoms that suggest a general medical condition but are not fully explained by any organic medical condition. Respondents are instructed to indicate how often each somatic symptom occurred (0 = not a problem to 4 = occurs daily) and how bothersome each was (0 = not a problem to 4 = extremely bothersome when occurs) during the past year, using separate 5-point scales. The average of the mean score for “how often” and for “how bothersome” was calculated to give a score for each item and an overall SSC score.
Survey methodology
We have been conducting population-based research in Olmsted County since 1988.24-29 Using the REP, we have drawn random samples of county residents and mailed them valid GI symptom questionnaires more than once.30 We have subsequently linked the data from these surveys to create a well characterised cohort. In 2003, a total of 4194 subjects who were originally randomly sampled from residents of Olmsted County were mailed the modified version of the validated BDQ. Subjects who had died, moved from Olmsted County or denied authorisation to use their medical records for research, as required by Minnesota law, were excluded.
The overall response rate was 55%. Responders were similar to non-responders in terms of gender, body mass index (BMI, kg/m2) and previous surgery (e.g. cholecystectomy), but a weak association with age was observed [odds ratio (OR) per year of age for responding was 1.02 [95% confidence interval (95% CI), 1.01 to 1.02)].31
The complete (inpatient and outpatient) medical records of the responders to this survey were also abstracted at the time. We used these existing data to perform the present study. Further, using HICDA codes, we also reviewed all subjects in the study to determine if there was any history of GI cancer, ovarian cancer or ascites.
Definitions of symptomatic groups
In the modified BDQ, there were two separate questions that measured bloating and visible distention. Subjects were classified into the following subgroups:
Bloating alone (bloating without distention)
Distention (bloating with distention)
Controls (neither bloating nor distention)
The site of bloating was next divided into three categories: upper (above the umbilicus), lower (below the umbilicus), or both (upper and lower) according to a separate question asking for the location of bloating. Figure 1 highlights the sampling methods used in our current study.
Figure 1.
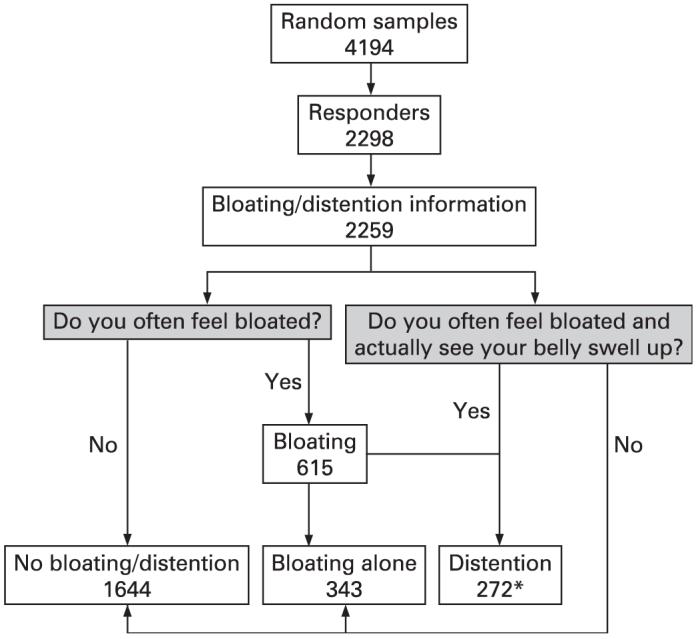
Flow chart of sampling techniques used in the study. *Note that there were 11 misclassified patients who said “yes” to the bloating with distention question but “no” to the bloating question who were added into the distention group. The exact wording of the questions assessing bloating and visible distention from the questionnaire is included in the shaded boxes.
We also classified subjects as having dyspepsia, functional constipation (FC) and IBS and its subtypes by the data from the BDQ questionnaire using modified Rome II criteria3 32 33 as follows:
-
▶
Dyspepsia. Two or more of the following symptoms: (a) frequent upper abdominal pain occurring more than six times in the previous year; (b) nausea once a week or more; (c) vomiting once a week or more; (d) early satiety; (e) loss of appetite in the past year.
-
▶
Irritable bowel syndrome. Abdominal pain or discomfort more than six times in the past year, and given this, two out of the following three features: (a) relieved with defaecation; (b) onset associated with a change in frequency of stool; and (c) onset associated with a change in form (appearance) of stool. IBS was sub-typed as (a) diarrhoea-predominant (IBS-D): IBS with loose or watery stools at least 25% and hard or lumpy stool <25% of bowel movements; (b) constipation-predominant (IBS-C): IBS with hard or lumpy stool at least 25% and loose or watery stools <25% of bowel movements; (c) mixed IBS (IBS-M): meet the criteria for both IBS-D and IBS-C; (d) unsubtyped IBS (IBS-U): IBS but not meeting the criteria for the other three categories.2 5 34
-
▶
Functional constipation. Two or more of the following and insufficient criteria for IBS: (a) fewer than three bowel movements per week; (b) strain often to have a bowel movement; (c) stools often hard; (d) incomplete evacuation.
-
▶
Gastro-oesophageal reflux disease.28 35 Weekly or more frequent heartburn or acid regurgitation.
If a subject met the criteria for more than one of the above disorders, we defined them as a “GI symptom complex overlap case”. If a subject did not meet the criteria of any of the above disorders, we defined them as being a “no FGID case”.
Predictors of bloating and distention
The a priori predictors considered for bloating and distention were age, gender, height and weight (for calculating BMI, kg/m2), SSC score, race, marital status, educational level, current smoking(yes/no), alcohol use (yes/no), past abdominal surgery; and in females, hysterectomy, pelvic inflammatory disease, number of live births or vaginal births, using both the questionnaire and chart review data.
Statistical analysis
A summary of demographics was compiled for the three groups (bloating alone, visible distention, and controls). Data were expressed as mean (standard error) for quantitative variables and proportions (%) for qualitative variables.
The age- and sex-adjusted prevalence of overall bloating as well as visible distention in Olmsted County was estimated adjusted to the population distribution of the 2000 United States white population. The 95% confidence intervals (CIs) were computed based on the binomial distribution.
Polychotomous logistic regression models were used to analyse risk factors. These models used a three category dependent variable: bloating alone, visible distention, and controls (neither) as the reference category. The odds ratios (ORs) for bloating alone, and for distension, were estimated for each potential risk factor in separate logistic regression models, adjusting for age, gender and SSC score in each model. The ORs and corresponding 95% CIs were computed from the coefficients in the logistic regression models. A two-sided α level of 0.05 was used to assess statistical significance. In addition, the odds for bloating and distention were also estimated with respect to SSC score and each of its constituent items using logistic regression models adjusting for just age and gender. The potential for specific FGIDs, including IBS subtypes and a GI complex overlap group to predict bloating and distension status were also examined in separate logistic regression models adjusting for age, gender, and SSC score. Furthermore, the association of gender and FGIDs with site of bloating or distension (upper abdomen, lower abdomen, both sites) was assessed using logistic regression models adjusting for age.
Alternatively, the distribution of IBS, IBS subtypes, dyspepsia, FC, and GORD was estimated in the subgroups of subjects with bloating alone, distention, and the controls. The univariate association of FGID subtypes with bloating or distention status was assessed using chi-square tests for two-way contingency tables.
RESULTS
Sample characteristics
Table 1 illustrates the distribution of demographic characteristic across the study groups and controls. Using diagnostic codes from Rochester Epidemiologic Project, we found 15 cases had a past history of GI cancer, ovarian cancer or ascites among subjects with bloating and/or distention. While one case had moderate ascites caused by liver cirrhosis in the distention group during the survey period, all others were disease-free from 2000 to 2005.
Table 1.
Distribution of demographic characteristics of participants by each study group
| Characteristic | Bloating alone (n = 343) | Distention (n = 272) | Control (n = 1644) |
|---|---|---|---|
| Mean age, years (SD) | 62 (13) | 60 (13) | 63 (12) |
| Female, % | 62.7 | 70.9 | 47.5 |
| White race, % | 97.7 | 99.3 | 98.1 |
| BMI, mean (SD) | 31.1 (9.0) | 30.1 (8.2) | 29.3 (7.3) |
| SSC score, mean (SD) | 0.8 (0.6) | 1.0 (0.6) | 0.5 (0.4) |
| Married, % | 75.5 | 71.0 | 81.3 |
| Current smoker,* % | 8.2 | 7.7 | 7.2 |
| Current alcohol use,* % | 42.9 | 43.0 | 46.5 |
| Education status, % | |||
| <High school | 8.6 | 5.9 | 4.8 |
| High school/some college | 58.7 | 57.6 | 51.6 |
| College graduate/professional training | 32.7 | 36.5 | 43.6 |
| Prior abdominal surgery, % | |||
| Abdominal exploration | 3.2 | 2.9 | 2.4 |
| Appendectomy | 32.4 | 32.3 | 27.9 |
| Cholecystectomy | 15.2 | 14.7 | 10.8 |
| Hernia repair | 7.0 | 6.3 | 7.5 |
| Hysterectomy(female) | 33.5 | 36.3 | 30.9 |
| Number of live births, mean (SD) | 1.8 (1.9) | 1.5 (1.6) | 1.8 (1.9) |
| Number of vaginal births, mean (SD) | 1.0 (1.6) | 1.0 (2.0) | 1.2 (1.7) |
| Pelvic inflammatory disease, % | 0 | 1.0 | 0.5 |
For either smoker or alcohol, it was a yes/no answer.
BMI, body mass index; SCC, Somatic Symptom Checklist.
Prevalence of bloating and distention
The overall age- and sex-adjusted prevalence of bloating was 19.0% (95% CI, 16.9 to 21.2), adjusting to the population distribution of the 2000 United States white population; for visible distention, the age and sex adjusted prevalence was 8.9% (95% CI, 7.2 to 10.6) (table 2). Females reported more bloating and more distention than males (p<0.001 for both), adjusting for age and SSC score. Younger age was also positively associated with distention (p<0.05).
Table 2.
Age-adjusted, sex-specific, and overall age and sex-adjusted prevalence rates (per 100) of bloating and visible distention among residents of Olmsted County, Minnesota
| Group | n | Overall* | Males† | Females‡ |
|---|---|---|---|---|
| Bloating alone | 343 | 10.2 (8.3 to 12.0) ‡ | 8.3 (5.9 to 10.6) | 11.9 (9.0 to 14.7) |
| Distention | 272 | 8.9 (7.2 to 10.6) | 5.1 (3.3 to 6.9) | 12.5 (9.5 to 15.5) |
| Bloating ± distention | 615 | 19.0 (16.9 to 21.2) | 13.4 (10.7 to 16.1) | 24.4 (21.1 to 27.7) |
Age and sex-adjusted to 2000 United States white population.
Age-adjusted to 2000 United States white population.
Numbers in parentheses are 95% CI.
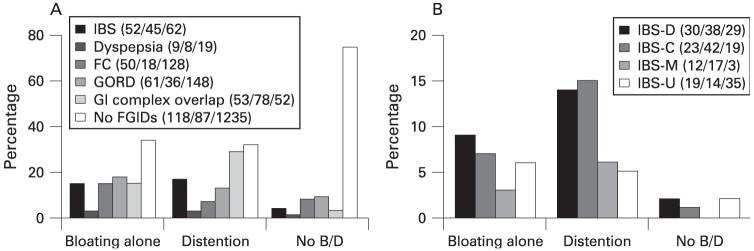
We also estimated the proportions of bloating and distention in each of the FGID categories and in GORD, as well as in subjects with none of these coexisting disorders. The results are summarised in fig 2. The proportion with bloating and, separately, visible distention was significantly higher in subjects with each FGID or GORD, compared to subjects with no FGIDs (tables 3 and 4). Notably, individuals with IBS-C or any GI symptom complex overlap had the highest proportion with bloating and especially visible distention.
Figure 2.
The proportion of abdominal bloating and visible distention among FGIDs, GORD as well as in subjects with none of these disorders (no FGIDs). The lower part of each column shows the proportion with distention. The GI overlap group included all subjects with complex symptoms meeting the criteria for more than one of irritable bowel syndrome (IBS), functional constipation (FC), functional dyspepsia (FD) and gastro-oesophageal reflux disease (GORD). GI, gastrointestinal; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhoea-predominant IBS.
Table 3.
Association of FGIDs (IBS, dyspepsia, FC, and/or GORD) with bloating and distention among residents of Olmsted County, Minnesota. Results are given as odds ratios*
| Bloating alone vs controls (95% CI) | Distention vs controls (95% CI) | Distention vs bloating alone (95% CI) | |
|---|---|---|---|
| Female† | 1.6 (1.2 to 2.1) | 2.3 (1.7 to 3.2) | 1.4 (1.0 to 2.1) |
| SSC‡ | 2.5 (1.9 to 3.2) | 3.4 (2.6 to 4.5) | 1.4 (1.1 to 1.8) |
| IBS§ | 4.7 (3.3 to 6.8) | 7.1 (4.9 to 10.2) | 1.5 (1.01 to 2.2) |
| Dyspepsia§ | 2.2 (1.2 to 3.9) | 4.1 (2.4 to 7.1) | 1.9 (1.1 to 3.2) |
| FC§ | 3.2 (2.2 to 4.5) | 1.7 (1.1 to 2.8) | 0.5 (0.3 to 0.9) |
| GORD§ | 2.5 (1.8 to 3.4) | 2.6 (1.9 to 3.7) | 1.1 (0.7 to 1.5) |
Multiple variable models adjusted for age.
Female vs male.
SSC score per unit.
Each FGID subtype included all cases with gastro-intestinal symptom complex overlap. The comparison was between IBS relative to no-IBS, dyspepsia relative to no-dyspepsia, FC relative to no FC, GORD relative to no-GORD.
FC, functional constipation; FGIDs, functional gastro-intestinal disorders; GORD, gastric-oesophageal reflux disease; IBS, irritable bowel syndrome; SSC, Somatic Symptom Checklist.
Table 4.
Association of IBS subtypes with bloating and distention among residents of Olmsted County, Minnesota. Results are given as the odd ratios (95% CI)*
| IBS subtype | Bloating alone vs controls | Distention vs controls | Distention vs bloating alone |
|---|---|---|---|
| IBS-D† | 5.4 (3.1 to 9.6) | 7.2 (4.0 to 13.0) | 1.3 (0.8 to 2.3) |
| IBS-C† | 6.1 (3.1 to 11.7) | 13.9 (7.4 to 25.9) | 2.3 (1.3 to 4.1) |
| IBS-M† | 12.3 (3.3 to 45.6) | 16.2 (4.4 to 60.0) | 1.3 (0.6 to 3.0) |
| IBS-U† | 2.8 (1.5 to 5.1) | 2.6 (1.3 to 5.1) | 0.9 (0.4 to 1.9) |
Multiple variable models model adjusted for age.
Each comparison was between each IBS subtype relative to no-IBS.
IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhoea-predominant IBS; IBS-M, mixed IBS (meets the criteria for both IBS-C and IBS-D); IBS-U, unsubtyped IBS (not meeting the criteria for the other three categories).
Risk factors for bloating and distention
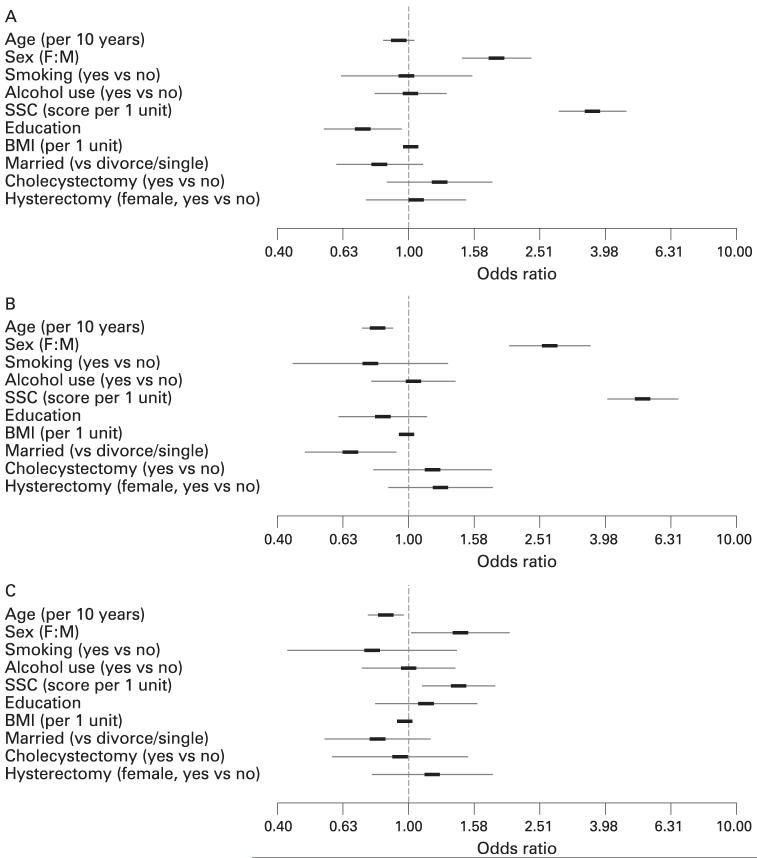
Using logistic regression adjusting for age, gender and SSC score, the risk factors identified for bloating alone and for visible distention are illustrated in fig 3. Notably, female gender and higher SSC score (per unit) significantly increased the odds for bloating and distention compared to controls. Moreover, female gender (OR, 1.5; 95% CI, 1.03 to 2.1) and higher SSC score (OR per unit, 1.4; 95% CI, 1.1 to 1.8) also increased the odds for visible distention compared to bloating alone. A weak association was also detected with greater BMI increasing the odds and higher education level decreasing the odds for bloating; older age and being married decreased the odds for visible distension. No significant association with bloating or distention was detected for race, current smoking or alcohol use, prior abdominal surgery (cholecystectomy, appendectomy, hernia repair, hysterectomy in females), number of live births, or number of vaginal births (fig 1).
Figure 3.
Potential risk factors for (A) bloating alone vs normal (B) visible distention vs normal, and (C) distention vs bloating alone among residents of Olmsted County, Minnesota. Logistic regression analysis was used to adjust for age, gender, somatic symptom checklist (SSC) score; age, gender and SSC score each adjusted for the other two factors; education refers to college graduate or professional training vs high school. BMI, body mass index; SSC, Somatic Symptom Checklist.
The results for each of the 17 non-GI symptom items in the Somatic Symptom Checklist using separate models adjusted for age and gender are given in table 5. Higher scores (ORs per unit) of each of the items in the SSC score were positively associated with bloating as well as distention compared to controls, and several items (10 out of 17) discriminated bloating alone from distension.
Table 5.
Association of individual components of Somatic Symptom Checklist (SSC) with bloating and distention among residents of Olmsted County, Minnesota. Results are given as odds ratios* (95% CI)
| Component of SSC score† | Bloating alone vs controls | Distention vs controls | Distention vs bloating alone |
|---|---|---|---|
| Heart palpitations (pounding or racing) | 1.7 (1.4 to 2.0) | 2.3 (1.9 to 2.7) | 1.3 (1.1 to 1.6) |
| Nervousness or shakiness | 1.8 (1.6 to 2.1) | 2.2 (1.9 to 2.6) | 1.2 (1.02 to 1.4) |
| Weakness | 1.6 (1.4 to 1.9) | 2.0 (1.7 to 2.3) | 1.2 (1.04 to 1.5) |
| Dizziness | 1.5 (1.2 to 1.7) | 1.9 (1.6 to 2.3) | 1.3 (1.1 to 1.6) |
| Fatigue (tiredness) | 1.6 (1.4 to 1.7) | 1.9 (1.8 to 2.2) | 1.2 (1.1 to 1.4) |
| Feeling anxious, fearful or afraid | 1.6 (1.3 to 1.8) | 1.9 (1.6 to 2.2) | 1.2 (1.02 to 1.5) |
| Depression (feeling sad or blue) | 1.5 (1.3 to 1.7) | 1.8 (1.6 to 2.1) | 1.2 (1.1 to 1.5) |
| Eye pain associated with reading | 1.4 (1.2 to 1.7) | 1.7 (1.5 to 2.0) | 1.2 (1.03 to 1.5) |
| Insomnia | 1.4 (1.2 to 1.5) | 1.7 (1.5 to 1.9) | 1.3 (1.1 to 1.4) |
| Joint pains | 1.4 (1.3 to 1.5) | 1.5 (1.3 to 1.6) | 1.1 (0.9 to 1.2) |
| General stiffness | 1.4 (1.3 to 1.6) | 1.5 (1.4 to 1.7) | 1.1 (0.95 to 1.2) |
| Hot or cold spells | 1.2 (1.04 to 1.3) | 1.4 (1.2 to 1.5) | 1.2 (1.01 to 1.3) |
| Asthma | 1.6 (1.3 to 1.9) | 1.9 (1.6 to 2.2) | 1.2 (0.98 to 1.5) |
| Trouble breathing | 1.6 (1.3 to 1.8) | 1.8 (1.6 to 2.1) | 1.2 (0.99 to 1.4) |
| Headaches | 1.5 (1.3 to 1.7) | 1.7 (1.4 to 1.9) | 1.1 (0.9 to 1.3) |
| Backaches | 1.5 (1.4 to 1.7) | 1.6 (1.5 to 1.8) | 1.1 (0.9 to 1.2) |
| High blood pressure | 1.3 (1.1 to 11.4) | 1.2 (1.0 to 1.4) | 0.9 (0.8 to 1.1) |
Models adjusted for age and gender.
Per unit higher.
In a multiple variable model incorporating the individual FGIDs and GORD along with age, gender and SSC score, each of the FGIDs and GORD were independent predictors of bloating alone, and of distension. In addition, significantly increased odds for distension versus bloating alone were detected in IBS and in dyspepsia, but decreased odds were observed for distension in FC (table 3).
A similar multiple variable model which subcategorised subjects meeting criteria for IBS into their four subtypes also indicated increased odds (relative to no IBS) for bloating alone, and for distension in each subtype, but only IBS-C increased the odds for distension versus bloating alone (table 4).
Using a separate multiple variable model, dramatically increased odds for bloating (OR, 7.6; 95% CI, 4.9 to 11.9) and especially for distention (OR, 12.7; 95% CI, 8.2 to 19.9) compared to controls, were detected in subjects identified with a GI complex overlap (relative to those meeting criteria for only one FGID, or GORD, or none). This overlap group also had increased odds for distention compared to bloating alone (OR, 1.7; 95% CI, 1.1 to 2.7). With respect to the FGID categories and their interactions with gender, female gender was significantly associated with an increased odds of distention (vs controls) in each FGID including IBS (OR, 2.8; 95% CI, 1.4 to 5.5), FC (OR, 4.5; 95% CI, 1.5 to 13.1) and dyspepsia (OR, 4.4; 95% CI, 1.4 to 13.6) as well as GORD (OR, 3.0; 95% CI, 1.7 to 5.2).
Abdominal bloating by site in different FGIDs
In females, a greater proportion reported bloating in the lower abdomen than at both sites, with the smallest proportion reporting bloating in the upper abdomen. Independent of gender, subjects with only IBS, and only FC, had increased odds for bloating in the lower abdomen (compared with both upper and lower sites, and compared with just upper alone) (table 6). Dyspepsia increased the odds for bloating in the upper abdomen. No significant association of the site of bloating with GORD was detected (table 6).
Table 6.
Abdominal bloating sites in different FGIDs. Results are given as odds ratios* (95% CI)
| Upper site vs general abdomen | Lower site vs general abdomen | Lower site vs upper site | |
|---|---|---|---|
| Female | 0.5 (0.3 to 0.7) | 2.1 (1.4 to 3.4) | 4.6 (2.9 to 7.4) |
| IBS† | 0.7 (0.3 to 1.4) | 2.1 (1.1 to 4.0) | 3.2 (1.5 to 6.8) |
| Dyspepsia† | 5.8 (1.5 to 22.4) | 1.5 (0.3 to 7.8) | 0.3 (0.1 to 1.03) |
| FC† | 0.8 (0.3 to 1.9) | 2.6 (1.3 to 5.4) | 3.3 (1.4 to 7.7) |
| GORD† | 1.2 (0.6 to 2.3) | 1.3 (0.7 to 2.6) | 1.1 (0.5 to 2.3) |
| GI complex overlap‡ | 0.9 (0.5 to 1.7) | 0.6 (0.4 to 1.1) | 0.7 (0.4 to 1.3) |
Multiple variable logistic models adjusted for age and SSC score.
IBS, dyspepsia, FC and GORD were cases with no GI complex symptom overlap.
GI complex overlap: subjects can be defined as more than one of the above disorders.
FC, functional constipation; FGIDs, functional gastro-intestinal disorders; GI, gastro-intestinal; GORD, gastro-oesophageal reflux disease; IBS, irritable bowel syndrome; SSC, Somatic Symptom Checklist.
Distribution of FGIDs and IBS subtypes in subjects with bloating and distention
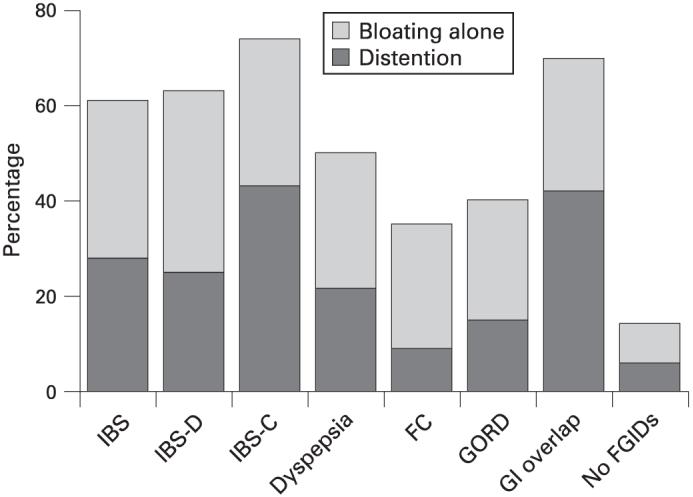
The distributions of FGIDs and GORD by bloating alone, distension and control groups are summarised in fig 4 (note that in fig 4A, subjects who satisfied criteria for more than one FGID subtypes were labelled as a GI complex overlap group). Overall, a significant association of bloating-distention status with FGIDs (p<0.001, fig 4A) and IBS subtype (p<0.001, fig 4B) was observed. In both the bloating alone and in the distention groups, the percentages of subjects reporting symptoms indicating IBS (including each of its subtypes, fig 4B), dyspepsia, and GORD were typically higher than in controls. In particular, a higher proportion of GI complex overlap and IBS-C subjects were observed in the distention group in contrast to the bloating alone group.
Figure 4.
(A) Distribution of only IBS, only dyspepsia, only FC, only GORD and GI complex overlap subjects as well as subjects with none of these disorders (no FGIDs) in groups with bloating alone, distention and no bloating/distention (no B/D) in Olmsted County, Minnesota. (B) Distribution of any IBS subtype (IBS-D; IBS-C, IBS-M and IBS-U) in group with bloating alone, distention and no bloating/distention (B/D) in Olmsted County, Minnesota. The numbers in brackets behind the series legend are absolute case numbers of each disorder in bloating/distention/no bloating or distention groups. FC, functional constipation; FGIDs, functional gastro-intestinal disorders; GI, gastro-intestinal; GORD, gastro-oesophageal reflux disease; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhoea-predominant IBS; IBS-M, mixed IBS (meets the criteria for both IBS-C and IBS-D); IBS-U, unsubtyped IBS (not meeting the criteria for the other three categories.
DISCUSSION
Abdominal bloating and visible distension are commonly reported by patients with FGIDs and by people in the community3 4 9 and the problem is often ranked as the most bothersome symptom.11 36 In the past, distention was considered a psychological symptom resulting from deliberate or unconscious protrusion of the abdomen37 but recent studies have concluded that abdominal distention is usually a real phenomenon.38 39 A considerable proportion of patients show a substantial increase in girth over the course of a day, which can reach up to 12 cm9 and this can be documented by objective ambulatory abdominal inductance plethysmography.38 39 In the present study, we report new data on the prevalence, risk factors and distribution of several FGIDs among subjects with abdominal bloating and visible distention in a population-based survey.
Our study showed that 19% of people experience abdominal bloating in the community and about half of the subjects with bloating also have visible distention. Why females report more bloating and have a higher risk of visible distention in the general population than males is unclear but similar findings were reported previously in IBS.7 10 11 Huerta-Franco et al40 reported that bloating is one of the most frequent menstrual symptoms, and a hormonal effect has been speculated.41-43 Another potential explanation may be differences in symptom expression by gender.4
It is controversial if psychological factors are important in the induction of bloating and distention.44 45 The SSC score is sensitive to psychosomatic distress and remains reliable over time.23 We observed that a higher overall SSC score, and higher scores on each of its 17 non-GI items, were positively associated with bloating and distention, suggesting that these symptoms may in part reflect the process of somatisation. Kroenke et al46 demonstrated that an increasing number of bodily complaints was a strong indicator of a non-organic disorder, while Neri et al47 reported that bloating discriminated IBS from those with organic disease. Further studies applying standard psychiatric interviews and the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria are needed to confirm the current observations.48
We observed that bloating and distention, although not considered of diagnostic value in most FGIDs, occurred in the majority of FGID cases identified in the community using modified Rome II criteria. IBS and each of its subtypes were strongly associated with bloating and distention. Especially notable was IBS-C, which was most strongly linked to visible distention; those with IBS-C were 14-fold more likely to have distention compared to controls, and twice as likely to have distention compared to bloating alone.
Other observations support our current findings that most IBS-C patients with bloating have visible distention.7 One possible explanation may be slower than normal colonic transit promoting increased bacterial overgrowth, and hence colonic fermentation and increased distention with stool and gas.11 49 On the other hand, some studies have shown that gas infusion of even up to 2 litres only results in a less than 2 cm change in girth (and was similar to that seen in healthy women).50 Tremolaterra et al51 reported that patients complaining of bloating and distention show impaired abdominal contraction in response to colonic gas loads, and even a paradoxical relaxation of the internal oblique muscle, suggesting a disorder in the abdominal wall accommodation reflex. Notably, we found that subjects with functional constipation were more likely to have bloating alone compared to distention, which might be related to the fact that patients with functional constipation feel more constantly distended and therefore do not perceive a diurnal variation in distension noticed by some IBS-C patients. In contrast, Chang et al7 reported in patients with IBS that bloating alone was more common in those with IBS-D. Houghton et al9 also suggested that bloating alone was more common in patients with IBS-D using objective measurement by abdominal inductance plethysmography. However, we could not confirm this in the general population. In our current study, IBS-D increased the risk for both bloating and distention compared to controls, but there was no increased risk for distention compared to bloating alone. Faster colonic transit,52 or perhaps a different bacterial flora in IBS-D may reflect this difference from IBS-C.
We also observed that dyspepsia was closely related to bloating and visible distention; about half of the subjects with dyspepsia had bloating symptoms and almost half of them had visible distention. Subjects with dyspepsia were at a 2-fold increased risk of distention compared to bloating alone. Impaired enterofundic and antrofundic relaxatory reflexes, and increased gastric perception of distention of a hypersensitive antrum, might explain the genesis of dyspeptic bloating.21 53 With respect to GORD, we observed an increased risk of bloating and distention compared to controls; however, GORD did not increase the risk of distention compared to bloating alone. Interestingly, we found that FGID symptom complex overlap was strongly association with bloating and especially with visible distention, suggesting the presence of more gut physiological derangements may lead to a higher risk.
A previous study suggested that the site of bloating may be hard to distinguish because patients have difficulty accurately localising this symptom.4 However, in the present study, 96.5% of bloaters defined their bloating site without apparent difficulty. Females reported bloating to occur more commonly in the lower abdomen, but whether this is related to any gynaecological factors is unknown. We also observed that, as expected, dyspepsia was associated with more upper abdominal bloating, which may support bloating originating from gastroduodenal dysfunction,54 while IBS and functional constipation had more lower abdominal bloating perhaps related to abnormalities of intestinal gas handling.50 55-57 However, we did not find any site of bloating was associated with GORD.
The mechanisms responsible for bloating and distention in the population are still largely unknown. We analysed putative risk factors for bloating and distention separately, but found that bloating and distention had similar risk factors. This suggests that bloating and visible distention may arise from distinctive but interrelated pathophysiological processes. Moreover, we found that bloating and distention were closely associated with somatic distress but the causal direction cannot be determined here.
The data resources of the Rochester Epidemiology Project and the unique medical delivery features in Olmsted County, Minnesota, allow the opportunity to perform high quality population-based research.20 We believe that the results from this study are valid and generalisable to the American white population.58 On the other hand, because a segment of the population of Olmsted County has been assessed by questionnaires on more than one occasion, it is conceivable that this might make them more vigilant regarding the presence of possible symptoms, although we know of no evidence that this is the case. Because bloating and distention are known to be common and usually of long duration, we chose to use a cross-sectional design which was relatively feasible and affordable to test our hypotheses; however, this study design cannot establish causal relationships. Our response rate was adequate and additional data comparing respondents and non-respondents suggest non-response bias is unlikely.31 We also confirmed that study subjects were cancer free during the survey period; while we did identify one case of ascites in the distention group, this would not have materially influenced our results.
In conclusion, bloating and distention are common in the community; these complaints are closely related to other FGIDs and somatisation, and have similar risk factors. Female gender, somatic symptoms, IBS-C subtype and dyspepsia, and GI symptom complex overlap increased the risk for distension over bloating alone. Further research on underlying mechanisms and effective treatments appear warranted based on the present results.
Acknowledgements
The authors wish to thank Susan M. Schlichter for her assistance in the preparation of the manuscript.
Footnotes
Competing interests: None.
Ethics approval: This study was approved by the Institutional Review Board of the Mayo Clinic.
REFERENCES
- 1.Houghton LA, Whorwell PJ. Towards a better understanding of abdominal bloating and distension in functional gastrointestinal disorders. Neurogastroenterol Motil. 2005;17:500–11. doi: 10.1111/j.1365-2982.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. In: Drossman DA, editor. ROME II.I The Functional Gastrointestinal Disorders. third edn. Degnon Associates, Inc.; Mclean, Virginia: 2006. pp. 487–555. [Google Scholar]
- 3.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talley NJ, Dennis EH, Schettler-Duncan VA, et al. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454–9. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. In: Drossman DA, Talley NJ, Thompson WG, Whitehead WE, Corazza GR, editors. Rome II. Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology, and Treatment. second edn. Degnon Associates, Inc; Mclean, VA: 2000. pp. 351–432. [Google Scholar]
- 6.Sandler RS, Stewart WF, Liberman JN, et al. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig Dis Sci. 2000;45:1166–71. doi: 10.1023/a:1005554103531. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Lee OY, Naliboff B, et al. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–7. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 8.Lea R, Whorwell PJ. Expert commentary - bloating, distension, and the irritable bowel syndrome. MedGenMed. 2005;7:18. [PMC free article] [PubMed] [Google Scholar]
- 9.Houghton LA, Lea R, Agrawal A, et al. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003–10. doi: 10.1053/j.gastro.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Smith RC, Greenbaum DS, Vancouver JB, et al. Gender differences in Manning criteria in the irritable bowel syndrome. Gastroenterology. 1991;100:591–5. doi: 10.1016/0016-5085(91)80002-q. [DOI] [PubMed] [Google Scholar]
- 11.Schmulson M, Lee OY, Chang L, et al. Symptom differences in moderate to severe IBS patients based on predominant bowel habit. Am J Gastroenterol. 1999;94:2929–35. doi: 10.1111/j.1572-0241.1999.01440.x. [DOI] [PubMed] [Google Scholar]
- 12.Talley NJ, Holtmann G, Agreus L, et al. Gastrointestinal symptoms and subjects cluster into distinct upper and lower groupings in the community: a four nations study. Am J Gastroenterol. 2000;95:1439–47. doi: 10.1111/j.1572-0241.2000.02075.x. [DOI] [PubMed] [Google Scholar]
- 13.Bijkerk CJ, Muris JW, Knottnerus JA, et al. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245–51. doi: 10.1111/j.0269-2813.2004.01862.x. [DOI] [PubMed] [Google Scholar]
- 14.Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol. 2004;99:2046–50. doi: 10.1111/j.1572-0241.2004.40266.x. [DOI] [PubMed] [Google Scholar]
- 15.Delgado-Aros S, Locke GR, 3rd, Camilleri M, et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99:1801–6. doi: 10.1111/j.1572-0241.2004.30887.x. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ, Quan C, Jones MP, et al. Association of upper and lower gastrointestinal tract symptoms with body mass index in an Australian cohort. Neurogastroenterol Motil. 2004;16:413–9. doi: 10.1111/j.1365-2982.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan SN. A prospective study of unexplained visible abdominal bloating. NZ Med J. 1994;107:428–30. [PubMed] [Google Scholar]
- 18.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–80. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 19.Maxton DG, Morris JA, Whorwell PJ. Ranking of symptoms by patients with the irritable bowel syndrome. BMJ. 1989;299:1138. doi: 10.1136/bmj.299.6708.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Talley NJ, Phillips SF, Melton J, 3rd, et al. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 22.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 23.Attanasio V, Andrasik F, Blanchard EB, et al. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7:247–57. doi: 10.1007/BF00845390. [DOI] [PubMed] [Google Scholar]
- 24.Talley NJ, Zinsmeister AR, Schleck CD, et al. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–68. [PubMed] [Google Scholar]
- 25.Talley NJ, Weaver AL, Zinsmeister AR, et al. Onset and disappearance of gastrointestinal symptoms and functional gastrointestinal disorders. Am J Epidemiol. 1992;136:165–77. doi: 10.1093/oxfordjournals.aje.a116483. [DOI] [PubMed] [Google Scholar]
- 26.Talley NJ, Zinsmeister AR, Schleck CD, et al. Smoking, alcohol, and analgesics in dyspepsia and among dyspepsia subgroups: lack of an association in a community. Gut. 1994;35:619–24. doi: 10.1136/gut.35.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talley NJ, Fett SL, Zinsmeister AR, et al. Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–9. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 28.Locke GR, 3rd, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 29.Locke GR, 3rd, Weaver AL, Melton LJ, 3rd, et al. Psychosocial factors are linked to functional gastrointestinal disorders: a population based nested case-control study. Am J Gastroenterol. 2004;99:350–7. doi: 10.1111/j.1572-0241.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 30.Halder SL, Locke GR, 3rd, Schleck CD, et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Choung RS, Locke GR, Zinsmeister AR. Epidemiology of slow and fast colonic transit using a stool form scale in a community. Alim Pharmacol Therap. 2007;26:1043–50. doi: 10.1111/j.1365-2036.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- 32.Talley NJ, Stanghellini V, Heading RC, et al. Functional gastroduodenal disorders. Gut. 1999;45(Suppl 2):II37–42. doi: 10.1136/gut.45.2008.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–24. doi: 10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 34.Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128:580–9. doi: 10.1053/j.gastro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Locke GR, 3rd, Talley NJ, Fett SL, et al. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–9. doi: 10.1016/s0002-9343(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 36.Lembo T, Naliboff B, Munakata J, et al. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–6. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez WC. Hysterical type of nongaseous abdominal bloating. Arch Intern Med. 1949;84:217–45. doi: 10.1001/archinte.1949.00230020020002. [DOI] [PubMed] [Google Scholar]
- 38.Reilly BP, Bolton MP, Lewis MJ, et al. A device for 24 hour ambulatory monitoring of abdominal girth using inductive plethysmography. Physiol Meas. 2002;23:661–70. doi: 10.1088/0967-3334/23/4/306. [DOI] [PubMed] [Google Scholar]
- 39.Lewis MJ, Reilly B, Houghton LA, et al. Ambulatory abdominal inductance plethysmography: towards objective assessment of abdominal distension in irritable bowel syndrome. Gut. 2001;48:216–20. doi: 10.1136/gut.48.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huerta-Franco MR, Malacara JM. Association of physical and emotional symptoms with the menstrual cycle and life-style. J Reprod Med. 1993;38:448–54. [PubMed] [Google Scholar]
- 41.Heitkemper MM, Jarrett M, Cain KC, et al. Daily gastrointestinal symptoms in women with and without a diagnosis of IBS. Dig Dis Sci. 1995;40:1511–9. doi: 10.1007/BF02285200. [DOI] [PubMed] [Google Scholar]
- 42.Heitkemper MM, Cain KC, Jarrett ME, et al. Symptoms across the menstrual cycle in women with irritable bowel syndrome. Am J Gastroenterol. 2003;98:420–30. doi: 10.1111/j.1572-0241.2003.07233.x. [DOI] [PubMed] [Google Scholar]
- 43.Houghton LA, Lea R, Jackson N, et al. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–4. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song JY, Merskey H, Sullivan S, et al. Anxiety and depression in patients with abdominal bloating. Can J Psychiatry. 1993;38:475–9. doi: 10.1177/070674379303800703. [DOI] [PubMed] [Google Scholar]
- 45.Johnsen R, Jacobsen BK, Forde OH. Associations between symptoms of irritable colon and psychological and social conditions and lifestyle. Br Med J (Clin Res Edn) 1986;292:1633–5. doi: 10.1136/bmj.292.6536.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroenke K, Spitzer RL, Williams JB, et al. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994;3:774–9. doi: 10.1001/archfami.3.9.774. [DOI] [PubMed] [Google Scholar]
- 47.Neri M, Laterza F, Howell S, et al. Symptoms discriminate irritable bowel syndrome from organic gastrointestinal diseases and food allergy. Eur J Gastroenterol Hepatol. 2000;12:981–8. doi: 10.1097/00042737-200012090-00003. [DOI] [PubMed] [Google Scholar]
- 48.Ustun B, Compton W, Mager D, et al. WHO study on the reliability and validity of the alcohol and drug use disorder instruments: overview of methods and results. Drug Alcohol Depend. 1997;47:161–9. doi: 10.1016/s0376-8716(97)00087-2. [DOI] [PubMed] [Google Scholar]
- 49.Di Stefano M, Miceli E, Missanelli A, et al. Role of colonic fermentation in the perception of colonic distention in irritable bowel syndrome and functional bloating. Clin Gastroenterol Hepatol. 2006;4:1242–7. doi: 10.1016/j.cgh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–9. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tremolaterra F, Villoria A, Azpiroz F, et al. Impaired viscerosomatic reflexes and abdominal-wall dystony associated with bloating. Gastroenterology. 2006;130:1062–8. doi: 10.1053/j.gastro.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 52.Vassallo M, Camilleri M, Phillips SF, et al. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102–8. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- 53.Knill-Jones RP. A formal approach to symptoms in dyspepsia. Clin Gastroenterol. 1985;14:517–29. [PubMed] [Google Scholar]
- 54.Caldarella MP, Azpiroz F, Malagelada JR. Antro-fundic dysfunctions in functional dyspepsia. Gastroenterology. 2003;124:1220–9. doi: 10.1016/s0016-5085(03)00287-7. [DOI] [PubMed] [Google Scholar]
- 55.Caldarella MP, Serra J, Azpiroz F, et al. Prokinetic effects in patients with intestinal gas retention. Gastroenterology. 2002;122:1748–55. doi: 10.1053/gast.2002.33658. [DOI] [PubMed] [Google Scholar]
- 56.Cann PA, Read NW, Brown C, et al. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983;24:405–11. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serra J, Azpiroz F, Malagelada JR. Mechanisms of intestinal gas retention in humans: impaired propulsion versus obstructed evacuation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G138–43. doi: 10.1152/ajpgi.2001.281.1.G138. [DOI] [PubMed] [Google Scholar]
- 58.Talley NJ, Zinsmeister AR, Van Dyke C, et al. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–34. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]