Abstract
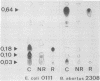
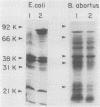
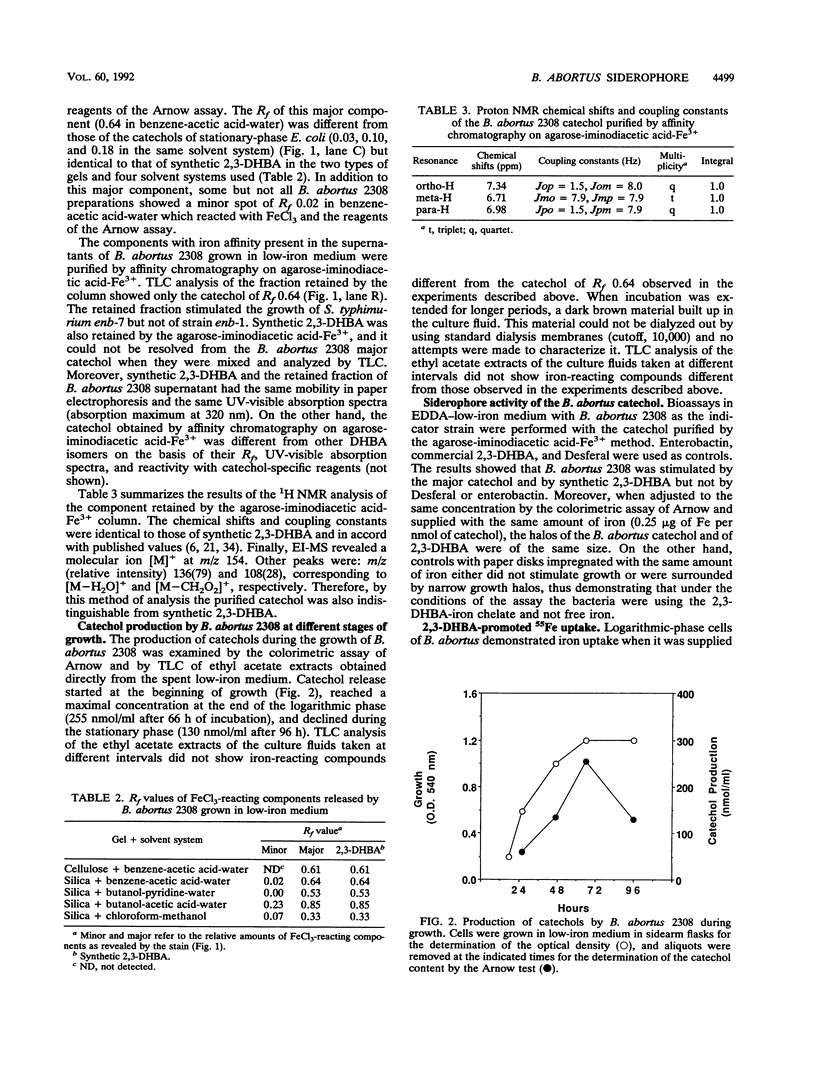
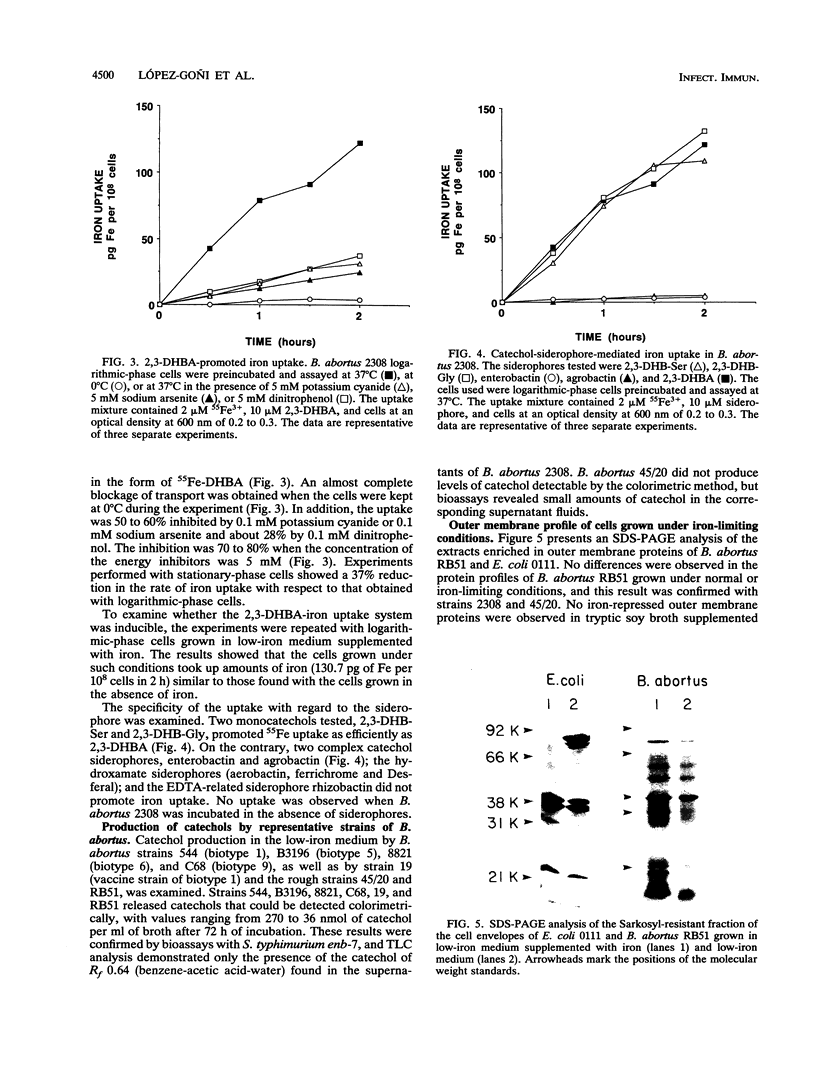
Brucella abortus grown in low-iron medium or in the presence of iron chelators [ethylenediamine-di(o-hydroxyphenylacetic acid) and 2,2-dipyridyl] showed reduced cell yields and released a material positive in chemical and biological assays for catechols. This material was purified from culture fluids of B. abortus 2308 by chromatography on agarose-iminodiacetic acid-Fe3+ and identified as 2,3-dihydroxybenzoic acid (2,3-DHBA) by thin-layer chromatography, paper electrophoresis, and UV-visible nuclear magnetic resonance and mass spectroscopy. No other major catechols were observed at different stages of growth, and 2,3-DHBA was also produced upon iron limitation by representative strains of B. abortus biotypes 1, 5, 6, and 9. Both synthetic 2,3-DHBA and the natural catechol relieved the growth inhibition of B. abortus 2308 by ethylenediamine-di(o-hydroxyphenylacetic acid), and 2,3-DHBA promoted 55Fe uptake by B. abortus 2308 by an energy-dependent mechanism. Two other monocatechols tested, 2,3-dihydroxybenzoyl-Ser and 2,3-dihydroxybenzoyl-Gly, also promoted 55Fe uptake. More complex catechol siderophores (agrobactin and enterobactin), hydroxamate siderophores (aerobactin, ferrichrome, and deferriferrioxamine mesylate [Desferal]), and an EDTA-related siderophore (rhizobactin) failed to mediate 55Fe uptake. B. abortus cells grown in low-iron medium or in medium with iron had similar rates of iron uptake when supplied with 55Fe-2,3-DHBA, and the release of 2,3-DHBA under iron starvation was not associated with the expression of new outer membrane proteins. These results suggest an uptake system in which only the synthesis of the siderophore is regulated by the iron available for growth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbin J. L., Bulen W. A. The isolation and identification of 2,3-dihydroxybenzoic acid and 2-N,6-N-di-92,3-dihydroxybenzoyl)-L-lysine formed by iron-deficient Azotobacter vinelandii. Biochemistry. 1969 Mar;8(3):757–762. doi: 10.1021/bi00831a002. [DOI] [PubMed] [Google Scholar]
- Douglas J. T., Rosenberg E. Y., Nikaido H., Verstreate D. R., Winter A. J. Porins of Brucella species. Infect Immun. 1984 Apr;44(1):16–21. doi: 10.1128/iai.44.1.16-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVENSON M. A., GERHARDT P. Nutrition of Brucellae: utilization of iron, magnesium, and manganese for growth. Proc Soc Exp Biol Med. 1955 Aug;89(4):678–680. doi: 10.3181/00379727-89-21914. [DOI] [PubMed] [Google Scholar]
- Feistner G. J., Beaman B. L. Characterization of 2,3-dihydroxybenzoic acid from Nocardia asteroides GUH-2. J Bacteriol. 1987 Sep;169(9):3982–3987. doi: 10.1128/jb.169.9.3982-3987.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., TUCKER L. A., WILSON J. B. The nutrition of Brucellae: utilization of single amino acids for growth. J Bacteriol. 1950 Jun;59(6):777–782. doi: 10.1128/jb.59.6.777-782.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano J. H., Miller D. R., Grady R. W., Cerami A. Inhibition of membrane peroxidation in thalassaemic erythrocytes by 2,3-dihydroxybenzoic acid. Br J Haematol. 1976 Mar;32(3):351–356. doi: 10.1111/j.1365-2141.1976.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport in Escherichia coli K-12. 2,3-Dihydroxybenzoate-promoted iron uptake. Arch Microbiol. 1977 Sep 28;114(3):231–239. doi: 10.1007/BF00446867. [DOI] [PubMed] [Google Scholar]
- Hantke K. Dihydroxybenzoylserine--a siderophore for E. coli. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):5–8. doi: 10.1016/0378-1097(90)90158-m. [DOI] [PubMed] [Google Scholar]
- Harding R. A., Royt P. W. Acquisition of iron from citrate by Pseudomonas aeruginosa. J Gen Microbiol. 1990 Sep;136(9):1859–1867. doi: 10.1099/00221287-136-9-1859. [DOI] [PubMed] [Google Scholar]
- Hu S. P., Felice L. J., Sivanandan V., Maheswaran S. K. Siderophore production by Pasteurella multocida. Infect Immun. 1986 Dec;54(3):804–810. doi: 10.1128/iai.54.3.804-810.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knosp O., von Tigerstrom M., Page W. J. Siderophore-mediated uptake of iron in Azotobacter vinelandii. J Bacteriol. 1984 Jul;159(1):341–347. doi: 10.1128/jb.159.1.341-347.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth H. Uber das Vorkommen von 2,3-Dihydroxybenzoesäure und ihrer Aminosäurederivate in Kulturmedien von Klebsiella oxytoca. Arch Mikrobiol. 1970;70(3):297–302. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Llinás M., Wilson D. M., Neilands J. B. Effect of metal binding on the conformation of enterobactin. A proton and carbon-13 nuclear magnetic resonance study. Biochemistry. 1973 Sep 25;12(20):3836–3843. doi: 10.1021/bi00744a007. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Moreno E., Stackebrandt E., Dorsch M., Wolters J., Busch M., Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990 Jul;172(7):3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):453–460. doi: 10.1016/0304-4165(70)90165-0. [DOI] [PubMed] [Google Scholar]
- Ong S. A., Peterson T., Neilands J. B. Agrobactin, a siderophore from Agrobacterium tumefaciens. J Biol Chem. 1979 Mar 25;254(6):1860–1865. [PubMed] [Google Scholar]
- Persmark M., Expert D., Neilands J. B. Ferric iron uptake in Erwinia chrysanthemi mediated by chrysobactin and related catechol-type compounds. J Bacteriol. 1992 Jul;174(14):4783–4789. doi: 10.1128/jb.174.14.4783-4789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persmark M., Expert D., Neilands J. B. Isolation, characterization, and synthesis of chrysobactin, a compound with siderophore activity from Erwinia chrysanthemi. J Biol Chem. 1989 Feb 25;264(6):3187–3193. [PubMed] [Google Scholar]
- Peters W. J., Warren R. A. The mechanism of iron uptake in Bacillus subtilis. Can J Microbiol. 1970 Dec;16(12):1285–1291. doi: 10.1139/m70-214. [DOI] [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Young L., Neshat S. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol. 1990 Dec;172(12):6991–6996. doi: 10.1128/jb.172.12.6991-6996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Neilands J. B., Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol. 1983 Apr;154(1):324–329. doi: 10.1128/jb.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Robertson D. C. Characterization of the electron transport system in Brucella abortus. J Bacteriol. 1975 Apr;122(1):139–144. doi: 10.1128/jb.122.1.139-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Synge C., Kimber B., Bayley P. M. Production of enterochelin by Escherichia coli 0111. Biochim Biophys Acta. 1977 Apr 27;497(2):548–557. doi: 10.1016/0304-4165(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Payne S. M. Effect of iron limitation on growth, siderophore production, and expression of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1982 Apr;150(1):148–155. doi: 10.1128/jb.150.1.148-155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. W., Freeman S., Minett W. G., Lambert P. A. Characterisation of a siderophore from Acinetobacter calcoaceticus. FEMS Microbiol Lett. 1990 Jun 15;58(1):29–32. doi: 10.1016/0378-1097(90)90097-a. [DOI] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARING W., ELBERG S., SCHNEIDER P., GREEN W. The role of iron in the biology of Brucella suis. I. Growth and virulence. J Bacteriol. 1953 Jul;66(1):82–91. doi: 10.1128/jb.66.1.82-91.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Brown M. R., Lambert P. A. Effect of iron deprivation on the production of siderophores and outer membrane proteins in Klebsiella aerogenes. J Gen Microbiol. 1984 Sep;130(9):2357–2365. doi: 10.1099/00221287-130-9-2357. [DOI] [PubMed] [Google Scholar]
- Winter A. J. Outer membrane proteins of Brucella. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):87–89. doi: 10.1016/0769-2609(87)90081-0. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Siderophore production by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):600–608. doi: 10.1128/iai.32.2.600-608.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]