Summary
The Mre11/Rad50 complex has been implicated in the early steps of DNA double-strand break (DSB) repair through homologous recombination in several organisms. However, the enzymatic properties of this complex are incompatible with the generation of 3’ single-stranded DNA for recombinase loading and strand exchange. In thermophilic archaea, the Mre11 and Rad50 genes cluster in an operon with genes encoding a helicase, HerA, and a 5’ to 3’ exonuclease, NurA, suggesting a common function. Here we show that purified Mre11 and Rad50 from Pyrococcus furiosus act cooperatively with HerA and NurA to resect the 5’ strand at a DNA end under physiological conditions in vitro. The 3’ single-stranded DNA generated by these enzymes can be utilized by the archaeal RecA homolog RadA to catalyze strand exchange. This work elucidates how the conserved Mre11/Rad50 complex promotes DNA end resection in archaea, and may serve as a model for DSB processing in eukaryotes.
Introduction
The rapid detection and subsequent repair of DNA double-strand breaks (DSBs) is critical for the survival of all organisms. Breaks in chromosomal DNA occur during replication, oxidative damage, programmed recombination events including meiosis and V(D)J recombination, and exposure to exogenous agents such as ionizing radiation (IR) and radiomimetic chemicals. Two distinct pathways exist to repair DSBs: non-homologous end joining (NHEJ) and homologous recombination (HR). NHEJ proteins process and religate the DNA ends, while the HR pathway uses a homologous template to repair DNA breaks and is considered a relatively error-free method of DSB repair.
The choice of DSB repair pathway varies by organism as well as by cell cycle stage. In bacteria, the predominant DSB repair pathway is HR (Kowalczykowski et al., 1994). Both NHEJ and HR are utilized in eukaryotic cells, however HR is preferred in S and G2 when sister chromatids are present (Sonoda et al., 2006). HR is presumably the preferred method of DSB repair in archaea as proteins involved in HR have been identified but NHEJ Ku70/80 homologs are absent in most archaea (Aravind and Koonin, 2001; Doherty et al., 2001).
HR proceeds in several distinct stages: the earliest step is processing of the DNA end to produce 3’ single-stranded DNA (ssDNA). Following or during 5’ strand resection, the 3’ ssDNA is bound by RecA-family recombinases into a filament that catalyzes homologous pairing and DNA strand exchange. The 3’ end then primes DNA synthesis, and resolution of Holliday junctions or strand annealing between newly-synthesized ends results in repair of the initial DSB (Seitz et al., 2001).
Much of our understanding of the initial DSB processing step in HR comes from studies of the E. coli RecBCD complex (Kowalczykowski et al., 1994; Kuzminov, 1999; Singleton et al., 2004; Smith, 2001). RecBCD exists as a heterotrimeric complex and exhibits DNA helicase, ATPase, and both 5’ and 3’ exonuclease activities. RecBCD binds with high affinity to DNA ends and translocates along the DNA duplex, usually accompanied by degradation of both strands, until it encounters the cis-element Chi on the 3’ strand. Binding of RecBCD to the Chi sequence results in pausing, nicking of the 3’ strand at Chi, and 5’ DNA strand resection (or further unwinding of the duplex). RecBCD also facilitates the loading of the RecA recombinase onto the 3’ ssDNA, which catalyzes strand exchange.
Despite the importance of RecBCD for DSB processing in bacteria, there are no apparent functional homologs of this complex in eukaryotes or archaea. In eukaryotes, the processing step is a critical decision point in HR and is controlled by cyclin-dependent kinases to occur in the S and G2 phases of the cell cycle (Aylon et al., 2004; Ira et al., 2004; Jazayeri et al., 2006). Genetic evidence from budding yeast suggests that the Mre11/Rad50/Xrs2 (MRX) complex is involved in the resection process in eukaryotic cells. The Mre11, Rad50, and Xrs2 genes were identified in S. cerevisiae through mutations which resulted in poor vegetative growth, reduced survival of ionizing radiation (IR), and spore inviability (Symington, 2002). Hypomorphic mutations in Rad50 (and functionally similar mutations later found in Mre11) were also found to specifically block resection of Spo11-induced DSBs during meiosis (Alani et al., 1990; Nairz and Klein, 1997; Tsubouchi and Ogawa, 1998). This evidence, combined with observations that MRX-null mutants exhibit a marked delay in DSB resection in vegetative cells (Ivanov et al., 1994), suggests that the MRX complex plays a direct role in DNA end processing in eukaryotes.
Homologs of Mre11 and Rad50 have been found in all organisms studied to date and exist as a stable complex. In eukaryotes, the Mre11/Rad50 (MR) complex also contains Nbs1 (Nibrin) in mammalian cells or Xrs2 in budding yeast to form MRN and MRX, respectively. However, the E. coli SbcCD complex and archaebacteria MR do not have an associated third component. Sequence analysis of Mre11/SbcD identified four N-terminal phosphodiesterase and two DNA-binding domains, and biochemical studies demonstrate that Mre11 exhibits manganese-dependent 3’-5’ exonuclease and ssDNA endonuclease activities in vitro (Connelly et al., 1999; Paull and Gellert, 1998; Trujillo and Sung, 2001). Rad50/SbcC is architecturally related to the Structural Maintenance of Chromosomes (SMC) family of proteins, with N- and C-terminal head domains which contain the Walker A and Walker B motifs, respectively, separated by a long coiled-coil region with a zinc hook. Biochemical characterization of Rad50 indicate that in addition to ATPase activity, it posseses adenylate kinase activity which is involved in DNA tethering (Bhaskara et al., 2007). The MR complex also plays a role in both NHEJ and HR pathways of DSB repair (D'Amours and Jackson, 2002).
Archaea constitute the third kingdom of life and consist of organisms that live in varied and extreme conditions. In all thermophilic archaea studied to date, the genes encoding Mre11 and Rad50 are found together in an operon, similar to E. coli SbcCD. In addition to Mre11 and Rad50, two other genes which encode an ATP-dependent, bidirectional DNA helicase (HerA/MlaA) and a 5’-3’ exonuclease (NurA) are found together with Mre11/Rad50 in almost all thermophilic archaea (Constantinesco et al., 2002; Constantinesco et al., 2004; Manzan et al., 2004). Besides their initial biochemical characterization, little is known about HerA and NurA in vivo activities. Based on this strong association and genetic evidence from eukaryotic cells, we hypothesize that Mre11 and Rad50 may function with the HerA and NurA gene products to catalyze DSB resection. To test this idea, we utilized proteins from the Euryarchaea hyperthermophile Pyrococcus furiosus (Pf) which grows optimally at 96°C under anaerobic conditions (Allers and Mevarech, 2005). The archaeal homologs of Mre11 and Rad50 were initially identified in P. furiosus (Hopfner et al., 2000a), and the crystal structures of the catalytic domains have been solved for these proteins (Hopfner et al., 2002; Hopfner et al., 2001; Hopfner et al., 2000b).
In this work, we show that purified, recombinant PfHerA and PfNurA interact physically and functionally to carry out helicase and nuclease activities on double-stranded DNA. We also show that at limiting levels of HerA and NurA, PfMre11/Rad50 is required for 5’ end resection and that this process is dependent upon the enzymatic activities of HerA, NurA, and Rad50 but not Mre11. We then demonstrate that all four proteins together with the RecA-homolog RadA are able to catalyze the formation of D-loop recombination intermediates from linear dsDNA and circular dsDNA in vitro. Our findings support a model where the MR complex is involved in the initial processing step required for the loading or activation of HerA-NurA to promote resection of the 5’ strand of the DSB and initiation of strand invasion.
Results
HerA and NurA physically and functionally interact
Based on the operon clustering of the Mre11, Rad50, HerA, and NurA genes in thermophilic archaea, we hypothesized that these gene products may interact physically and/or functionally. To investigate this possibility, we cloned the HerA and NurA genes from the Pyrococcus furiosus genomic DNA, expressed each protein separately in E. coli, purified the proteins to ≥95% homogeneity (Fig. S1A), and performed gel filtration, nuclease, and helicase assays. We observed that P. furiosus HerA fractionates as a large complex by gel filtration (Fig. S2, top panel), consistent with electron microscopy results showing that a homolog of HerA from M. thermoautotrophicus exists in a hexameric ring structure (Manzan et al., 2004). The P. furiosus NurA gel filtration profile is consistent with either a monomer or dimer stoichiometry (Fig. S2, middle panel). A strong, ATP-independent interaction was observed between HerA and NurA when incubated together (Fig. S2, bottom panel), and based on this result we estimate that between 1–3 NurA monomers bind the HerA hexamer. No direct interaction was observed between purified, recombinant P. furiosus Mre11/Rad50 (MR) and either HerA or NurA (data not shown).
Consistent with the physical interaction between HerA and NurA, we found that the catalytic activities of these enzymes were mutually interdependent. The 5’ to 3’ exonuclease activity of NurA was strictly dependent on manganese when assayed alone, similar to previous results with NurA from Sulfolobus acidocaldarius (Constantinesco et al., 2002), but in the presence of HerA the two enzymes exhibited robust exonuclease and helicase activity in physiological levels of magnesium (Fig. S3 and Fig S4C). Nucleolytic degradation of linear DNA was dependent on the ATP-related functions of HerA and nuclease-activity of NurA (Fig. S5).
The Mre11/Rad50 complex stimulates DNA degradation with HerA and NurA
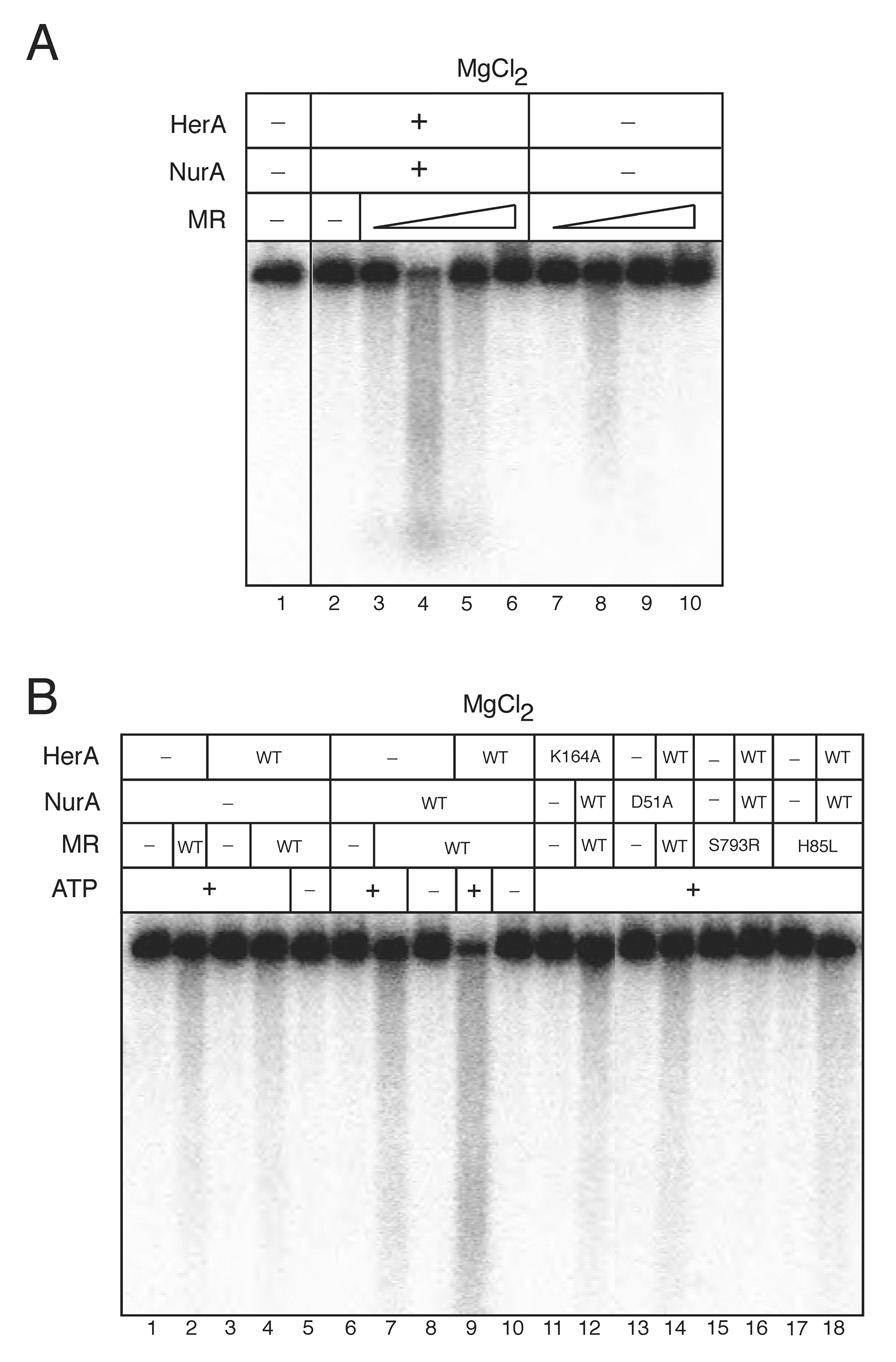
Based on the genomic structure of the operon containing the Mre11, Rad50, HerA, and NurA genes in thermophilic archaea, we hypothesized that Mre11/Rad50 (MR) may act cooperatively with HerA and NurA. To investigate this possibility, nuclease assays were performed with limiting concentrations of HerA and NurA (2.7 nM HerA hexamer, 19 nM NurA monomer) on a 2.5 kb linear DNA substrate (Fig. 1A). When purified MR was titrated into the reaction, we observed a 19-fold stimulation in DNA degradation in the presence of 3.3 nM MR (assuming an M2R2 complex) (Fig. 1A, lane 2 vs. 4 and Fig S6). Under these conditions, 3.3 nM MR demonstrates weak nuclease activity in magnesium in the absence of HerA and NurA (Fig. 1A, lane 8). Efficient degradation of dsDNA required all four proteins and ATP (Fig. 1B, lanes 1–10). To investigate the requirements for individual catalytic activities, we utilized a nuclease-deficient mutant of NurA (D51A; Fig. S4A), a helicase-deficient mutant of HerA (K164A; Fig. S4B (Constantinesco et al., 2004)), a nuclease-deficient mutant of Mre11 (H85L, “Mre11-3”; (Arthur et al., 2004)), and an ATPase- and adenylate kinase-deficient mutant of Rad50 (S793R; (Bhaskara et al., 2007; Hopfner et al., 2000b; Moncalian et al., 2004)). As shown in Fig. 1B, efficient degradation of the linear DNA substrate required the catalytic activities of all four enzymes. However, the nuclease function(s) of Mre11 appear to be partially dispensable for the cooperative degradation activity, as the reactions containing a nuclease-deficient Mre11 H85L mutant showed DNA degradation greater than that observed in reactions containing the Rad50 S793R mutant, but still considerably less than with wild-type (WT) Mre11 (Fig. 1B, lanes 9, 15–18). Taken together, these results indicate that all four gene products interact functionally to degrade dsDNA.
Figure 1. HerA and NurA degrade dsDNA cooperatively with Mre11/Rad50 in magnesium.
(A) Nuclease assays were performed using 2.5 kb internally [32P]-labeled dsDNA. Reactions contained 2.7 nM wild-type HerA and 19.2 nM wild-type NurA, 5 mM MgCl2, 1 mM ATP, and 100 mM NaCl. Wild-type MR complex was included in the reaction at 0.3, 3.3, 33, and 330 nM. HerA molar concentrations are given as a hexamer of 370 kDa, NurA concentrations are given as a monomer of 52 kDa, and MR complex is given as a 2:2 stoichiometric complex of 306 kDa.
(B) Reactions performed as in (A) with HerA wild-type or K164A, NurA wild-type or D51A, and 3.3 nM MR wild-type, M(H85L)R, or MR(S793R) as indicated in 80 mM NaCl.
The directionality of Mre11/Rad50/HerA/NurA resection is 5’ to 3’
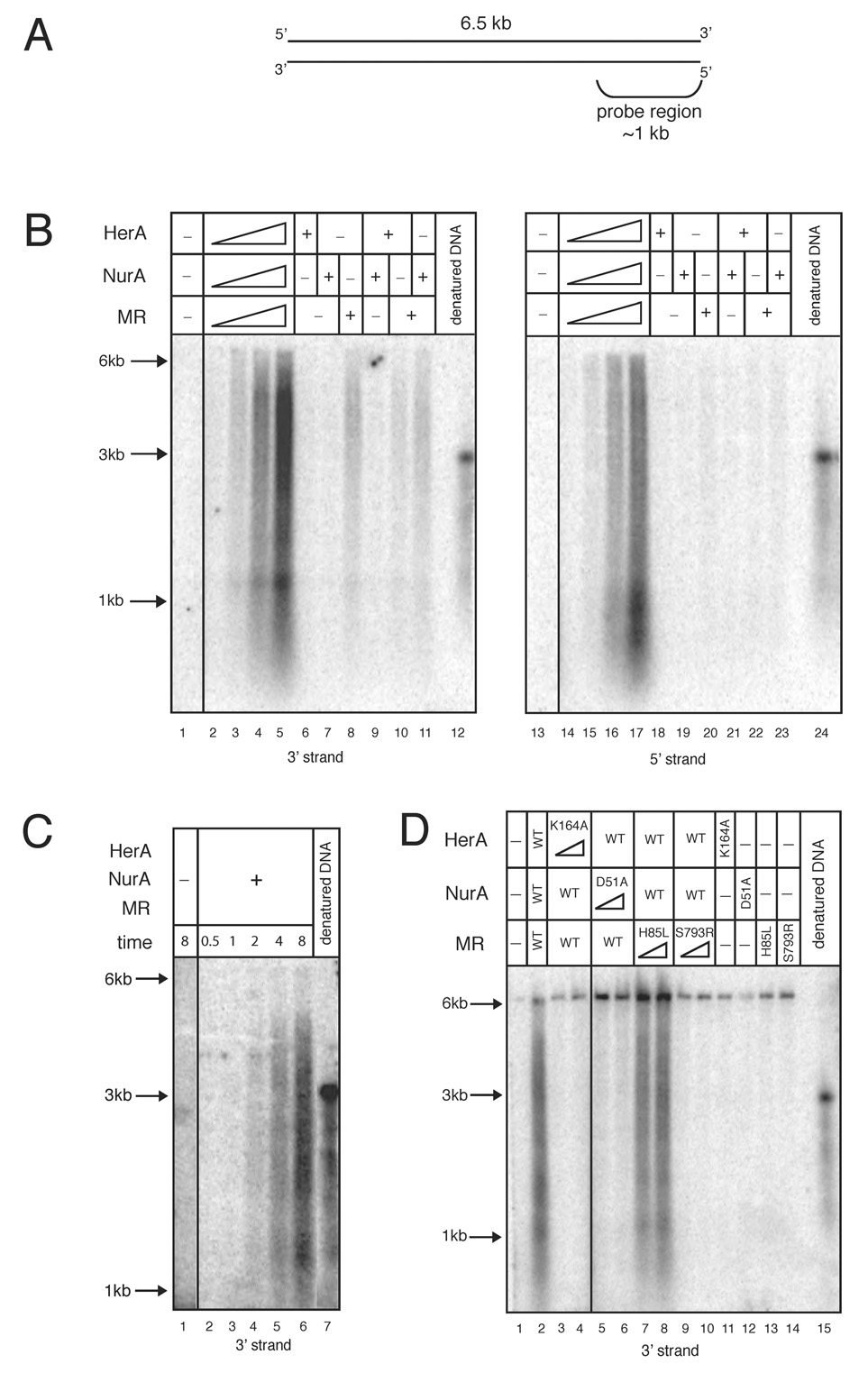
To determine the polarity of the DNA resection catalyzed by Mre11, Rad50, HerA, and NurA (PfMRHN), we used a 6.5 kb linearized plasmid DNA substrate and analyzed the reaction products using non-denaturing Southern hybridization with RNA probes specific for either the 3’ or 5’ strand of one end of the linear plasmid (Fig. 2A). In this reaction with all four proteins present, the 3’ strand of the DNA end was exposed as the DNA substrate was degraded (Fig. 2B, lanes 2–5, “3’ strand”). The resection of the 5’ end occurred rapidly as products were seen as early as 2 minutes (Fig. 2C, lane 4). We also observed significant levels of 5’ ssDNA generated in the reaction, although less efficient than the production of 3’ ssDNA (Fig. 2B, lanes 14–17, “5’ strand”). Southern blot analysis of the resection reactions using HerA, NurA, and MR catalytic mutants demonstrated that the nuclease activity of NurA and the ATP-dependent function of HerA are both required for production of 3’ ssDNA (Fig. 2D, lanes 3–6). In agreement with the results utilizing labeled DNA substrates, mutation of the Mre11 nuclease domain reduces the efficiency of 5’ strand processing but the ATP-related activities of Rad50 are essential for this process (Fig. 2D, lanes 7–10).
Figure 2. Mre11, Rad50, HerA, and NurA function cooperatively to resect the 5’ strand of a DSB.
(A) Schematic of the linearized plasmid substrate used in nuclease reactions analyzed by Southern hybridization. The DNA length and location of the probe region are indicated.
(B) Nuclease assays were performed with a 6.5 kb linearized plasmid DNA substrate and analyzed by non-denaturing Southern hybridization with strand-specific RNA probes. Reactions contained 0.3, 1.4, 2.7, and 5.4 nM wild-type HerA, 1.9, 9.5, 19.2, and 38.4 nM wild-type NurA, and 0.3, 1.7, 3.3, and 6.6 nM wild-type MR in 5 mM MgCl2, 1 mM ATP, and 100 mM NaCl. “+” indicates 5.4 nM HerA, 38.4 nM NurA, and 6.6 nM MR wild-type proteins. Reactions were incubated at 65°C for 15 min and analyzed in native agarose gels. The denatured DNA blot control consists of NaOH-denatured substrate DNA, which runs at a position equivalent to ~3 kb non-denatured dsDNA. DNA molecular weight ladder positions of 6, 3, and 1 kb are indicated. The probe used in the left panel recognizes the 3’ strand on one end of the DNA, while the probe used in the right panel recognizes the 5’ strand.
(C) Time course reaction as in (B) with 5.4 nM HerA, 38.4 nM NurA, and 6.6 nM MR wild-type proteins. Reactions were stopped at 0.5, 1, 2, 4, or 8 mins and analyzed with the 3’ strand-specific probe.
(D) Nuclease assays as in (B) but with 5.4 or 10.8 nM HerA K164A, 38.4 or 76.8 nM NurA D51A, and 6.6 or 13.2 nM M(H85L)R and MR(S793R) as indicated. “WT” indicates 5.4 nM HerA, 38.4 nM NurA, and 6.6 nM MR wild-type proteins. Reactions were analyzed with the 3’ strand-specific probe.
To determine whether the resection process catalyzed by PfMRHN is a processive reaction, we carried out competition experiments and analyzed the products by Southern hybridization. We found that adding up to 50-fold molar excess competitor linear DNA did not decrease the level of 5’ strand degradation after resection had been initated. However, when the same concentration of competitor DNA was added at the beginning of the reaction, resection was completely abolished (data not shown). These results demonstrate that 5’ strand resection by PfMRHN occurs through a processive mechanism.
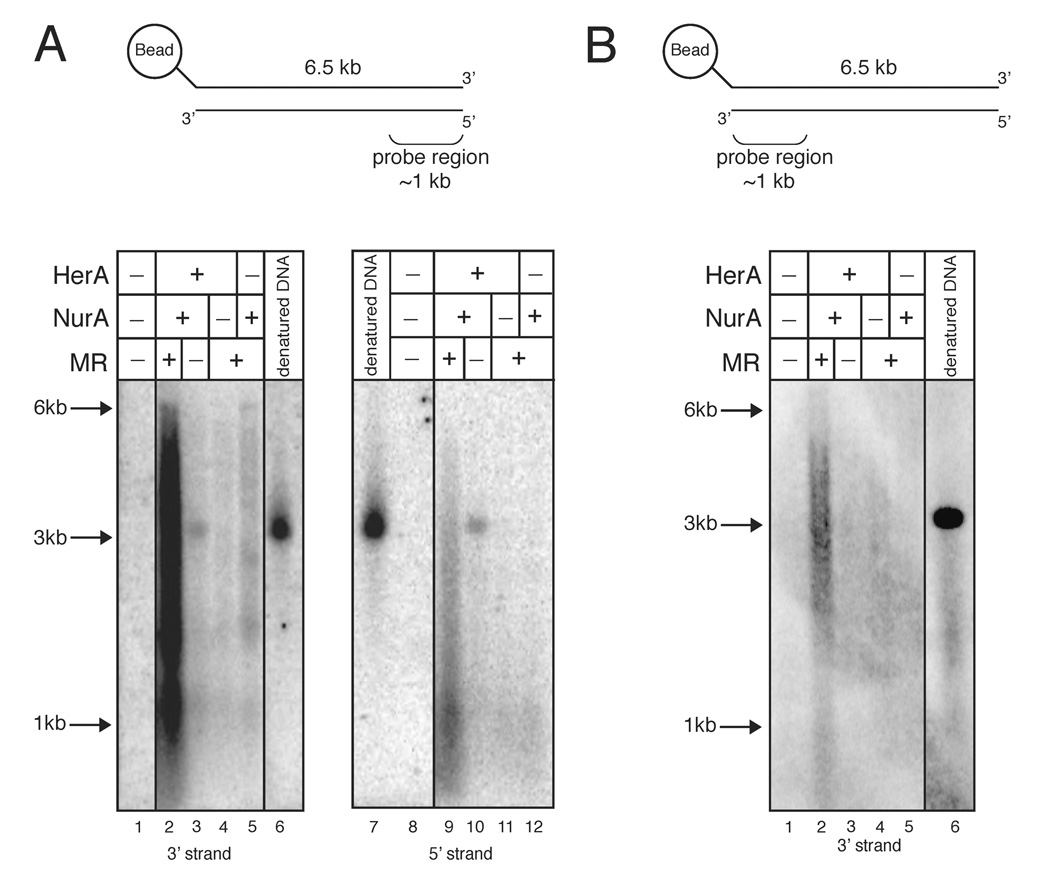
We considered two possibilities for the generation of both 5’ and 3’ ssDNA in the resection reaction: bidirectional but uncoupled degradation of each DNA end, or resection of the 5’ strand from both ends of the DNA. The latter situation would occur if the enzymes were present at saturating levels in the reaction. Consistent with this hypothesis, we observed that increasing the concentration of DNA in the reactions increased the ratio of 3’ vs. 5’ ssDNA generated (data not shown). To determine more conclusively whether the generation of 5’ ssDNA is the result of degradation from the distal 5’ strand, we constructed a substrate with a biotin attached at the distal 5’ end. The biotinylated DNA was then attached to streptavidin-coated magnetic beads to block access to the distal 5’ strand, and used as a substrate in the resection assay. As shown in Fig. 3A, the generation of 5’ ssDNA was greatly reduced in comparison to the 3’ ssDNA product. This result strongly supports the conclusion that the cooperative activities of Mre11, Rad50, HerA, and NurA, resect the 5’ strand at a DNA DSB.
Figure 3. Mre11, Rad50, HerA, and NurA resects the 5’ strand and not the 3’ strand.
(A) Nuclease assays were performed as in Fig. 2 except that the linearized 6.5 kb plasmid substrate was attached to a magnetic bead via a biotin/streptavidin linkage to the 5’ strand of the DNA distal to the probe region (see schematic at top). Assays were performed with 5.4 nM wild-type HerA, 38.4 nM wild-type NurA, and 6.6 nM wild-type MR as indicated for 30 min and analyzed in native agarose gels.
(B) Nuclease assays were performed as in (A) but the reaction products were analyzed using a probe specific for the 3’ strand adjacent to the 5’ biotin/bead (see schematic at top).
The products of the resection reaction shown in Fig. 3A probed for the 3’ strand range in size from nearly full-length (6.5 kb) down to less than 1 kb in the native agarose gel; however, complete resection of the bottom strand of the substrate should not yield products smaller than ~3 kb (the migration of full-length ssDNA). 2-dimensional gel analysis of these products (first dimension native, second dimension denaturing) indicated that a subset of the products were in fact shorter than full-length (data not shown). To explain this, we hypothesized that the enzymes in the reaction may be removing a subset of the DNA molecules from the bead by cleaving the 5’ strand proximal to the biotin-streptavidin linkage. To test this hypothesis, we performed the same reaction and a non-denaturing Southern blot but probed for the 3’ strand on the DNA end attached to the bead, a strand which should only be in single-stranded form if the bead is removed. This analysis is shown in Fig. 3B, clearly indicates that the 5’ end of the top strand is resected. This processing is less efficient than the processing of the open 5’ strand, but nevertheless provides evidence for 5’ strand conjugate removal in this system.
MR generates short 3’ overhangs through 5’ strand endonucleolytic activity
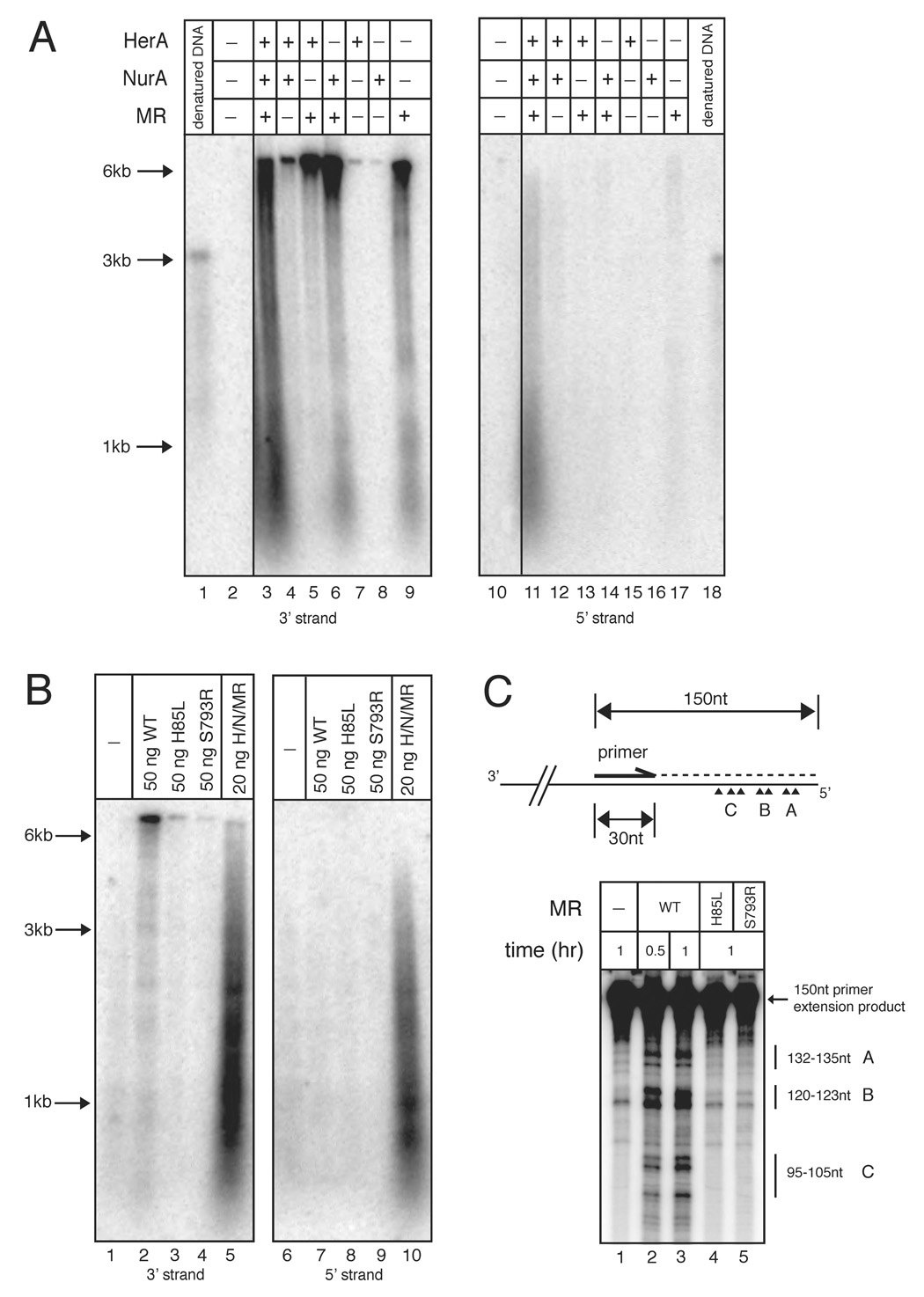
The role of the MR complex in the resection process is of significant interest since it is conserved in all organisms and has been implicated in the removal of 5’ covalent protein complexes during meiotic recombination in eukaryotes. To investigate the role of MR in the resection process, we performed the resection assay with a 6.5 kb DNA substrate in the absence of HerA and NurA but an increased level of MR to determine whether MR alone has detectable resection activity. Interestingly, 16.5 nM MR complex alone produced a signal on the blot specific for the 3’ strand, but the DNA product migrated close to the size of the 6.5 kb substrate, indicating that the product of the MR reaction contained 3’ ssDNA but was not extensively degraded (Fig. 4A, lane 9, 17 and 4B, lane 2, 7). Analysis of this product by primer extension indicated that approximately 15–55 nt of the 5’ strand are removed by MR and were found in three distinct groups ~15 nt apart (Fig. 4C and Fig.S7B, lanes 2, 3). Formation of these products was dependent on both the nuclease activity of Mre11 and the ATP-associated functions of Rad50 (Fig. 4B, lanes 3, 4, 4C and Fig.S7B, lanes 4, 5). No detectable 5’ ssDNA was observed, which indicates that MR plays a role in producing short 3’ single-stranded overhangs through either an exo- or endo-nucleolytic event. This processing by MR was enhanced by the presence of NurA while HerA alone did not affect MR activity (Fig. 4A, lanes 5, 6).
Figure 4. Mre11/Rad50 nuclease activity generates short 3’ overhangs.
(A) Nuclease assays were performed as in Fig. 2 but with 13.4 nM wild-type HerA, 96.2 nM wild-type NurA, and 16.5 nM wild-type MR as indicated. Reactions were incubated at 65°C for 1 hr.
(B) Reactions performed as in (A) but with 16.5 nM MR wild-type, M(H85L)R, or MR(S793R) proteins. Reactions in lanes 5 and 10 contained 5.4 nM HerA, 38.4 nM NurA, and 6.6 nM MR wild-type proteins. Reactions in lanes 1–4 and 6–9 were incubated at 65°C for 1 hr. Reactions in lanes 5 and 10 were incubated at 65°C for 15 min.
(C) (Top) Schematic of primer extension reaction. For simplicity, only the bottom strand of pTP163 is shown. The lengths of the full-length primer extension product and primer are indicated. The internally labeled primer extension product is indicated by the dashed line. Major MR resection sites are indicated as arrow heads and grouped into A, B, and C subgroups containing 2 or 3 cut sites. (Bottom) P. furiosus Mre11/Rad50 resection reactions were performed with 2.3 nM pTP163 linearized with SacI and 165 nM Mre11/Rad50 at 65°C for 30 min or 1 hr as indicated. Reactions were then used in primer extension reactions and the primer extension products were analyzed on 10% polyacrylamide denaturing sequencing gels. The position of the 150 nt primer extension product and the various resected products are indicated. Single-stranded DNA markers are indicated. For the full-size gel image, see Fig. S7.
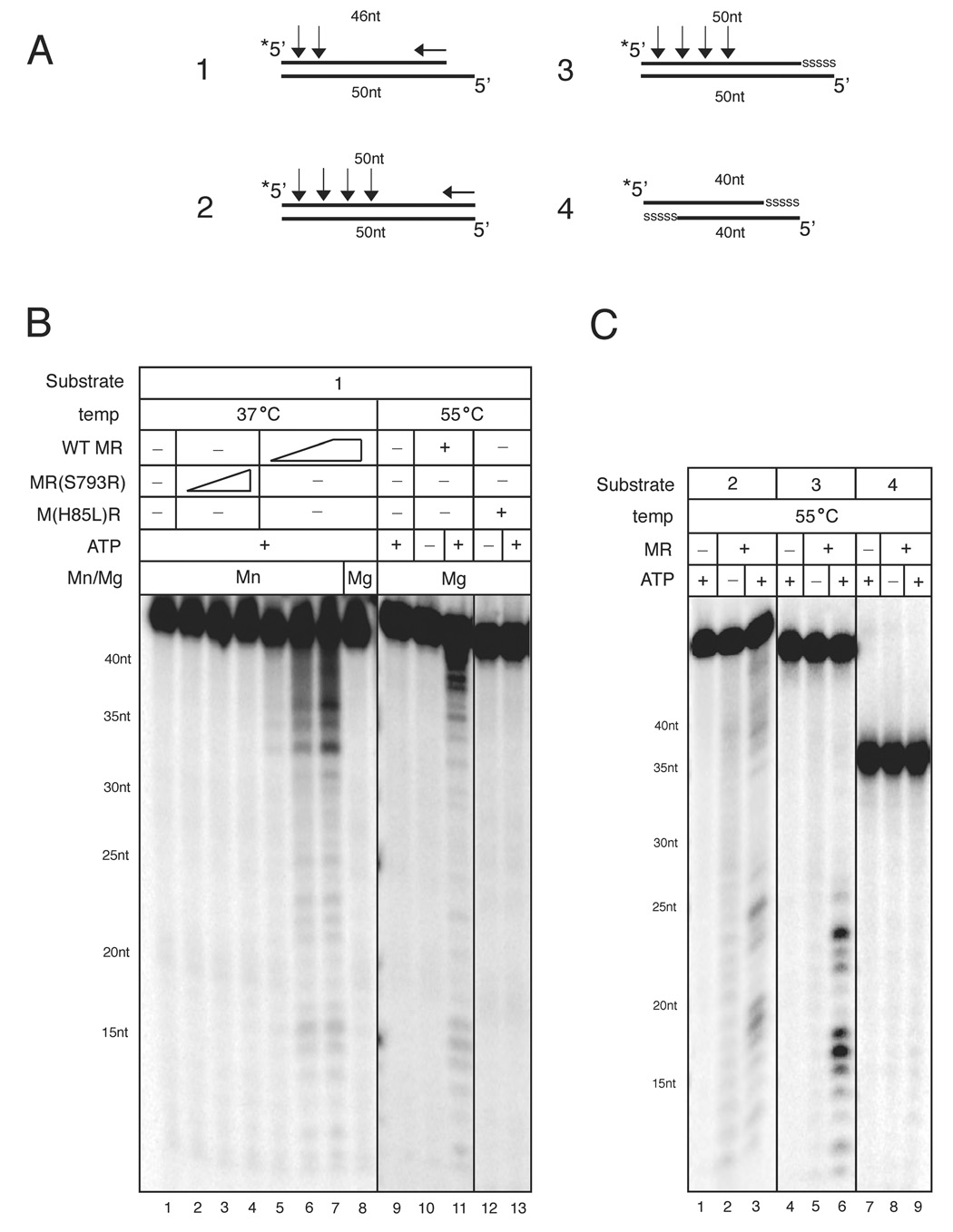
These results suggest that the P. furiosus MR complex has the ability to cleave the 5’ strand of a DNA end under physiological conditions in magnesium. In contrast, previous reports have only observed Mre11 3’–5’ exonuclease activity and this was dependent on manganese in the reaction (Hopfner et al., 2000a; Paull and Gellert, 1998; Trujillo et al., 1998; Usui et al., 1998). To examine this issue in greater detail we utilized 5’ [32P]-labeled dsDNA oligonucleotide substrates (Fig. 5A) and found that MR degraded DNA in a 3’-5’ direction on this substrate, as previously reported. At lower temperatures (37°C), MR was active in manganese but inactive in magnesium (Fig. 5B, lanes 7–8). However, at a temperature closer to physiological conditions for P. furiosus (55°C), MR demonstrated weak but detectable nuclease activity in magnesium which was dependent on ATP (Fig. 5B, lane 10, 11). This activity is Mre11-dependent because the H85L mutant does not generate these products (Fig. 5B, lane 12–13).
Figure 5. Mre11/Rad50 5’ endonucleolytic activity occurs in magnesium and is dependent on 3’-5’ exonuclease activity.
(A) Schematic of the oligonucleotide substrates is shown. The 5’ [32P]-label is indicated by an asterisk, and the length of each oligonucleotide is indicated. Phosphorothioate bonds are shown as “sssss.” The positions of the nucleolytic cuts from data shown in (B) and (C) are indicated by arrows. Vertical arrows indicate major endonuclease cleavage sites and the horizontal arrows denote 3'-5' exonuclease activity.
(B) Nuclease assays were performed with MR wild-type or MR(S793R) and 2 nM Substrate 1 at 37°C for 30 min (lanes 1–8) or 55°C for 1 hr (lanes 9–13). Reactions at 37°C contained 11, 55, or 275 nM MR protein, 70–80 mM NaCl, 0.5 mM ATP, 1 mM MnCl2, or 5 mM MgCl2 as indicated. Reactions at 55°C were similar except they contained 60 mM NaCl, 2 mM MgCl2, and 11 nM MR proteins as indicated.
(C) Nuclease assays were performed with 1 nM Substrates 2, 3, or 4 as indicated. Reactions contained 58 nM wild-type MR in 60 mM NaCl and 2 mM MgCl2, and were 35 incubated at 55°C for 1 hr. Reaction products were analyzed on 15% denaturing sequencing gels. Single-stranded DNA markers are indicated.
We observed that Mre11 nuclease products in magnesium differed slightly from manganese nuclease products in that two distinct types of product were generated. The first type of product was close in size to the original substrate, while the second set of products appeared to be much smaller oligonucleotides (~12–25nt). To determine if any of these products were dependent on the 3’-5’ resection of the substrate, we designed an oligonucleotide substrate containing five phosphorothioate bonds at the 3’ end of the top strand (Fig. 5A, substrate 3). This type of substrate is refractory to human Mre11 3’–5’ exonuclease activity in vitro (data not shown). As shown in Fig. 5C, when the 3’ end of the labeled strand is blocked, only the smaller type of product is observed after MR incubation (Fig. 5C, lanes 4–6). The formation of these products is dependent upon the nuclease and ATP-dependent functions of MR as these products are not observed in reactions containing the Mre11 and Rad50 mutants (Fig. S8, lanes 7, 8). This suggests that the smaller products are not the result of 3’ exonuclease activity on the top strand but have to be products of endonuclease activity. Consistent with endonucleolytic cut sites close to the 5’ end, we observed products ranging from 20–30 nt when Substrate 3 was labeled at the 3’ end (Fig. S8, lane 4). Endonucleolytic cuts proximal to the 5’ end would explain the absence of products between 30 and 50 nt. With a substrate containing phosphorothioates at both 3’ ends, neither product species were observed (Fig. 5C, lanes 7–9). Together these data confirm that the MR complex can cleave DNA in magnesium, and that this cleavage activity consists of both a weak 3’-5’ exonuclease activity as well as endonucleolytic cleavage activity on the 5’ strand at a break. This result suggests that this novel 5’ endonucleolytic activity of Mre11 is responsible for the generation of limited 3’ ssDNA on the plasmid substrate in Fig. 4.
3’ ssDNA generated by PfMRHN is utilized by RadA in strand exchange
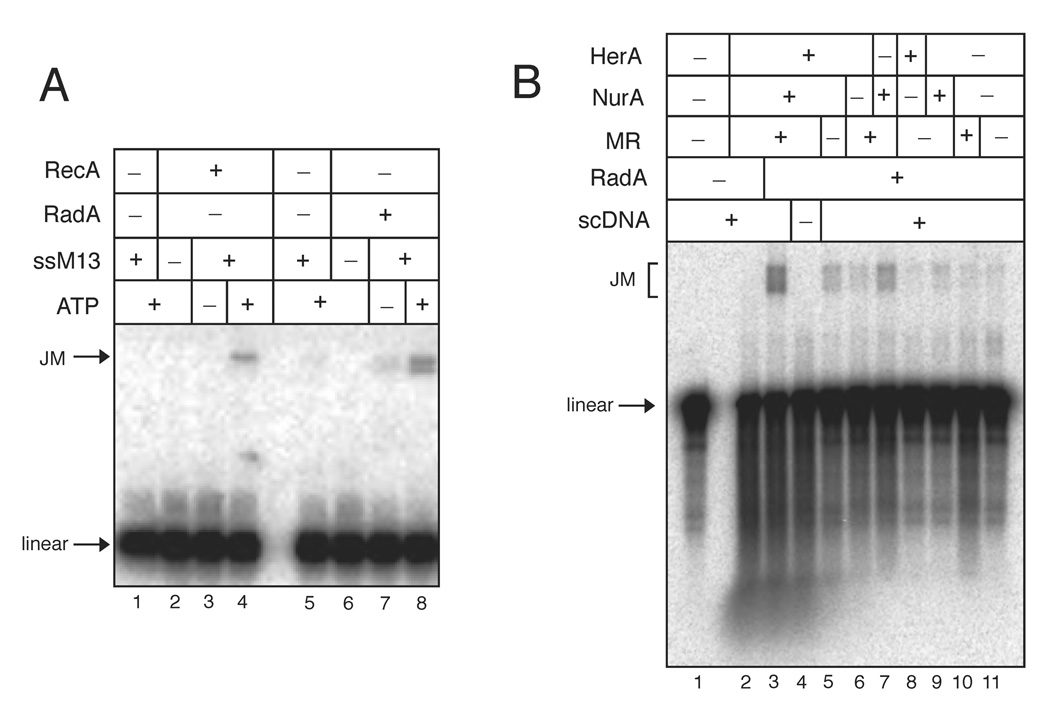
Following the initiation and processing events of DNA ends, the 3’ ssDNA is coated with strand-exchange factor(s), which catalyze strand invasion and homologous pairing. To examine whether strand invasion can occur in a concerted way with archaeal proteins, we purified the RecA/Rad51-homolog RadA from P. furiosus genomic DNA (Fig. S1B) and characterized its strand-exchange activity using a joint-molecule (JM) assay. As shown in Fig. 6A, RadA catalyzed the formation of joint-molecules with internally-labeled dsDNA and homologous circular single-stranded M13 DNA in an ATP-dependent manner at 50°C (lanes 5–8), similar to previous reports of RadA activity in vitro (Komori et al., 2000; Seitz et al., 1998). In comparison, RecA-catalyzed JM reactions performed at 37°C are shown in lanes 1–4.
Figure 6. Mre11, Rad50, HerA, and NurA together with RadA catalyze homologous strand exchange.
(A) Joint molecule reactions were performed with 100 nM RecA at 37°C (lanes 1–4) and 50 nM RadA at 50°C (lanes 5–8). Reactions contained 1.8 nM linear [32P]-labeled 415 bp DNA, 0.2 nM circular M13 ssDNA, and 2 mM ATP as indicated and were separated by native agarose gel electrophoresis. Positions of the linear dsDNA (linear) and the joint molecule product (JM) are indicated.
(B) Strand invasion experiments were performed with 13.2 nM MR, 10.8 nM HerA, 76.8 nM NurA, and 52.6 nM RadA as indicated. Reactions contained 0.1 nM [32P]-labeled linear 1.4 kb dsDNA and 1.7 nM supercoiled dsDNA as indicated and were separated by native agarose gel electrophoresis. Positions of the linear DNA and the joint molecule products (JM) are indicated.
If Mre11, Rad50, HerA, and NurA work together cooperatively to process DNA for RadA loading and strand invasion, it should be possible to perform a concerted reaction with all of these proteins, starting from a double-stranded linear DNA substrate and producing a D-loop strand invasion product with negatively supercoiled DNA. We performed this reaction with labeled linear dsDNA and found that HerA, NurA, and MR together with RadA are able to catalyze the formation of joint molecule (D-loop) products in the presence of supercoiled DNA and ATP (Fig. 6B and Fig. S9). We observed low levels of D-loop formation (up to 20% of the level seen with all proteins present) in the absence of any one of the three components, suggesting that extensive 5’ strand resection is not necessary for initiating strand exchange. This is consistent with previous findings that short ssDNA (54 nt) is sufficient for RadA-catalyzed strand exchange (Seitz et al., 1998). Therefore the cooperative activities of HerA, NurA, and MR are sufficient to process DNA ends such that RadA is able to bind and initiate homologous pairing and strand exchange.
Discussion
The Mre11/Rad50 (MR) complex is implicated in both non-homologous end joining (NHEJ) and homologous recombination (HR) double-strand break (DSB) repair pathways in eukaryotes and is conserved in all three biological kingdoms. In almost all thermophilic archaea, the Mre11 and Rad50 genes exist in an operon with the genes encoding the HerA helicase and the NurA nuclease (Constantinesco et al., 2004; Manzan et al., 2004). Based on this genomic organization and the absence of RecBCD homologs, we hypothesized that Mre11, Rad50, HerA, and NurA are involved in DNA end processing for DSB repair through HR.
We show here that purified, recombinant HerA and NurA from Pyrococcus furiosus interact physically and that this association stimulates NurA 5’-3’ exonuclease and HerA DNA helicase activities. We also demonstrate that in the presence of low levels of HerA and NurA (2.7 hexamer and 19 nM monomer, respectively), the addition of PfMR (3.3 nM M2R2) stimulated the degradation of linear dsDNA up to 19-fold. This highly cooperative reaction occurred at 65°C and generated 3’ single-stranded DNA (ssDNA) tails which could be utilized by PfRadA in vitro to catalyze strand invasion. Taken together, this evidence suggests that Mre11, Rad50, HerA, and NurA function cooperatively in the creation of 3’ ssDNA and illustrate how Mre11/Rad50 complexes in higher organisms may also function in DSB resection.
HerA and NurA
PfHerA and NurA clearly act as a functional unit. The helicase activity of HerA is stimulated approximately 3-fold by NurA, and NurA 5’ to 3’ exonuclease activity is completely dependent on the presence of HerA when assayed in physiological magnesium conditions (Fig. S3 and Fig. S5). The two proteins interact in the absence of DNA and, based on gel filtration studies, we estimate between 1 and 3 NurA monomers bind HerA (Fig. S2), which we infer to be a hexamer based on previously published results with MlaA from M. thermoautotrophicus (Manzan et al., 2004). Two other groups have reported that HerA and Mre11 from Sulfolobus tokodaii and S. acidocaldarius interact directly while HerA and NurA do not (Quaiser et al., 2008; Zhang et al., 2008). However, we did not observe any direct protein-protein interactions between PfMR and either PfHerA or PfNurA (data not shown). Since we observed a strong functional interaction between these four proteins in reactions containing DNA (Fig. 1A), it is possible that alternative protein-binding interfaces may be exposed in response to DNA-induced conformational changes. Interestingly, the optimal concentration of MR and HerA in the reaction were approximately equal (~3 nM), suggesting a potential stoichiometric activation mechanism.
Catalytic activities of Mre11 and Rad50 in strand resection
The cooperative degradation of linear DNA by 5’ strand resection required all four of the proteins in the operon, but only absolutely required the enzymatic activities of HerA, NurA, and Rad50 (Fig. 1B and 2B). The nuclease function of Mre11 appears to be partially dispensable for this cooperative DNA degradation, which is consistent with earlier findings that Mre11 nuclease activity is not required for mitotic DSB processing in budding yeast (Moreau et al., 1999). We hypothesize that Mre11 nuclease function may only be absolutely required if the 5’ strand at a break is blocked by an adduct or a covalently attached protein. Consistent with this possibility is the observation that Spo11 conjugates persist in yeast strains expressing nuclease-deficient Mre11 mutants (Moreau et al., 1999).
Although we did not test for the removal of proteins conjugates in our assays, we did observe a catalytic activity with the PfMR complex that would explain a conserved requirement for Mre11/Rad50 complexes in 5’ conjugate removal. We found that MR alone at higher concentrations catalyzed the formation of a 3’ single-stranded tail by removing approximately 15–55 nt from the 5’ strand (Fig. 4 and Fig. S7B). This product was abolished in reactions containing either the nuclease-deficient Mre11 H85L mutant or the signature motif Rad50 S793R mutant (Fig. 4B, 4C and Fig. S7B). The most likely explanation for this event is the removal of the 5’ end through a combined unwinding/endonucleolytic cut which requires both Mre11 nuclease activity and Rad50 ATP-dependent functions. Thus, the role of Mre11 nuclease activity in resection may be to remove the terminal 15–55 nt of the 5’ strand at a DSB, but this may only be essential when the 5’ strand has an adduct or protein conjugate. Interestingly, a recent study indicates that the structure of a DNA end influences the rate and efficiency of DSB processing in budding yeast (Barlow et al., 2008). Cells expressing an Mre11-nuclease deficient mutant exhibit a delay in the formation of RPA filaments on ssDNA in G1 phase, which may indicate a requirement for Mre11 nuclease activity to remove conjugates or damaged bases.
Metal ion specificity of Mre11
The ability of MR to cleave DNA using magnesium instead of manganese is a critical observation as the intracellular magnesium concentration is orders of magnitude higher than manganese in most organisms. A recent report analyzing the thermophilic archaea Thermus thermophilus showed that intracellular free magnesium and manganese levels are 1.53 mM and 0.9 µM, respectively (Kondo et al., 2008). Previous to this report, purified Mre11 and Mre11/Rad50 complexes from several species have only been shown to be active in manganese (Connelly et al., 1997; Hopfner et al., 2000a; Paull and Gellert, 1998; Trujillo and Sung, 2001). From our observations we suggest two explanations for this discrepancy. First, it appears that the MR-dependent nuclease activity we have observed in magnesium is more efficient on long DNA substrates such as plasmid DNA compared to oligonucleotide substrates, but most in vitro studies on these complexes have been performed with oligonucleotides. Second, when using long DNA substrates, it is technically difficult to document a loss of 15–55 nt on one strand, but the strand-specific Southern assay we use here is capable of identifying these cleavage events. The crystal structure of PfMre11 shows two metal-binding pockets in the active site which are involved in catalysis (Hopfner et al., 2001), suggesting that manganese may be bound to one site constitutively, or both sites may bind magnesium in our assays. We do not know if our PfMR contains manganese and contributes to our observed magnesium activity in vitro; however, exonuclease assays performed in the absence of divalent cations do not demonstrate detectable nuclease activity (data not shown).
Despite the inefficiency of oligonucleotide cleavage by PfMR in magnesium, we were able to observe endonucleolytic cleavage events on the 5’ strand of a short duplex. When the 3’ bottom strand was blocked by phosphorothioate nucleotides, this 5’ strand cutting was abolished (Fig. 5C). Thus, it appears that Mre11 3’-5’ degradation of the 3’ end is required for the endonucleolytic cutting of the 5’ strand.
Joint molecule formation in concert with RadA
Consistent with the hypothesis that Mre11, Rad50, HerA, and NurA are involved in DSB-end processing during the initial stages of HR, we observed that these proteins together with the RadA recombinase were able to catalyze the formation of joint molecules starting with linear dsDNA and supercoiled plasmid DNA (Fig. 6B). This indicates that the processed ends are suitable for RadA binding and that the nucleoprotein filament formed is competent for homologous pairing and strand exchange. In the case of RecBCD in E. coli, the RecB subunit makes direct protein-protein interactions with RecA that are important for the recruitment of RecA to ssDNA produced by RecBCD (Spies and Kowalczykowski, 2006). With PfMR, HerA, and NurA, we do not currently know if RadA is actively loaded onto the 3’ ssDNA and if so, which component(s) are involved.
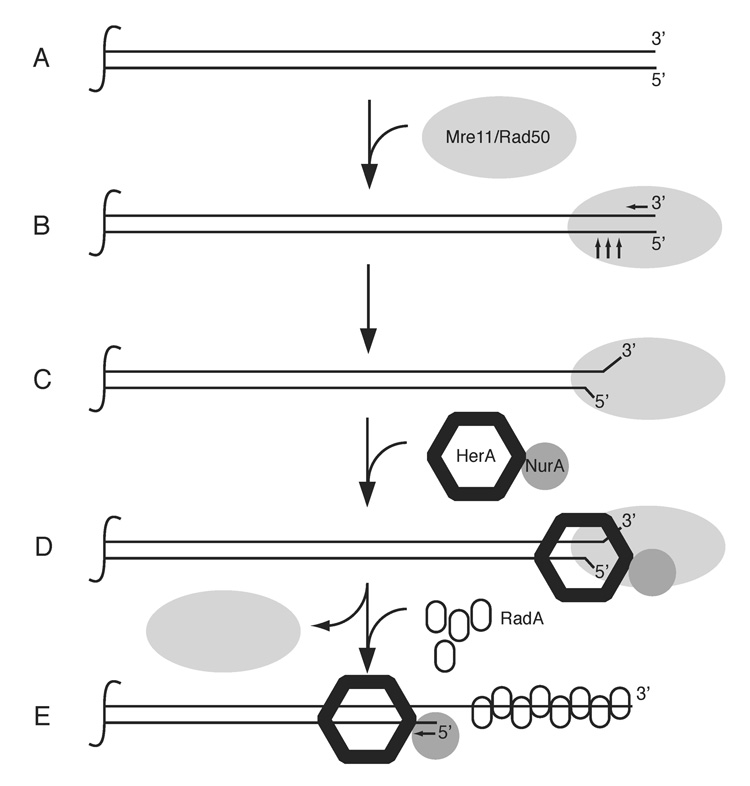
Based on the work described here, we propose a model for the function of Mre11, Rad50, HerA, and NurA in the processing of DNA ends, where the ATP-related functions of Rad50 are necessary for initiating or activating HerA/NurA, and the NurA nuclease together with HerA helicase activities produce the long 3’ single-strand tails necessary for RadA loading (Fig. 7). We do not yet know how PfMR activates HerA/NurA and whether this is through a specific DNA structure or protein-protein interactions on the DNA. Our inability to detect physical interactions between PfMR and HerA/NurA suggests that the role of PfMR is to generate a specific DNA structure that is optimal for entry of HerA/NurA. Based on previous observations with human MRN (Paull and Gellert, 1999), we propose that this DNA structure may contain unwound DNA strands. Alternatively, it may be a combination of an unwound DNA structure and a protein surface that is recognized by HerA/NurA. Recent evidence from S. cerevisiae suggest that an unwinding event coupled with endonucleolytic cleavage may generate single-stranded DNA in this organism (Zierhut and Diffley, 2008).
Figure 7. Schematic model of PfMRHN resection process.
The Mre11/Rad50 complex binds to DSB ends and processes the 3’ and 5’ ends (A & B). MR may also facilitate opening of the processed DNA ends to aid in the recruitment of HerA/NurA (C & D). The HerA/NurA complex catalyzes 5’ strand resection in a processive fashion producing 3’ ssDNA for RadA binding (E). We do not know the specific structure of the DNA end bound by MR or whether MR leaves the processed DNA end. However, we suggest the dissociation of MR from the DNA ends based on data in yeast demonstrating loss of MRX at resected ends (Lisby et al., 2004). The HerA/NurA complex is shown with one NurA monomer, but may contain up to three NurA monomers per HerA hexamer based on our gel filtration studies.
The RecBCD system in bacteria is unique in its regulation by the Chi sequence in chromosomal DNA (Amundsen et al., 2007; Spies et al., 2003). In archaea and in eukaryotes there has been no indication of a Chi-like sequence regulating recombination frequency. Consistent with this, our studies with PfMR and HerA/NurA on different plasmid substrates have not indicated any preference for specific sequences. The strand specificity of the resection argues that a polarity-switching signal like Chi should not be required in this system.
Archaea are excellent systems for the study of DNA repair due to their relative simplicity compared to eukaryotic organisms. Also, many of the proteins in archaea involved in DNA repair show higher sequence and structural similarity to their eukaryotic counterparts than to bacterial proteins, including RadA/RecA/Rad51, Mre11/SbcD, and Rad50/SbcC (Allers and Mevarech, 2005; Seitz et al., 2001). HerA and NurA homologs are absent in eukaryotes; however, there are likely functional homologs which may include Sgs1, Exo1, and Dna2 (Mimitou and Symington, 2008; Zhu et al., 2008). Studies in budding yeast suggest that the MRX complex is involved in the initial stages of DNA resection while Sgs1, Exo1, and Dna2 are redundantly necessary for the extensive 5’ strand resection (Mimitou and Symington, 2008; Zhu et al., 2008). The model proposed by Ira and Symington is consistent with ours and supports an evolutionarily conserved function for the Mre11/Rad50 complex. Our results with Mre11/Rad50 are consistent with the known activities of the MRX and MRN complexes, and thus provides a conceptual framework for understanding the roles of this enzyme in homologous recombination in higher organisms.
Experimental Procedures
Expression constructs
Protein expression and purification
Nuclease assays with labeled DNA substrates
Nuclease assays with oligonucleotide substrates contained 25 mM MOPS pH 7.0, 1 mM DTT, and 1–2 nM [32P]-labeled oligonucleotide duplex with or without 0.5 mM ATP in 10 µl reactions and were incubated at 37°C for 30 min or 55°C for 1 hr as indicated. Reactions were stopped by the addition of 0.2% SDS and 10 mM EDTA and were analyzed on 15% polyacrylamide denaturing sequencing gels. See Supplemental Data for oligonucleotide sequences. Nuclease reactions with internally-labeled DNA substrates were similar to those described for oligonucleotide assays, except 0.06 nM 2.5 kb DNA was used. Reactions were analyzed by native electrophoresis in 1.2% Tris-acetate-EDTA (TAE; 40 mM Tris-acetate, 1 mM EDTA) agarose gels for 1.5 hrs at 3.9 V/cm, dried, and scanned on a phosphorimager (Bio-Rad or GE). Preparation of oligonucleotide and internally-labeled substrates are described in the Supplemental Data. All DNA concentrations are in moles of molecules.
Nuclease assays analyzed by non-denaturing Southern hybridization
The unlabeled DNA substrate consisted of a 6.5 kb plasmid (pTP163) linearized at a single site with SacI (NEB). 10 µl reactions contained 0.2 nM DNA in 25 mM MOPS pH 7.0, 1 mM DTT, 100 mM NaCl, 5 mM MgCl2, and 1 mM ATP and were incubated at 65°C for varying lengths of time as indicated. Reactions were terminated by the addition of 0.2% SDS and 10 mM EDTA, split into two equal volume (one for the blot with the 3’ strand-specific probe and the other for the 5’ strand-specific probe), and were separated by native agarose gel electrophoresis in 0.8% TAE agarose gels for 14 hrs at 0.9 V/cm. Nuclease reactions with the 5’ Biotin substrate were carried out as above except that proteinase K was also added to 1 µg and incubated for 15 min at 37°, the beads were then treated with 2% SDS and 10 mM EDTA and incubated for 5 min at 65°C before separation by gel electrophoresis. Preparation of the biotin-DNA substrate and processing of gels and membranes for Southern hybridization is described in the Supplemental Data. All DNA concentrations are in moles of molecules.
Primer extension
Primer extension reactions were carried out in 25 mM MOPS pH 7.0, 1 mM DTT, 1 mM ATP, 5 mM MgCl2, and 100 mM NaCl at 65°C for 30 min or 1 hr as indicated. Reactions contained 2.3 nM pTP163 digested with SacI and 165 nM MR complex as indicated. Reactions were stopped by the addition of 20 mM EDTA and placed on ice. Half of the reaction was used in primer extension reactions containing [α-32P]dATP (NEN). Primer extension products were analyzed on 10% polyacrylamide denaturing sequencing gels.
Joint molecule and strand invasion assays
Joint-molecule reactions contained 1.8 nM 415 bp [32P]-labeled dsDNA in 20 mM Tris pH 7.4, 10 mM MgCl2, 2 mM DTT, 50 mM NaCl, 50 µg/ml BSA, and 2 mM ATP as indicated in 19 µl reactions. RadA was incubated with the labeled DNA at 50°C for 20 min, then 1 µl ssM13 DNA was added (final 0.2 nM) and incubated an additional hour. RecA reactions were incubated at 37°C for 40 min in the presence of both linear and single-stranded M13 DNA. Reactions were stopped by the addition of 0.2% SDS, 10 mM EDTA, and 0.5 µg/ml Ethidium bromide (EtBr), and were separated by native gel electrophoresis in 1.2% TAE gels for 1.25 hrs at 3.5 V/cm.
Strand invasion reactions contained 25 mM MOPS pH 7.0, 2 mM DTT, 2 mM ATP, 10 mM MgCl2, 100 mM NaCl, and 0.1 nM linear [32P]-labeled dsDNA in 9 µl reactions. Reactions were incubated at 65°C for 15 min before the addition of 1 µl of 17 nM supercoiled DNA and incubated for an additional 15 min. Reactions were stopped with 0.2% SDS, 10 mM EDTA, and 0.5 µg/ml EtBr, and separated by native agarose gel electrophoresis in 0.7% Tris-borate EDTA (TBE; 89 mM Tris-borate, 2 mM EDTA) gels for 2 hrs at 4.3 V/cm. Preparation of the internally-labeled substrates and supercoiled plasmid DNA are described in the Supplemental Data.
Supplementary Material
Acknowledgments
We are grateful to J. Carney for the P. furiosus Mre11/Rad50 expression plasmid, to L. Symington for personal communication of unpublished results, and to members of the Paull lab for comments about this project. This work was supported by NIH grant R01 CA094008 and DOD pre-doctoral fellowship to B.B.H
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- Allers T, Mevarech M. Archaeal genetics—the third way. Nat Rev Genet. 2005;6:58–73. doi: 10.1038/nrg1504. [DOI] [PubMed] [Google Scholar]
- Amundsen SK, Taylor AF, Reddy M, Smith GR. Intersubunit signaling in RecBCD enzyme, a complex protein machine regulated by Chi hot spots. Genes Dev. 2007;21:3296–3307. doi: 10.1101/gad.1605807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. Prokaryotic homologs of the eukaryotic DNA-end-binding protein Ku, novel domains in the Ku protein and prediction of a prokaryotic double-strand break repair system. Genome Res. 2001;11:1365–1374. doi: 10.1101/gr.181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur LM, Gustausson K, Hopfner K-P, Carson CT, Stracker TH, Karcher A, Felton D, Weitzman MD, Tainer J, Carney JP. Structural and functional analysis of Mre11-3. Nucl Acids Res. 2004;32:1886–1893. doi: 10.1093/nar/gkh343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JH, Lisby M, Rothstein R. Differential regulation of the cellular response to DNA double-strand breaks in G1. Mol Cell. 2008;30:73–85. doi: 10.1016/j.molcel.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara V, Dupre A, Lengsfeld B, Hopkins BB, Chan A, Lee JH, Zhang X, Gautier J, Zakian V, Paull TT. Rad50 adenylate kinase activity regulates DNA tethering by Mre11 /Rad50 complexes. Mol Cell. 2007;25:647–661. doi: 10.1016/j.molcel.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly JC, de Leau ES, Leach DRF. DNA cleavage and degradation by the SbcCD protein complex from escherichia coli. Nucleic Acids Res. 1999;27:1039–1046. doi: 10.1093/nar/27.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly JC, de Leau ES, Okely EA, Leach DR. Overexpression, purification, and characterization of the SbcCD protein from Escherichia coli. J Biol Chem. 1997;272:19819–19826. doi: 10.1074/jbc.272.32.19819. [DOI] [PubMed] [Google Scholar]
- Constantinesco F, Forterre P, Elie C. NurA, a novel 5′-3′ nuclease gene linked to rad50 and mre11 homologs of thermophilic Archaea. EMBO Rep. 2002;3:537–542. doi: 10.1093/embo-reports/kvf112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinesco F, Forterre P, Koonin EV, Aravind L, Elie C. A bipolar DNA helicase gene, herA, clusters with rad50, mre11 and nurA genes in thermophilic archaea. Nucleic Acids Res. 2004;32:1439–1447. doi: 10.1093/nar/gkh283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amours D, Jackson SP. The Mre11 complex: at the crossroads of dna repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Jackson SP, Weller GR. Identification of bacterial homologues of the Ku DNA repair proteins. FEBS Lett. 2001;500:186–188. doi: 10.1016/s0014-5793(01)02589-3. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BA, Karche A, Henderson B, Bodmer JL, McMurray CT, et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;418:562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Karcher A, Craig L, Woo TT, Carney JP, Tainer JA. Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell. 2001;105:473–485. doi: 10.1016/s0092-8674(01)00335-x. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Karcher A, Shin D, Fairley C, Tainer JA, Carney JP. Mre11 and rad50 from pyrococcus furiosus: cloning and biochemical characterization reveal an evolutionarily conserved multiprotein machine. J Bacteriol. 2000a;182:6036–6041. doi: 10.1128/jb.182.21.6036-6041.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000b;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov EL, Sugawara N, White CI, Fabre F, Haber JE. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:3414–3425. doi: 10.1128/mcb.14.5.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GCM, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Komori K, Miyata T, DiRuggiero J, Holley-Shanks R, Hayashi I, Cann IK, Mayanagi K, Shinagawa H, Ishino Y. Both RadA and RadB are involved in homologous recombination in Pyrococcus furiosus. J Biol Chem. 2000;275:33782–33790. doi: 10.1074/jbc.M004557200. [DOI] [PubMed] [Google Scholar]
- Kondo N, Nishikubo T, Wakamatsu T, Ishikawa H, Nakagawa N, Kuramitsu S, Masui R. Insights into different dependence of dNTP triphosphohydrolase on metal ion species from intracellular ion concentrations in Thermus thermophilus. Extremophiles. 2008;12:217–223. doi: 10.1007/s00792-007-0118-6. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski SC, Dixon DA, Eggleston AK, Lauder SD, Rehrauer WM. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzminov A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol Mol Biol Rev. 1999;63:751–813. doi: 10.1128/mmbr.63.4.751-813.1999. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Manzan A, Pfeiffer G, Hefferin ML, Lang CE, Carney JP, Hopfner KP. MlaA, a hexameric ATPase linked to the Mre11 complex in archaeal genomes. EMBO Rep. 2004;5:54–59. doi: 10.1038/sj.embor.7400037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Sae2/CtIP, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008 doi: 10.1038/nature07312. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncalian G, Lengsfeld B, Bhaskara V, Hopfner KP, Karcher A, Alden E, Tainer JA, Paull TT. The rad50 signature motif: essential to ATP binding and biological function. J Mol Biol. 2004;335:937–951. doi: 10.1016/j.jmb.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Moreau S, Ferguson JR, Symington LS. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz K, Klein F. mre11S--a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes & Dev. 1997;11:2272–2290. doi: 10.1101/gad.11.17.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Gellert M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes & Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaiser A, Constantinesco F, White MF, Forterre P, Elie C. The Mre11 protein interacts with both Rad50 and the HerA bipolar helicase and is recruited to DNA following gamma irradiation in the archaeon Sulfolobus acidocaldarius. BMC Mol Biol. 2008;9:25. doi: 10.1186/1471-2199-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz EM, Brockman JP, Sandler SJ, Clark AJ, Kowalczykowski SC. RadA protein is an archaeal RecA protein homolog that catalyzes DNA strand exchange. Genes Dev. 1998;12:1248–1253. doi: 10.1101/gad.12.9.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz EM, Haseltine CA, Kowalczykowski SC, Paul B. Adv Appl Microbiol. Academic Press; 2001. DNA recombination and repair in the Archaea; pp. 101–169. [DOI] [PubMed] [Google Scholar]
- Singleton MR, Dillingham MS, Gaudier M, Kowalczykowski SC, Wigley DB. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature. 2004;432:187–193. doi: 10.1038/nature02988. [DOI] [PubMed] [Google Scholar]
- Smith GR. Homologous recombination near and far from DNA breaks: alternative roles and contrasting views. Annu Rev Genet. 2001;35:243–274. doi: 10.1146/annurev.genet.35.102401.090509. [DOI] [PubMed] [Google Scholar]
- Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair (Amst) 2006;5:1021–1029. doi: 10.1016/j.dnarep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Spies M, Bianco PR, Dillingham MS, Handa N, Baskin RJ, Kowalczykowski SC. A molecular throttle: the recombination hotspot chi controls DNA translocation by the RecBCD helicase. Cell. 2003;114:647–654. doi: 10.1016/s0092-8674(03)00681-0. [DOI] [PubMed] [Google Scholar]
- Spies M, Kowalczykowski SC. The RecA binding locus of RecBCD is a general domain for recruitment of DNA strand exchange proteins. Mol Cell. 2006;21:573–580. doi: 10.1016/j.molcel.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Symington LS. Role of RAD52 Epistasis Group Genes in Homologous Recombination and Double-Strand Break Repair. Microbiol Mol Biol Rev. 2002;66:630–670. doi: 10.1128/MMBR.66.4.630-670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KM, Sung P. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50/Mre11 complex. J Biol Chem. 2001;13:13. doi: 10.1074/jbc.M105482200. [DOI] [PubMed] [Google Scholar]
- Trujillo KM, Yuan SS, Lee EY, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J Biol Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- Tsubouchi H, Ogawa H. A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis. Mol Cell Biol. 1998;18:260–268. doi: 10.1128/mcb.18.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Ohta T, Oshiumi H, Tomizawa J, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wei T, Hou G, Zhang C, Liang P, Ni J, Sheng D, Shen Y. Archaeal DNA helicase HerA interacts with Mre11 homologue and unwinds blunt-ended double-stranded DNA and recombination intermediates. DNA Repair (Amst) 2008;7:380–391. doi: 10.1016/j.dnarep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases dna2 and exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut C, Diffley JF. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008;27:1875–1885. doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.