NOVEMBER 2007 was marked by the loss of Seymour Benzer, long considered the father of the field of neurogenetics. Benzer's scientific contributions are broad and span from physics (his Ph.D. training) to molecular biology (defining the linearity of the gene) to behavioral genetics (establishing the field). Benzer's unswerving devotion to science led him to continue running a vibrant laboratory at the California Institute of Technology in Pasadena, California, until his death at the age of 86. Quite astonishing was his ability to recreate himself multiple times in widely different disciplines and to achieve remarkable scientific insights and discoveries in each of these fields.
I had the privilege of being a postdoctoral scientist with Benzer—or Seymour as he was generally known—in the late 1980s to early 1990s and am one of a large number of scientists whom he mentored in his long career. In many respects, these scientists whom Seymour trained and sent off to pursue rewarding careers are collectively one of his greatest and most enduring accomplishments and a testament to his commitment to science.
For his contributions first in defining the linearity of the gene using bacteriophage genetics and subsequently in establishing the field of behavioral genetics, Benzer received many awards and prizes. These include the Lasker Award (1971), the Harvey Prize from Israel (1977), the Jaffe Prize from the Royal Society of London (1982), the National Medal of Science (1983), the Thomas Hunt Morgan Medal from the Genetics Society of America (1986), the Wolf Prize for Medicine from Israel (1991), the Crafoord Prize of the Royal Swedish Academy of Sciences (1993), the Mendel Award from the Genetical Society of Great Britain (1994), the International Prize for Biology from Japan (2000), the Bower Award for Achievement in Science (2004), the Gairdner International Award (2004), and the Albany Prize in Medicine and Biomedical Research (2006). On his resumé, Benzer organized his scientific contributions into three periods (reminiscent of Picasso, another 20th-century figure known for a long and varied output): the physics period, the molecular biology period, and the behavioral biology period.
The physics period:
Seymour was born in 1921 in New York, grew up in Brooklyn, and attended Brooklyn College. Although interested in biology, he majored in physics because he did not want to have take the general biology requirement, which at the time focused mostly on taxonomy, a field of little interest to him that was required for the biology major (Holmes 2006). He received his Ph.D. in 1947 from Purdue University, pursuing research on the metalloid germanium. His work on germanium, for which he was awarded several patents, was instrumental in the development of semiconductors for transistors, used in a wide range of electronic applications. Germanium was the critical component of semiconductors in electronics prior to the switch to silicon.
Seymour was hired as a professor in the physics department at Purdue in 1947. However, he developed an interest in biophysics, and, upon advice from Salvador Luria, he took the bacteriophage course at Cold Spring Harbor in the summer of 1948 (Figure 1) (Holmes 2006), which triggered his first major change in disciplines. Reminiscence and history of the phage course, which was established by Max Delbrück, with whom Seymour eventually worked, can be found in a previous Perspectives (Susman 1995).
Figure 1.—
Seymour Benzer at the laboratory phage bench during the Cold Spring Harbor bacteriophage course, summer of 1948 (Holmes 2006). (Photo courtesy of Carol Miller and the estate of Seymour Benzer)
The molecular biology period:
Seymour was one of a number of physicists to become enamored by the fundamental questions of biology, intrigued by how the basis of life could be encoded. This shift of physicists into biology was initially stimulated by the amazing discovery by Hermann Muller, in experiments performed around 1926–1928, that X rays caused inherited genetic mutations, which culminated in the Nobel Prize in 1946 (for an account of these experiments, see Crow and Abrahamson 1997). How was it possible that X rays—in the domain of physics—could cause heritable changes to the code of life? The physicists intrigued by this question included Erwin Shrödinger, who in 1944 wrote the book What Is Life?, which is credited with catalyzing the move of many colleagues into the biological sciences. Seymour still had his copy of this book on the shelves just outside his office when I was in the lab. Physicists that shifted fields included Max Delbrück, with whom Seymour was a postdoctoral scientist from 1949 to 1951. Seymour subsequently worked as a Fulbright Fellow with André Lwoff, François Jacob, and Jacques Monod at the Pasteur Institute from 1951 to 1952 and later with Francis Crick (another physics refugee) and Sydney Brenner at Cambridge from 1957 to 1958.
In his work with the bacteriophage T4, Seymour used phage genetics—studies of mutants and recombination—to define whether genes were linear. If we consider the time, seminal proof that DNA encoded the basis of life was presented by Hershey and Chase (1952), and DNA structure was presented as a double helix by Watson and Crick (1953). Seymour started working on phage with the Cold Spring Harbor course in 1948 and published his work on the fine structure of the rII gene of T4 in 1959 and 1961 (the 1961 article has the image of the rII gene often included in college textbooks, Benzer 1959, 1961). Thus, at the time of Seymour's phage work, questions regarding how DNA encoded biological function, including the fundamental nature of the gene, were at the forefront of biology.
Key aspects of the bacteriophage work illustrate central features of Seymour's ability to design scientific investigations; these features played out again and again in his scientific career, to great success. One of these is the “simple assay.” Seymour was an enthusiast of developing and using simple assays to approach any biological question of interest. Why use a complicated, time-consuming assay if a simple one would address the question equally well? Using a simpler assay would give more time and opportunity to delve in greater depth into the specific scientific question, as well as allow more time and opportunity to address additional questions of great interest.
In the work on T4, Seymour genetically mapped >2400 mutations in the rII gene (Benzer 1959, 1961). The fact that he was able to map so many mutations allowed him to conclude two fundamental features about the gene: (1) the sequence of a gene is linear and (2) by being able to map and determine the recombination distance between mutations that map very near each other (and knowing how much DNA was present in the T4 phage), Seymour could deduce that the smallest unit of recombination was between two adjacent DNA base pairs. These are now concepts about genes and recombination that we regard as given, but these, like all other fundamental principles, had to be proven to provide the foundation for further hypotheses regarding the nature of the gene and gene function.
How did Seymour determine how to generate and map so many mutations to define these principles of the gene? A key point is that Seymour was able to choose an effective experimental strategy for analysis of the large numbers of mutations required for these fine-structure mapping studies. First, Seymour, among other prominent scientists of the day, including Francis Crick, Sydney Brenner, Salvador Luria, and Max Delbrück, recognized the power of bacteriophage for elucidating principles of DNA and the genetic code. It is a numbers game: using phage, it was possible for Seymour to generate thousands of mutations over the ∼3000 bp of DNA that compose the rII gene. This scale of analysis is simply not possible in systems where one cannot easily screen thousands of progeny within a reasonable time frame, cost, and space for intragenic recombination or mutational events. With phage, Seymour could.
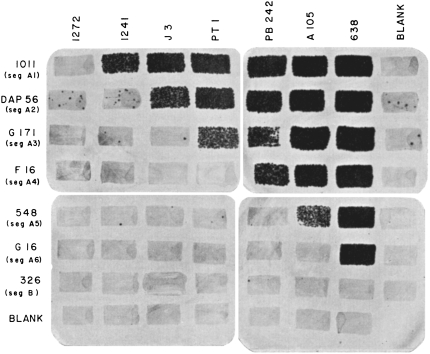
Second, one of Seymour's most elegant and repeated insights is to see a simple way to achieve a scientific goal—or perhaps because he was able to see simplicity, he was able to visualize how to provide important insights. In his rII work, Seymour realized clever ways to select for rII mutants (generate a situation where only the rare mutants can grow), clever ways to select for rII recombinants (generate a situation where individual mutants cannot grow, but wild-type recombinants can grow), and clever ways to map the rII mutants into smaller subdomains (a series of deletions, the “big seven” deficiencies, that spanned the rII genes; Figure 2) that saved enormous amounts of time, effort, and resources. Although Seymour mapped a first set of ∼150 rII mutants relative to each other using recombination (Benzer 1959), he then realized some of his mutations were deletions (isolated as rII mutants not able to recombine within a particular interval and that did not revert) and could order them in a sequential nested series (Benzer 1961). This allowed rapid mapping of many individual rII mutants with just a limited number of crosses to a deletion interval. Only then, did he perform the more time-consuming pairwise recombination crosses to place the mutations in linear sequence. By this approach, Seymour was able to map thousands of mutations in sequence along the rII region. Such strategies, devised and exploited to great effect by Seymour and other phage geneticists at the time, led to the development of the widely used selection methods of modern molecular cloning.
Figure 2.—
Bacteriophage plaques illustrating deletion crosses that Seymour used for placing rII mutations in sequence. It was the insight of deletion mapping that allowed Seymour to map in linear sequence thousands of mutations of the rII genes. Each row shows a given mutant tested against reference deletions that span the rII locus. Plaques with growth are dark. Plaques appearing in the blanks are due to revertants present in the mutant stocks. The results show each of these mutations to be located in a different segment of the rII gene (Benzer 1961). (Courtesy of Carol Miller and the estate of Seymour Benzer)
Seymour contributed his recollections and extensive notes on his phage work to a book by Frederic Holmes on this important period of his scientific career, Reconceiving the Gene, published in 2006 (Holmes 2006). Seymour's interest in documenting this important period of his life may have been stimulated in part by the success of Time, Love and Memory, the recollections of his adventures and journey in behavioral genetics (Weiner 1999). Seymour often noted to us that, at the time, after he and others had worked out many of the details of the gene and gene structure, he decided that it was time to move on to new horizons in the erroneous belief that all the “big problems” of DNA had been solved. Although he never expressed regret for his decision to move on from the study of gene structure, he noted how wrong he was, because so many interesting discoveries were still to be made.
Seymour's recollection of his insight into the simplicity of manipulating phage that allowed him to do these studies is interesting. He noted that he thought he had a eureka moment when the phage failed to grow as anticipated, and he immediately had the insight into how to use this biological property to advantage to define the nature of the gene [recollected in Phage and the Origins of Molecular Biology (Benzer 1972)]. However, when reviewing his notebooks, he found with irritation that it had taken him several months for this insight to sink in (Holmes 2006).
When I was in the lab studying the eyes absent (eya) gene, Seymour made a touching reference to his phage work. eya mutants showed fascinating interallelic genetic interactions (Leiserson et al. 1994). Billy Leiserson (the graduate student with whom I collaborated) and I generated huge tables of crosses between all the different eya alleles to assess the extent and degree of interallelic complementation. One afternoon in the Benzer lunchroom, Billy and I pulled out these tables to show Seymour. Seymour promptly got excited and with a “you need to make very, very large tables, crossing mutants with each other,” jumped up and proceeded to stride down the hall, open one of his many cabinets and—stunning us—pull out his T4 bacteriophage notebooks to show us how to generate such tables!!! Billy and I were astounded at being confronted with the very notebooks and pages in which Seymour had recorded, so many years ago, the T4 phage complementation studies. It was a striking reminder of the extraordinary scientific abilities of this man who had the courage to reinvent himself so many times throughout his career.
The behavioral biology period:
Several observations likely led Seymour to move from phage to behavioral genetics. As noted, Seymour often said that with the elucidation of the structure of DNA and the fast pace of subsequent work to elucidate details of gene structure, he felt there was little more to be done and that it was time to find a new field in which to make new discoveries. But he also is often quoted telling the story of the birth of his second child—that he and his wife were taken aback at the difference in personalities between his first and second daughters—such different personalities, yet the same parents. How did this happen?
From knowing Seymour I would argue that he was simply fascinated by behavior; although I cannot know if this was a developed interest or if he had this interest from the start, I would guess that behavior was something he always noticed and was always fascinated by. And then it came to the forefront in his work, most elegantly summarized in his own words, from the 1971 Albert Lasker Basic Medical Research Award Lecture (Benzer 1971). Seymour, the scientist who defined the linearity of the gene, was intrigued by the question of how that one-dimensional information becomes translated into the multi-dimensional complexity of behavior:
When an individual develops from an egg, the one-dimensional information contained in the linear sequence of genes on the chromosomes is somehow translated into a two-dimensional blastula, which later folds to produce a precise three-dimensional array of sense organs, central nervous system, and muscles. Finally, the ensemble interacts to produce behavior, a phenomenon which requires four dimensions, at the least, to describe. The genes contain the information for the circuit diagram, but little is known about the relationship between this primary information and the end result. How the tags of specificity are parceled out among the neurons so that they form the proper network, or even what kinds of molecules carry the specificity are, at present, complete mysteries. The problem of tracing the emergence of multidimensional behavior from the genes is a challenge that may not become obsolete so soon. (Benzer 1971, From the gene to behavior, JAMA 218: 1015, copyright ©1971, American Medical Association, all rights reserved)
And thus the adventures in behavior began (Figure 3).
Figure 3.—
Seymour and the fly. Seymour Benzer making eye contact with his favorite research organism of the behavioral biology period at his desk in his office at Caltech. (Courtesy of the Archives, California Institute of Technology)
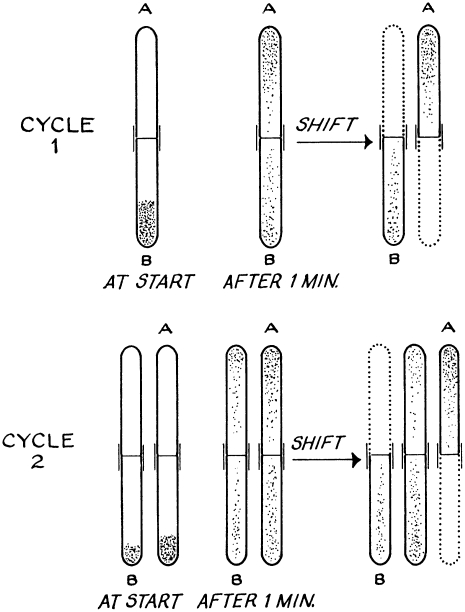
The journey was launched with the countercurrent device (Figure 4) (Benzer 1967). Seymour designed this apparatus to separate flies on the basis of the strength of their phototactic response, that is their motivation and ability to move toward light. Wild-type flies display robust phototaxis, with most of the flies, after repeated chances to go toward light, ending up in the final tubes. Mutants with altered vision or motility partitioned with altered distribution patterns, such as the nonphototactic SB8 (SB for “strange behavior”) mutant (Benzer 1967). An interesting aside is the language that Seymour used in this article, which often included sentences and phrases that read like a physicist “partitioning” behavior and not a biologist describing behavior, for example, “fractionating” a population of Drosophila according to their visual light preference (Benzer 1967).
Figure 4.—
Seymour's countercurrent approach to isolating behavioral fly mutants on the basis of the tendency of the animal to walk toward light. Countercurrent distribution procedure for fractionating a Drosophila population. In each cycle, the flies are partitioned between two alternatives. Dotted lines indicate new tubes introduced at the end of each cycle. Only the first 2 cycles of 15 are shown (Benzer 1967). (Courtesy of Carol Miller and the estate of Seymour Benzer)
This entrée into behavioral genetics supplied the Benzer laboratory with an initial complement of mutants. Already by the time of the Lasker Lecture, he and his colleagues had noted numerous mutants with a range of behavioral aberrations, many of which kept the laboratory busy for the next several decades (Benzer 1971). From these early phototaxis screens, Yoshiki Hotta as a postdoctoral scientist isolated Seymour's beloved drop-dead (drd) fly as a mutant that catastrophically died (Y. Hotta, personal communication); drd returns in a prominent role in later years when Seymour's interest in aging and neurodegenerative disease came to the forefront.
The behavioral mutants isolated or studied by Seymour and colleagues spanned a dramatic range of behaviors that are reflected in humans: from flies defective in vision (nonphototactic, negative phototactic), to locomotion (sluggish, uncoordinated), to stress sensitivity (Shaker, freaked-out), to sexual dynamics (savoir-faire, fruitless), to nerve and muscle abnormalities (photoreceptor degeneration, drop-dead), to learning and memory (rutabaga, dunce) (Benzer 1971). Among Seymour's mutants, the period (per) mutants are perhaps the most striking example illustrating the impact of his work: that a tiny fly might pave the way to understanding the complexities of human behavior. Seymour said that when he started his work on behavioral genetics, there were two camps: those who agreed that of course genes influence behavior and those who said he was crazy. This polarized response became one of Seymour's criteria for pursuing an idea: if half the people think you are crazy, then it is probably a good idea.
per, from fly behavior to human behavior:
The per mutants were isolated by Ron Konopka, a graduate student who had an interest in light regulation of organismal behavior. Circadian rhythms, however, must have been of great interest to Seymour, as it is clear from recollections of his life and knowledge from his colleagues that his own clock was off: he was a self-described night owl, arriving late in the lab and returning to work very late at night. The per mutants were isolated on the basis of altered eclosion timing (Konopka and Benzer 1971). Adult flies typically emerge from the pupal case in the early morning. By screening for mutant fly lines with aberrant eclosion timing that emerged earlier, later, or randomly compared to normal, the plan was to isolate mutants with an altered circadian property. This screen led to the isolation of three independent behavioral mutants that turned out to be alleles of the same gene: the arrhythmic per0, perl with an abnormally long rhythm, and pers with an abnormally short rhythm.
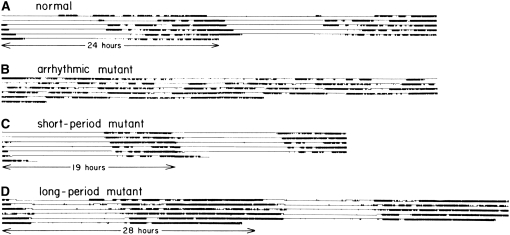
That the per mutants emerged as adults at altered time of day indicated that the circadian timing of a single lifetime event was altered. To determine whether other rhythms were altered, Yoshiki Hotta designed the first contraptions to monitor circadian locomotor activity, using parts from the local Radio Shack store (Y. Hotta, personal communication) (Konopka and Benzer 1971). Although the methods of monitoring activity have become more sophisticated since then, the principle underlying this new technology is similar to the one used by Hotta and the basic output, the actogram, looks very similar. The trait of daily circadian locomotor rhythm served as the basis for the study of rhythms and mutant screens for many years to come in fly as well as rodent studies and later in fly studies of sleep (Hendricks et al. 2000; Shaw et al. 2000; Panda et al. 2002). Using such a device, Konopka and Benzer (1971) showed that the per mutants not only were altered in a one-time rhythmic event (eclosion), but also displayed similarly altered daily locomotor rhythms in free-running conditions (Figure 5).
Figure 5.—
Locomotor rhythms of normal and per circadian mutants, measured with the activity monitor designed and built by Yoshiki Hotta in the Benzer laboratory. Locomotor activity rhythms, monitored in infrared light, for individual rhythmically normal or mutant flies previously exposed to a light:dark cycle of 12 hr:12 hr. Locomotor activity registered by event recorder. Records read from left to right, each line representing the start of a successive interval. For visual continuity, each successive interval is also replotted to the right of the immediately preceding interval (Konopka and Benzer 1971). (Courtesy of Carol Miller and the estate of Seymour Benzer)
Although Seymour clearly hoped that his studies would lead to insight into our own behavior, he probably never anticipated the remarkable conservation of the components and mechanism of the circadian clock. Benzer and Konopka reported the per mutants in 1971 (Konopka and Benzer 1971), per was cloned independently by the Young and Rosbash groups in 1984 (Bargiello et al. 1984; Reddy et al. 1984), mammalian per homologs were identified in 1997 (Sun et al. 1997; Tei et al. 1997), and in 1997 the mouse Clock mutation, identified from a forward genetic screen, was found to occur in a core component of the clock that functions with per (King et al. 1997). In 2001, mutations in human Per2 were defined as a cause of familial advanced sleep phase syndrome—a 4-hr advanced phase circadian disorder (Toh et al. 2001). It is now clear that the fundamental mechanism of the circadian clock as a negative feedback loop, in which per and homologs are central players, is conserved over >600 million years (Panda et al. 2002). This is truly remarkable, and it is humbling to reflect that this was gleaned from studies initiated in 1967 with the simple fruit fly. Underscoring the pioneering aspect of Konopka and Benzer's work is the fact that the second circadian mutant in Drosophila was not isolated until >25 years later using the same screening approach that revealed per (Sehgal et al. 1994).
The adventures of Seymour's lab led him through studies of various aspects of the fly at its many stages of development and adult life. In the lecture that he gave at the University of Pennsylvania in 2004, when he received the Bower Award and Prize for Achievement in Science from the Franklin Institute, he presented his journey with the fly as one that progressed around the “clock” of the fly's lifetime, from egg to larvae to pupae to adult to interactions between males and females to life span and aging, with the per studies defining the actual circadian clock being among the initial discoveries.
Highlights of his work over these years include many important findings. Among these were studies that led to the blossoming of the fly as a model system to glean insight into cell–cell interactions of mammalian development. When the fly was established as an experimental system, the fact that one individual Drosophila looked exactly like another in such exquisite detail that every bristle on the animal could have a name led many to the idea that developmental events must be predetermined by a defined developmental program. As a graduate student with Seymour, Don Ready tested this idea by generating individually marked cells within the eye of the fly to determine the relatedness between cells of any one developmental unit, or ommatidium (Ready et al. 1976). Such genetic mosaics would generate adult cells that were marked for lineage, such that one could determine if all of the cells that compose a single unit of the highly regular eye were related in lineage. This strict lineage model was especially attractive because it was easy to imagine how the eight photoreceptor cells of each ommatidium could be generated by precisely three cell divisions of a single neural progenitor cell. But these studies gave the unexpected result of “no”; that is, the cells of this highly precise and regular adult structure, a neurocrystalline lattice, were in fact not related, but had the potential to develop into any of the cells of the pattern, despite the exquisite regularity of the final structure. This finding indicated that the cellular identities were not predetermined. That is, they were not defined by lineage, but rather cell fate in the fly eye was defined by cell–cell interactions—the same principle that is the basis of mammalian development. An explosion of research followed in the fly, and in particular the fly eye, using the genetics of the organism to define the biological pathways and molecular mechanisms that are the basis of cell–cell developmental interactions.
Adventures with an old friend:
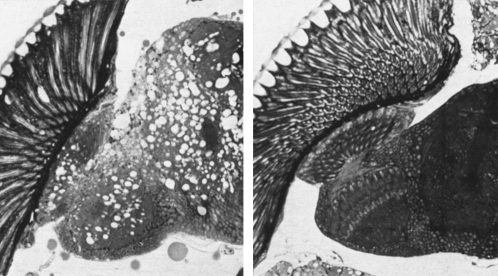
Seymour's work on the fly encompassed many features of the animal's behavior and development that have significance in human biology. One event that took place that was particularly influential to Seymour's later work on maintenance of the brain, aging, and disease was a renewed interest in drd by Robert Buchanan when he joined the lab as a postdoctoral scientist in the late 1980s. As noted, drd had been isolated in the late 1960s by Yoshiki Hotta in countercurrent screens. The drd mutant had the intriguing property that the adult fly was born seemingly normal, but within 2 weeks, all the mutant animals catastrophically died: they would become sluggish and uncoordinated and then within several hours drop dead (hence the name). Analysis of the animal revealed a brain full of holes, intriguingly reminiscent of human neurodegenerative disease (Figure 6).
Figure 6.—
Holes in the brain of the drop-dead mutant. The loss of brain integrity in the drop-dead mutant (left) compared to normal (right). Horizontal sections through the head of the fly Drosophila, showing the eye (at extreme left), optic ganglia (at left of center), and brain (at right). Right, normal fly. Left, drop-dead mutant at stage of pronounced behavioral staggering (Benzer 1973). (Courtesy of Carol Miller and the estate of Seymour Benzer)
With drd and the other behavioral mutants, one fundamental question that Seymour and colleagues addressed early on was where was the site of the gene action. That is, an animal may die or show altered behavior due to problems in many tissues, but was the brain or nervous system involved? Hotta and Benzer (1972) used elegant and spectacularly detailed mosaic analyses to map the sites of action of genes responsible for the various behavioral mutants. They did this by generating so-called gynandromorphic animals (half male–half female). These were animals where half the tissue had a single X chromosome, was male, and expressed the mutation on that X chromosome, whereas the other half of the animal had two X chromosomes and was female and wild type. By detailed examination of adult mosaic animals for both expression of the mutant characteristic and analysis of which tissue was male and mutant, and which female and normal, Hotta and Benzer constructed a fate map on the Drosophila blastoderm for adult external body parts (Hotta and Benzer, 1972). They projected on that map those areas that would give rise to the internal structures—where most of their mutations in fact mapped—of the brain, nervous system, and muscle. Seymour [who loved coming up with new scientific terms—he mentioned to us that he was always disappointed that cistron, which he proposed during his phage period (Benzer 1959)—did not catch on as the term for a gene] proposed the term “sturt” for units of measure of the fate map, with one unit being the 1% probability that the two structures in question will be of different somatic genotype in the mosaic. Sturt was the nickname of Alfred Sturtevant, his senior colleague at the California Institute of Technology (Caltech) who conceived the idea of a fate map, which Hotta and Benzer extended to the analysis of behavioral phenotypes.
In these studies, Hotta and Benzer mapped drd gene action to the brain. But a second remarkably intriguing property of the drd gene was also revealed in these studies. The remarkable finding was that gynandromorphic animals with a brain that was half mutant and half normal were typically entirely biologically normal: that is, the wild-type half of the fly's brain could rescue the mutant half of the brain that was missing drd function. This indicated that drd encoded or regulated a nonautonomous activity that is capable of rescuing neighboring mutant tissue—reminiscent of mammalian neural survival factors and functional recovery from stroke and other brain injuries in humans.
Interest in drd was revived by Bob Buchanan who showed that drd had glia that failed to mature and wrap brain neurons properly (Buchanan and Benzer 1993). Subsequently, it was revealed that drd also had tracheal defects (Kretzschmar 2005); thus, one scenario is that, due to defective glia and/or trachea, the drd brain becomes anoxic and the fly dies due to massive loss of integrity of the brain. Seymour loved and was intrigued by drd and what the mutant might reveal about human brain maintenance and function, an interest that also became keen with collaboration with his second wife Carol Miller, Chief of Neuropathology at the University of Southern California (Miller and Benzer 1983).
Eyes absent has a polyglutamine repeat:
A second event that occurred during this time period was the cloning of the eya gene by Billy Leiserson and myself. eya was of interest because the eye progenitor cells underwent cell death rather than developing into eye cells. Our studies of eya also illustrate Seymour's principle of a simple assay. We were studying eya cell death using complicated and time-consuming approaches (epon embedding and tissue sectioning). But Seymour suggested a simple technique of Spreij (1971), acridine orange staining, a quick and rapid technique for imaging dying cells. We tried it, it worked, and after I showed such images at the third Molecular Neurobiology of Drosophila meeting at Cold Spring Harbor in 1989, acridine orange became a popular technique widely used for rapidly visualizing and studying cell death (in flies and beyond), allowing the isolation of mutants in cell-death genes due to its simplicity.
Another finding that led Seymour further toward human disease research occurred when we sequenced the eya gene. At the time, we found that eya encoded a novel protein with only a single feature recognizable at the time—a long domain of the amino acid glutamine (Bonini et al. 1993). Such polyglutamine stretches had been noted in several Drosophila proteins with developmental roles, the first being the Notch gene where it was coined an opa repeat and led to a cloning nightmare: while walking through Notch, the CAG repeat region was discovered when clones containing the CAG repeat hybridized to many regions of the genome, disrupting the ability to walk smoothly through the Notch region (Wharton et al. 1985). We published the eya work in February 1993; in March, the Huntington's disease gene was finally identified and the mutation revealed to be an expanded CAG-repeat domain encoding the amino acid glutamine (Huntington's Disease Collaborative Research Group 1993). The renewed interest in drd and the discovery of a polyglutamine domain in eya further kindled Seymour's and my own interest in brain maintenance and aging. In a remarkably prescient statement, ∼20 years before these events, Seymour wrote about drd:
This syndrome [that is, drd staggering, a brain “shot full of holes,” catastrophic death] recalls the many kinds of hereditary brain degeneration in man. For instance, the gene for Huntington's disease leads to degeneration which appears to start in a specific brain region and is followed by more general deterioration, production of incapacitation, and death… . In fact, the distribution of incidence [of death] vs. age for drop-dead is roughly similar to that for Huntington's disease, one day in the life of a drop-dead fly being roughly equivalent to a decade for an affected human. (Benzer, 1971, From the gene to behavior, JAMA 218: 1022, copyright ©1971, American Medical Association, all rights reserved)
Seymour's work continued with additional discoveries of genes that influence maintenance of the brain and genes and mechanisms that affect life span. Many of his latter prizes were awarded for providing the foundation of insight into human disease by using the fly as a launching pad and illustrative system (Figure 7).
Figure 7.—
Receiving the Albany Prize in 2006. Seymour receiving the Albany Prize in Medicine and Biomedical Research in 2006. (AP photo/T. Roske)
Although many of Seymour's seminal discoveries were made in the 1960s and 1970s, their impact has become most apparent during the last decade with the tremendous focus on behavioral genetics in the mouse and human. An ongoing project that is fascinating in reference to Seymour's pioneering studies is the Dog Genome Project. It has been clear for years that different dog breeds have different behaviors, reflecting in part early programs aimed at developing different breeds with distinct and advantageous work habits. One goal of the Dog Genome Project has been to perform specific crosses between dogs with different behavioral characteristics to define genes for those traits—not only physical characteristics (a large dog with big ears vs. a small dog with a long tail), but also those noteworthy behaviors that clearly differ between breeds of dog, such as loyalty, fetching ability, herding, aggression, friendliness, laziness, workaholic tendencies. Many of these are traits or tendencies that we recognize in ourselves or in others we know. Indeed, the behavioral repertoire of the dog in many ways closely resembles ourselves, with pharmacological studies underscoring the striking relevance of such studies to human physiology. The idea and global acceptance of a Dog Genome Project with the goal of defining genes that regulate behavior can be attributed to Benzer: we accept the concept that genes influence dog behavior to the extent that an entire genome project has been built on such a premise. This approach will certainly yield genes that regulate these elusive behaviors that we find so fascinating. This underscores Seymour's enormous scientific influence to this day and contrasts with the skepticism that he received when he initially pioneered this research on the genetic basis of behavior.
Seymour's gifts as a scientist:
Seymour had many extraordinary qualities that made him exceptional as a scientist. One quality was his unceasing interest in new experiences. One could certainly say this of his constant interest in new foods and restaurants, but also of his passion for scientific knowledge. Seymour reinvented himself many times, from physicist, to phage biologist, to behavioral geneticist. He often mentioned serendipitous events. He was in the lunch line at Cold Spring Harbor when someone came by announcing that there was one spot left in the Woods Hole embryology course. Was anyone interested? He said yes and was off. His career is remarkable for the range of scientific courses that he took, from the Cold Spring Harbor phage course in the summer of 1948 to the Woods Hole embryology course in the summer of 1959 to the Woods Hole neurobiology course in the summer of 1966 to the Cold Spring Harbor leech course in 1975. In today's scientific climate, which tends to discourage a winding path in favor of a narrow focus, one is struck by his remarkable ability to continually explore new areas and repeatedly make remarkable achievements as a scientist.
Seymour had the gift of vision: he was the type of person who could see both the forest and every branch of every tree. His visionary abilities are elegantly displayed in the attention to detail in his articles that address fundamental problems of great interest in biology. Among the most striking of these features include the phage studies (Benzer 1961), the detail of the countercurrent studies (Benzer 1967), and the mosaic studies mapping the site of behavioral gene function (Hotta and Benzer 1972). His vision was also quite remarkable. He could look at a scientific problem and appreciate the details and also see the most important and critical essence of the question that should be addressed (whether or not it was feasible to do). I recall a situation of this type with eya, where he indicated what the key question was. My response was okay, but it was not possible to do. And his answer? Well, that was not his problem, but that was the key question to focus on.
I also think that he liked to work in uncrowded fields, and this allowed him to think freely about the questions. He would often pose to us, why do what everyone else is doing? Do something new, do something different. He encouraged his students to think beyond the horizons. It would be more challenging, but also more fun, with more chance to discover the new and unexpected. As is also clear from his legacy, he cared very deeply about training scientists and about the future careers of his students. He left a living legacy in both the students that he trained and in the many scientists with whom he interacted and inspired.
In the end, one finds remarkable the vast expanse that Seymour's work impacts, from the development of transistors, to defining the linearity of the gene, to defining genes that influence who we are and how we act. As one who also worked with him, I am struck by how much Seymour simply followed his passionate interests. Seymour's fascination was behavior. He noted it all around him and remarked on it all the time; my recollections of the famous Monday lab meetings include so many conversations spent remarking on various aspects of society and society's reaction to events. Thus, in studying the fly's behavior, Seymour was following his passions, and, with his many gifts for science, he made remarkable discoveries along his journey. Most of all, Seymour was always the first to say how much he enjoyed science. When he tentatively raised the idea that the fly may be able to reveal insight into our own behavior, he ended with a most important point:
Experience thus far with the fly as a model system for unraveling the path from the gene to behavior is encouraging. In any case, it is fun. (Benzer, 1971, From the gene to behavior, JAMA 218: 1022, copyright ©1971, American Medical Association, all rights reserved)
Acknowledgments
I gratefully thank Carol Miller for allowing use of figure material and Nick Lawrence for finding and sending slides and original reprints. The author thanks Tony Cashmore, Rich Spielman, Mark Fortini, Amita Sehgal, Yoshiki Hotta, Derek Lessing, and Julide Bilen for input and comments.
This tribute is based on a presentation given by the author at the Memorial Symposium for Seymour Benzer, held at the California Institute of Technology, March 14, 2008, and is dedicated, with love, to Seymour Benzer.
References
- Bargiello, T. A., F. R. Jackson and M. W. Young, 1984. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312 752–754. [DOI] [PubMed] [Google Scholar]
- Benzer, S., 1959. On the topology of the genetic fine structure. Proc. Natl. Acad. Sci. USA 45 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer, S., 1961. On the topography of the genetic fine structure. Proc. Natl. Acad. Sci. USA 47 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer, S., 1967. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc. Natl. Acad. Sci. USA 58 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer, S., 1971. From the gene to behavior. JAMA 218 1015–1022. [PubMed] [Google Scholar]
- Benzer, S., 1972. Adventures in the rII region, pp. 157–165 in Phage and the Origins of Molecular Biology, edited by J. Cairns, G. Stent and J. D. Watson. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Benzer, S., 1973. Genetic dissection of behavior. Sci. Am. 229 24–37. [DOI] [PubMed] [Google Scholar]
- Bonini, N. M., W. M. Leiserson and S. Benzer, 1993. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72 379–395. [DOI] [PubMed] [Google Scholar]
- Buchanan, R. L., and S. Benzer, 1993. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron 10 839–850. [DOI] [PubMed] [Google Scholar]
- Crow, J. F., and S. Abrahamson, 1997. Seventy years ago: mutation becomes experimental. Genetics 147 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks, J. C., S. M. Finn, K. A. Panckeri, J. Chavkin, J. A. Williams et al., 2000. Rest in Drosophila is a sleep-like state. Neuron 25 129–138. [DOI] [PubMed] [Google Scholar]
- Hershey, A. D., and M. Chase, 1952. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J. Gen. Physiol. 36 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, F. L., 2006. Reconceiving the Gene: Seymour Benzer's Adventures in Phage Genetics. Yale University Press, New Haven/London.
- Hotta, Y., and S. Benzer, 1972. Mapping of behaviour in Drosophila mosaics. Nature 240 527–535. [DOI] [PubMed] [Google Scholar]
- Huntington's Disease Collaborative Research Group, 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72 971–983. [DOI] [PubMed] [Google Scholar]
- King, D. P., Y. Zhao, A. M. Sangoram, L. D. Wilsbacher, M. Tanaka et al., 1997. Positional cloning of the mouse circadian clock gene. Cell 89 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka, R. J., and S. Benzer, 1971. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 68 2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar, D., 2005. Neurodegenerative mutants in Drosophila: A means to identify genes and mechanisms involved in human diseases? Invert. Neurosci. 5 97–109. [DOI] [PubMed] [Google Scholar]
- Leiserson, W. M., N. M. Bonini and S. Benzer, 1994. Transvection at the eyes absent gene of Drosophila. Genetics 138 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, C. A., and S. Benzer, 1983. Monoclonal antibody cross-reactions between Drosophila and human brain. Proc. Natl. Acad. Sci. USA 80 7641–7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda, S., J. B. Hogenesch and S. A. Kay, 2002. Circadian rhythms from flies to human. Nature 417 329–335. [DOI] [PubMed] [Google Scholar]
- Ready, D. F., T. E. Hanson and S. Benzer, 1976. Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 53 217–240. [DOI] [PubMed] [Google Scholar]
- Reddy, P., W. A. Zehring, D. A. Wheeler, V. Pirrotta, C. Hadfield et al., 1984. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38 701–710. [DOI] [PubMed] [Google Scholar]
- Sehgal, A., J. L. Price, B. Man and M. W. Young, 1994. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science 263 1603–1606. [DOI] [PubMed] [Google Scholar]
- Shaw, P. J., C. Cirelli, R. J. Greenspan and G. Tononi, 2000. Correlates of sleep and waking in Drosophila melanogaster. Science 287 1834–1837. [DOI] [PubMed] [Google Scholar]
- Spreij, T., 1971. Netherlands J. Zool. 21 221–264. [Google Scholar]
- Sun, Z. S., U. Albrecht, O. Zhuchenko, J. Bailey, G. Eichele et al., 1997. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90 1003–1011. [DOI] [PubMed] [Google Scholar]
- Susman, M., 1995. The Cold Spring Harbor Phage Course (1945–1970): a 50th anniversary remembrance. Genetics 139 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei, H., H. Okamura, Y. Shigeyoshi, C. Fukuhara, R. Ozawa et al., 1997. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389 512–516. [DOI] [PubMed] [Google Scholar]
- Toh, K. L., C. R. Jones, Y. He, E. J. Eide, W. A. Hinz et al., 2001. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291 1040–1043. [DOI] [PubMed] [Google Scholar]
- Watson, J. D., and F. H. Crick, 1953. Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid. Nature 171 737–738. [DOI] [PubMed] [Google Scholar]
- Weiner, J., 1999. Time, Love, Memory: A Great Biologist and His Quest for the Origins of Behavior. Alfred A. Knopf/Random House, New York.
- Wharton, K. A., B. Yedvobnick, V. G. Finnerty and S. Artavanis-Tsakonas, 1985. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell 40 55–62. [DOI] [PubMed] [Google Scholar]