Abstract
The auxiliary spliceosomal protein SCNM1 contributes to recognition of nonconsensus splice donor sites. SCNM1 was first identified as a modifier of the severity of a sodium channelopathy in the mouse. The most severely affected strain, C57BL/6J, carries the variant allele SCNM1R187X, which is defective in splicing the mutated donor site in the Scn8amedJ transcript. To further probe the in vivo function of SCNM1, we constructed a floxed allele and generated a mouse with constitutive deletion of exons 3–5. The SCNM1Δ3-5 protein is produced and correctly localized to the nucleus, but is more functionally impaired than the C57BL/6J allele. Deficiency of SCNM1 did not significantly alter other brain transcripts. We characterized an ENU-induced allele of Scnm1 and evaluated the ability of wild-type SCNM1 to rescue lethal mutations of I-mfa and Brunol4. The phenotypes of the Scnm1Δ3-5 mutant confirm the role of this splice factor in processing the Scn8amedJ transcript and provide a new allele of greater severity for future studies.
SODIUM channel modifier 1 (Scnm1) is an auxiliary splice factor that was identified by its role in the strain-specific lethality of the sodium channel mutation Scn8amedJ (Buchner et al. 2003). A direct role for SCNM1 in splicing the Scn8amedJ transcript was subsequently demonstrated in a cell culture assay (Howell et al. 2007). The Scn8amedJ mutation is a 4-bp deletion in the splice donor site of exon 3, nucleotides +5 to +8, generating a weak site with a C nucleotide at the consensus +5G position (Kohrman et al. 1996). In the presence of a wild-type Scnm1 gene, only 10% of the Scn8amedJ transcripts are correctly spliced and encode an active channel, while ∼90% of the transcripts skip exon 2 and exon 3. In the presence of the C57BL/6J-specific Scnm1R187X allele, the amount of full-length transcript is reduced to 5% and the Scn8amedJ mice do not survive (Buchner et al. 2003). It is not clear from the earlier studies whether Scnm1R187X is a null allele or retains partial function.
SCNM1 is a 229-amino-acid protein with a bipartite nuclear localization signal near the N terminus and one C2H2 zinc finger of the U1C type (Figure 1A). It is an accessory component of the U1 splicesome protein complex and interacts with the spliceosomal Sm and U1-70K proteins (Howell et al. 2007). Along with other members of the U1C protein family, SCNM1 is thought to contribute to the recognition of different subsets of nonconsensus splice donor sites (Roca et al. 2005). By analogy with the effect of Scnm1R187X on the splicing of Scn8amedJ, variants of these proteins in the human population could modify the severity of disorders caused by splice donor site mutations.
Figure 1.—
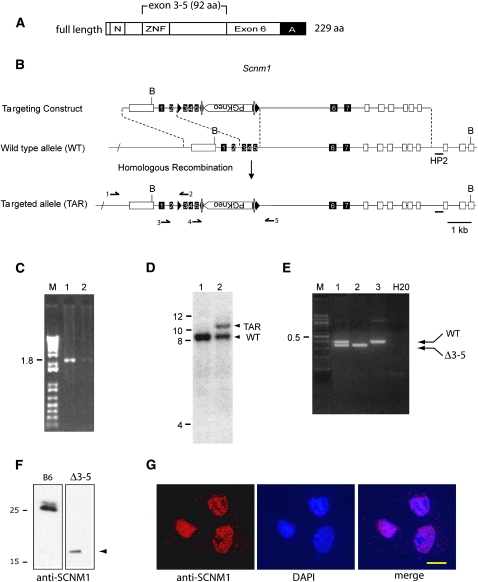
Targeting of Scnm1. (A) Full-length SCNM1 (NP_081289) and the region targeted for deletion. N, nuclear localization signal; ZNF, zinc finger; A, acidic domain. (B) The targeting construct contains loxP sites that flank Scnm1 exons 3–5 and a neomycin resistance cassette flanked by FRT sites in intron 5. The 5′ homologous arm is 2 kb in length and includes exon 1 of the upstream gene AK011517 (open box). The 3′ homologous arm is 5.6 kb in length and contains exons 5–7 of Scnm1, as well as exons 4–9 of the adjacent downstream gene Tmod4 (open boxes). B, BamHI sites; HP2, Southern blotting probe. Arrows 1–5 indicate primer locations. (C) Amplification of embryonic stem cell DNA using primers 1 and 2 to detect correct targeting of the 5′ arm of the construct. (D) Southern blot of BamHI-digested embryonic stem cell DNA using probe HP2 to detect correct targeting of the 3′ arm of the construct. (E) Genotyping of Scnm1Δ3-5 mice using primers 2, 3, and 5. (F) Endogenous SCNM1 proteins in brain nuclear extracts from C5BL/6J wild-type and Scnm1Δ3-5 homozygous mice detected by Western blot using anti-SCNM1 antiserum (Howell et al. 2007). (G) Immunocytochemistry of Scnm1Δ3-5 homozygous fibroblasts demonstrates nuclear localization of the truncated SCNM1 protein detected in F. DNA is stained with DAPI. Bar, 20 um.
LUC7L2 is a mammalian homolog of yeast Luc7p, a component of the U1 snRNP with a role in splicing nonconsensus splice donor sites (Fortes et al. 1999). SCNM1 interacts directly with LUC7L2, and the two proteins cooperate to increase the splicing efficiency of an Scn8amedJ minigene in cultured cells (Howell et al. 2007).
Auxiliary splice factors can be used to restore the activity of genes with splice-site mutations. For example, in cells from a cystic fibrosis patient with an intronic mutation that generates a cryptic splice donor site, overexpression of Htra2-β1 increased the level of correctly spliced transcript and restored function (Nissim-Rafinia et al. 2004).
Inactivation of a splice factor itself may lead to disease. Germline inactivation of the SR proteins ASF/SF2, SC35, and SRp20 results in embryonic lethality (Xu et al. 2005). The neuron-specific auxiliary splice factor NOVA-1 and its paralog NOVA-2 regulate alternative splicing of 41 synapse-related transcripts (Ule et al. 2006). Loss of NOVA-1 due to autoimmune disease in cancer patients leads to ataxia, seizures, and dementia. Inactivation of mouse NOVA-1 is lethal, but inactivation of NOVA-2 is not (Huang et al. 2005). Patients with mutations in the splice factors PRPF31, PRPF8, and PRPF3 develop photoreceptor degeneration, although the proteins are ubiquitously expressed. They are associated with the spliceosomal complex U4/U6.U5 tri-snRNP, which is involved in the activation of the spliceosome for lariat formation and intron removal (Pacione et al. 2003). Mutation of PRPF31 inhibits pre-mRNA splicing of Rhodopsin, leading to retinal apoptosis and autosomal dominant retinitis pigmentosa (Yuan et al. 2005).
To further characterize the function of SCNM1, we examined two new alleles, an internal deletion of 92 residues generated by gene targeting in embryonic stem (ES) cells (Figure 1A) and an N-ethyl-N-nitrosourea (ENU)-induced missense mutation identified by screening a DNA repository (Michaud et al. 2005). We examined the effect of the deletion allele using expression arrays and the role of Scnm1R187X in the strain-specific lethality of two mouse mutants.
MATERIALS AND METHODS
Generation of a floxed allele of Scnm1:
The targeted allele of Scnm1 was constructed as shown in Figure 1. Two genomic fragments (5.0 and 4.4 kb) containing Scnm1 and flanking regions were amplified from the C57BL/6J BAC clone RP23-11G21 (http://bacpac.chori.org) and cloned into pSP72 (Promega, Madison, WI). Restriction sites were engineered by Quikchange multi-site mutagenesis (Stratagene, La Jolla, CA) and used to incorporate two loxP sites, two Flp recombinase (FRT) sites, and a PGKneo cassette from plox-2FRT-PGKneo (kindly provided by David Gordon, University of Colorado).
The targeting construct was electroporated into C57BL/6J ES cell line BL/6-III (Schuster-Gossler et al. 2001) by the University of Michigan Transgenic Animal Model Core (T. Saunders; http://www.med.umich.edu/tamc). Correct targeting of the construct was evaluated in neomycin-resistant ES cell clones by PCR and Southern blotting. The 5′ region of the targeting construct was amplified using a forward primer upstream of the targeting construct and a reverse primer downstream of the first loxP site (Figure 1B, primers 1 and 2), which generated a 1.8-kb PCR product after correct integration (Figure 1C). The 3′ region was evaluated by Southern blot hybridization of BamHI-digested DNA using probe HP2 to detect a wild-type product of 8.5 kb and a targeted product of 10.4 kb (Figure 1D).
Two euploid clones were expanded, micro-injected into albino C57BL/6J-Tyrc-2J blastocysts, and implanted into pseudopregnant C57BL/6J females. Male chimeric founders with patches of white hair were mated with C57BL/6J females. Germline transmission of the targeted allele was detected by PCR of genomic DNA using primers 3 and 2 flanking the first loxP site (Figure 1B), generating a 187-bp wild-type product and a 220-bp product from the targeted allele. FRT sites were removed in vivo by crossing male Scnm1TAR/+ mice with a strain expressing the FLPE recombinase [B6:SJL-Tg(ACTFLPe)9205Dym/J, Jackson Laboratory stock 003800]. The floxed allele was detected by PCR using primers 4 and 5 flanking the second loxP site (Figure 1B), which generated a 220-bp wild-type product and a 305-bp floxed allele product.
Generation of the Scnm1Δ3-5 allele:
Scnm1flox/+ mice were crossed with mice carrying a ubiquitously expressed EIIa-CRE transgene [B6.FVB-Tg(EIIa-cre)C5379Lmgd/J, Jackson Laboratory stock 003724] (Lakso et al. 1996). The deleted allele (Δ3-5) was detected by PCR using primers 2, 3, and 5 (Figure 1B), which generate a 380-bp wild-type product and 330 bp from the deleted allele (Figure 1E). All experimental procedures were approved by the University of Michigan Committee for the Use and Care of Animals. Animals were housed and cared for in accordance with National Institutes of Health guidelines.
ENU mutant alleles of Scnm1:
DNA and cDNA samples from the Cryopreserved Mutant Mouse Bank, derived from 4000 G1 male offspring of ENU-treated B6 mutant mice (Michaud et al. 2005), were screened by temperature-gradient capillary electrophoresis to detect variants in the exons of Scnm1 at the Oak Ridge National Laboratory. Mice were recovered from cryopreserved sperm by intracytoplasmic sperm injection, as previously described (Michaud et al. 2005).
Culture and immunocytochemistry of Scnm1Δ3-5 fibroblasts:
Skin fibroblasts were cultured from tail biopsies taken from homozygous Scnm1Δ3-5 mice. Cells were isolated in the presence of collagenase type II (Worthington Biochemical, Lakewood, NJ) in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 15% fetal calf serum (Invitrogen) and incubated at 37°, 5% CO2. Immunocytochemistry was performed as previously described (Howell et al. 2007).
Gene expression and splicing profiling:
RNA was extracted from fresh tissue using TRIzol reagent (Invitrogen) and purified using RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturers' protocols. RNA was prepared from two Scnm1Δ3-5 homozygotes and two Scnm1R187X homozygous littermates. Gene expression microarray analysis using Affymetrix Mouse 430 2.0 GeneChips (Affymetrix, Santa Clara, CA) was carried out at the University of Michigan Comprehensive Cancer Center Affymetrix and Microarray Core. Three arrays for each genotype were analyzed using Affymetrix and Limma software packages of Bioconductor. Splicing microarrays that interrogate 1339 genes for 1639 splicing events (Srinivasan et al. 2005) were analyzed in Manny Ares' lab at the Center for Molecular Biology of RNA, University of California, Santa Cruz. The Mouse G-Protein Coupled Receptor Splicearray (469 genes) and Ion Channel Splicearray (330 genes) (44K format) were analyzed by ExonHit Therapeutics (Gaithersburg, MD).
Quantitation of correctly spliced Scn8a transcripts:
RNA was extracted as described above from brain of Scn8amed homozygous mice carrying various Scnm1 alleles. The control was brain RNA from wild-type C3HeB/FeJ mice, genotype Scn8a+/+, Scnm1+/+. Five micrograms of total RNA was treated with DNase 1 (Invitrogen) and reverse transcribed in a 20-μl reaction using the SuperScript first-strand synthesis system (Invitrogen) according to the manufacturer's protocol. Correctly spliced Scn8a transcripts were specifically amplified with a forward primer in exon 1 (5′-CCG ACA GTT TCA AGC CTT TCA CCC-3′) and a reverse primer in exon 2 (5′-AGG ACT TAG AAT GTA CAA GGC AGG-3′). Only correctly spliced Scn8a transcripts are amplified with these primers as the exon 3 splice-site mutation in Scn8amedJ results in a majority of incorrectly spliced transcripts lacking exon 2 and exon 3 (Kohrman et al. 1996). RT–PCR products were examined on agarose gels.
To quantitate the correctly spliced Scn8a transcript in each cDNA, we used the ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA). Reactions were carried out in a 96-well plate (Applied Biosystems) with a final reaction volume of 30 μl. The Taqman probe Mm01300417_m1 (Applied Biosystems) that spans the exon 1–2 junction was used according to manufacturer's protocol. TATA box binding protein (Tbp) transcript was measured for normalization (Mm00446971_m1, Applied Biosystems). Samples were assayed in triplicate or quadruplicate. The fold change in Scn8a gene expression was calculated as 2−ΔΔCt, where ΔΔCt = (Ct, Scn8a − Ct, Tbp) sample − (Ct, Scn8a − Ct, Tbp)wild-type sample.
sample − (Ct, Scn8a − Ct, Tbp)wild-type sample.
Protein interaction and yeast two-hybrid screen:
The yeast two-hybrid screen of a mouse embryo cDNA library was carried out as previously described (Howell et al. 2007). Wild-type Scnm1 cDNA from strain C3HeB/FeJ was cloned into the mammalian expression vector pCMVTnT (Promega). Full-length I-mfa cDNA from C57BL/6J brain was cloned into pCMV-MYC (Clontech Laboratories, Mountain View, CA). Transfection in COS7 cells, immunoprecipitation, and Western blotting were carried out as previously described (Howell et al. 2007). Protein was extracted 48 hr after transfection using RIPA buffer (Sigma-Aldrich, St. Louis) containing 1 mm phenylmethylsulfonyl fluoride (Roche Diagnostics, Indianapolis).
RESULTS
Targeted disruption of Scnm1:
To generate a targeted allele on a homogenous C57BL/6J background for subsequent phenotypic analysis, targeting was carried out in ES cell line BL/6-III, and the targeting construct was derived from a C57BL/6J BAC clone. To avoid disrupting the expression of the nearby upstream and downstream genes in this gene-rich region, we introduced the loxP sites into intron 2 and intron 5 of Scnm1 (Figure 1B). This produced a target for CRE recombinase that results in removal of most of the coding sequence of SCNM1 (Figure 1A). Correct targeting of ES cells was detected by PCR for the 5′ flank (Figure 1C) and by Southern blotting for the 3′ flank (Figure 1D). Germline transmission of the targeted construct was obtained from two chimeric founders derived from the same ES cell clone. The PGK-neo cassette was removed by crossing male Scnm1TAR/+ mice with an FLPE transgenic mouse to generate the Scnm1flox allele (Figure 1B).
Floxed mice were crossed with an EIIa-CRE transgenic line expressing Cre recombinase in the zygote (Lakso et al. 1996). Chimeric offspring were intercrossed to obtain mice with germline deletion of exons 3–5 of Scnm1, which were identified by genotyping as shown in Figure 1E.
Phenotype of Scnm1Δ3-5 mice with ubiquitous deletion of exons 3–5:
Normal Mendelian inheritance was observed in crosses between Scnm1Δ3-5/+ heterozygotes (Table 1). In the first two generations, the body weight of Scnm1Δ3-5 homozygotes was 50% of littermates. However, this effect disappeared when the CRE transgene segregated away from Scnm1Δ3-5. Other examples of CRE transgenes that cause reduced body weight were recently described (Naiche and Papaioannou 2007). Homozygous Scnm1Δ3-5 mice were viable and fertile, with normal grip strength and swimming and beam-walking ability. No visible abnormalities were detected in mice after aging >1 year (data not shown).
TABLE 1.
Strain background does not influence survival of homozygous Scnm1Δ3-5 mice
| No. of F2 offspring with the indicated genotype
|
Likelihood of Mendelian segregation (P-value) | |||
|---|---|---|---|---|
| Cross | +/+ | Δ3-5/+ | Δ3-5/Δ3-5 | |
| B6.Scnm1Δ3-5/+ × B6 | 21 | 44 | 22 | 0.98 |
| B6.Scnm1Δ3-5/+ × 129S6 | 12 | 28 | 17 | 0.63 |
| B6.Scnm1Δ3-5/+ × C3H | 22 | 45 | 13 | 0.19 |
F2 litters were obtained by crossing heterozygous Scnm1Δ3-5/+ F1 mice. Scnm1Δ3-5 homozygotes were recovered at the expected frequency of 25% from crosses with three inbred strains. P-values for agreement with Mendelian ratios were calculated by χ2 test for goodness of fit. B6, C57BL/6J; 129S6, 129S6/SvEvTac; C3H, C3HeB/FeJ.
Western blots from Scnm1Δ3-5 homozygotes demonstrated that the mutant protein is stable in vivo, but is present at reduced levels (Figure 1F). The mutant protein migrates more slowly than predicted by its 10.3-kDa calculated molecular weight as previously reported for other SCNM1 isoforms (Howell et al. 2007). Immunohistochemistry of cultured fibroblasts from Scnm1Δ3-5 homozygotes demonstrated that the mutant protein, which retains the nuclear localization signal (Figure 1A), is correctly localized to the nucleus (Figure 1G).
Viability of Scnm1Δ3-5 homozygotes on other strain backgrounds:
Scnm1Δ3-5/+ mice were crossed to strains 129S6 and C3H, and homozygous F2 mice were generated. In both crosses, homozygotes were born in the predicted Mendelian ratios (Table 1), and no abnormal phenotypes were observed.
The Scnm1Δ3-5 allele reduces splicing of the Scn8amedJ transcript:
To determine the functional effect of the targeted deletion allele, we compared the amount of correctly spliced Scn8a transcript in brains of Scn8amedJ homozygous mice carrying various Scnm1 alleles. F2 mice with the indicated genotypes were obtained by intercrossing Scn8amedJ/+, Scnm1Δ3-5/R187X double heterozygous C57BL/6J mice (Table 2). All genotypes were recovered in the predicted Mendelian ratios (data not shown). Correctly spliced transcripts were quantitated with a Taqman assay using a probe spanning the cDNA junction between exon 1 and exon 2 as described in materials and methods.
TABLE 2.
Effect of the Scnm1Δ3-5 targeted allele on splicing of the Scn8amedJ transcript
| Genotype | No. of mice | % correctly spliced Scn8a transcripts | Previous estimates (%) |
|---|---|---|---|
| Scn8a+/+ | 3 | 100 ± 16 (12) | 100 by definition |
| Scn8amedJ/medJ, Scnm1+/+ | 1 | 8.1 ± 0.7 (4) | 12a; 10b |
| Scn8amedJ/medJ, ScnmR187X/R187X | 2 | 3.8 ± 0.4 (8) | 6.4a; 5b |
| Scn8amedJ/medJ, Scnm1Δ3-5/R187X | 1 | 3.4 ± 0.3 (4) | NA |
| Scn8amedJ/medJ, Scnm1Δ3-5/Δ3-5 | 2 | 1.4 ± 0.1 (8) | NA |
Mice were generated from intercrosses of (B6.Scn8a1medJ/+, Scnm1Δ3-5/R187X) mice and of (B6.Scn8a1medJ/+, Scnm1R187X/+) mice. Scn8a transcripts in brain RNA were quantitated with a Taqman assay; the probe spanned the exon1/exon 2 junction to specifically detect correctly spliced Scn8a transcripts. Values represent 2(−ΔΔCt) × 100, mean ± SD (n), where n is the number of replicate assays (four per mouse). NA, not applicable.
Scn8amedJ/medJ mice carrying the wild-type Scnm1+/+ allele produced ∼8.1% of correctly spliced transcript relative to wild-type mice, consistent with previous estimates using other assays (Table 2). This was reduced by another 50% to give 3.8% correctly spliced transcript in mice homozygous for the B6 allele, Scnm1R187X, and a similar level in individuals heterozygous for Scnm1R187X and the Δ3-5 allele (Table 2). In mice homozygous for the targeted allele Scnm1Δ3-5, there was a further reduction to 1.5% correctly spliced Scn8amedJ transcript (Table 2 and Figure 2A). The data demonstrate that the Scnm1Δ3-5 allele is more severe than the previously described alleles and indicate that the Scnm1R187X allele retains partial function in splicing of the Scn8amedJ transcript. It is not clear whether Scnm1Δ3-5 is a complete null or retains a low level of residual activity, but this allele is significantly less active than the Scnm1R187X allele previously described in strain C57BL/6J (Buchner et al. 2003).
Figure 2.—
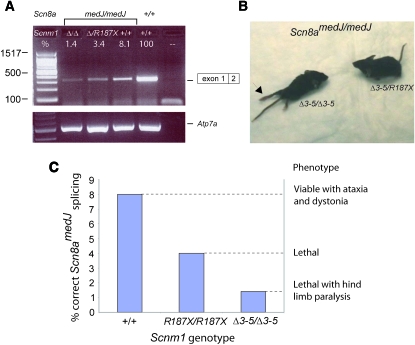
Correlation of molecular and neurological phenotypes for the indicated Scnm1 genotypes in combination with the Scn8amedJ/medJ mutation. (A) Semiquantitative RT–PCR of correctly spliced Scn8amedJ transcripts is consistent with the quantitative RT–PCR data in Table 2. 100% is defined as the transcript level in wild-type Scnm1+/+ Scn8a+/+ mice. Numerical values above the gel are from Table 2. (B) Hind-limb paralysis in the Scn8amedJ/medJ, Scnm1Δ3-5/Δ3-5 double homozygote producing 1.4% of normal transcript abundance (left). Some hind-limb function is retained in the Scn8amedJ/medJ, Scnm1Δ3-5/R187X animal with one copy of the partially functional R187 allele and 3.4% of normal transcript abundance (right). (C) Summary of transcript levels and phenotypes of Scn8amedJ homozygotes with various Scnm1 genotypes.
Exacerbation of the Scn8amedJ movement disorder by the Scnm1Δ3-5 allele:
The Scn8a gene encodes sodium channel Nav1.6, the major sodium channel at nodes of Ranvier in myelinated neurons of adult mice. The neurological phenotype of homozygotes for the Scn8amedJ mutation is modified by the Scnm1 genotype, with a direct correlation between the percentage of correctly spliced Scn8amedJ transcripts and clinical severity (reviewed in Meisler et al. 2004). In the presence of the wild-type allele of Scnm1, Scn8amedJ homozygotes have a normal life span with a movement disorder that includes ataxic gait, visible tremor, and chronic dystonia. In the Scnm1R187X/R187X background of C57BL/6J mice, Scn8amedJ homozygotes survive for only 1 month, with significant muscle weakness but retention of hind-limb function. To determine the clinical effect of the reduced Scn8amedJ splicing described above, we generated double homozygous mice with the genotype Scn8amedJ/medJ, Scnm1Δ3-5/Δ3-5 (Table 3). The eight double homozygotes all developed hind-limb paralysis that was not observed in littermates who were Scnm1Δ3-5/R187X or homozygous for Scnm1R187X (Table 3 and Figure 2B). These data demonstrate that the residual 1.4% of full-length Scn8a transcripts in Scn8amedJ/medJ, Scnm1Δ3-5/Δ3-5 mice is insufficient for normal hind-limb innervation, and confirms the greater severity of the Δ3-5 allele. The relationship between hind-limb phenotype and transcript level is summarized in Figure 2C.
TABLE 3.
Hind-limb paralysis is observed only in Scn8amedJ homozygotes that are also homozygous for Scnm1Δ3-5
|
medJ/medJ
|
||||
|---|---|---|---|---|
| R187X/R187X | R187X/Δ3-5 | Δ3-5/Δ3-5 | P-value | |
| Cross 1 | 9 | 20 | 5 | 0.37 |
| Cross 2 | NE | 2 | 3 | 0.57 |
| Total | 9 | 22 | 8 | |
| Hind-limb paralysis | 0 | 0 | 8 | |
P-values are for agreement with Mendelian predictions. Scnm1 genotypes are indicated for all Scn8amedJ homozygotes from both crosses. Hind-limb paralysis was observed in all Scnm1Δ3-5, Scn8amedJ double homozygotes and not in mice with other genotypes. Cross 1, (Scnm1Δ3-5/R187X × Scn8amedJ/+)F2; cross 2, (Scnm1Δ3-5/ Δ3-5, Scn8amedJ/+) × (Scnm1Δ3-5/R187X, Scn8amedJ/+). NE, none expected.
Expression array analysis of Scnm1Δ3-5 tissues:
To identify additional transcripts with altered expression caused by the Scnm1Δ3-5 allele, we analyzed brain RNA from Scnm1Δ3-5 homozygotes and wild-type controls by hybridization with Affymetrix Mouse 430 2.0 GeneChips. The greatest change observed was a 3.8-fold reduction in the Scnm1 transcript itself, which may reflect reduced stability of the Scnm1Δ3-5 transcript. Decreased transcript abundance was confirmed by RT–PCR (data not shown) and is likely to contribute to the reduced amount of mutant protein (Figure 1F). The 20 genes with the largest changes are listed in Table 4; they include 8 genes with nucleic-acid-binding domains and two splice factors. In view of the small magnitude of the observed changes, confirmation of biological significance would require further study.
TABLE 4.
The 20 transcripts with greatest differences in abundance between RNA from Scnm1R187X/R187X mice and from Scnm1Δ3-5/Δ3-5 mice
| Gene | Fold change | Effect of Δ3-5 | t-statistic | P-value |
|---|---|---|---|---|
| Sodium channel modifier 1 (Scnm1) | 3.8 | Decrease | −15.29 | 0.0001 |
| RIKEN cDNA B230343J05 gene | 2.4 | Increase | 15.31 | 0.0001 |
| Glucocorticoid induced transcript 1 | 2.1 | Increase | 7.01 | 0.0022 |
| Synaptotagmin binding, cytoplasmic RNA interacting | 2.1 | Increase | 7.63 | 0.0016 |
| Eph receptor A5 | 2.0 | Increase | 7.82 | 0.0014 |
| ELOVL family member 7, elongation of long chain fatty acids (yeast) | 2.0 | Decrease | −7.72 | 0.0015 |
| RIKEN cDNA E030025D05 gene | 1.9 | Increase | 7.52 | 0.0017 |
| Gap junction membrane channel protein α-12 | 1.9 | Decrease | −10.55 | 0.0005 |
| Endothelial differentiation, sphingolipid G-protein-coupled receptor, 8 | 1.9 | Decrease | −10.88 | 0.0004 |
| SRY-box containing gene 4 | 1.8 | Increase | 7.54 | 0.0017 |
| IQ motif and WD repeats 1 | 1.8 | Increase | 8.62 | 0.0010 |
| Myelin-associated oligodendrocytic basic protein | 1.8 | Decrease | −16.86 | <0.0001 |
| Splicing factor, arginine/serine-rich 7 | 1.7 | Increase | 9.03 | 0.0008 |
| RIKEN cDNA 4632409L22 gene | 1.7 | Increase | 10.68 | 0.0004 |
| RNA-binding motif protein 3 | 1.7 | Decrease | −11.27 | 0.0004 |
| Immunoglobulin heavy chain 6 (heavy chain of IgM) | 1.7 | Decrease | −7.35 | 0.0018 |
| UDP galactosyltransferase 8A | 1.7 | Decrease | −12.56 | 0.0002 |
| Gelsolin | 1.7 | Decrease | −15.77 | <0.0001 |
| Gelsolin | 1.7 | Decrease | −24.15 | <0.0001 |
| CD38 antigen | 1.7 | Decrease | −9.1 | 0.0008 |
RNA was hybridized to Affymetrix Mouse 430 2.0 GeneChips. Three replicates for each genotype were assayed, including two biological replicates. The decreased abundance of Scnm1 may reflect instability of the Δ3-5 transcript.
To determine whether the Scnm1Δ3-5 mutation changes the ratios of alternative transcripts from genes with weak donor splice sites, we interrogated brain and testis RNA using microarrays with probes to exon junctions (Srinivasan et al. 2005). Experiments included two Scnm1Δ3-5 homogygotes and two littermate controls, with three replicates for each genotype and dye-swap controls. No statistically significant changes were identified (data not shown). RNA samples were also hybridized to microarrays containing exon junction probes for 799 G-coupled protein receptors and ion channel genes (ExonHit Therapeutics). No substantial changes were observed. It thus appears that reduced expression of Scnm1 alone does not produce major changes in transcript processing.
Can SCNM1 rescue defective splicing of a Brunol4 null allele?
The spontaneous neurological mutant frequent-flyer (Ff) was caused by a transgene insertion in intron 1 of Bruno-like 4 (Brunol4), which encodes an RNA-binding protein involved in RNA processing. There is no detectable Brunol4 transcript in frequent-flyer homozygous mice, apparently due to disrupted splicing by the inserted transgene (Yang et al. 2007). Brunol4Ff homozygotes are not viable on strain C57BL/6J but have higher rates of survival on other inbred strains (Yang et al. 2007). To determine whether impaired splicing by the Scnm1R187X allele accounts for the greater lethality on C57BL/6J, we generated B6.Brunol4Ff homozygous mice carrying a wild-type SCNM1 cDNA transgene. This transgene, under the regulation of the chicken β-actin promoter, rescued the lethality of Scn8amedJ/medJ, Scnm1R187X/R187X mice (Buchner et al. 2003). Brunol4Ff/Ff mice were born at the expected overall frequency of 25% (11/41) (Table 5). The 11 homozygotes included 5 transgenic and 6 nontransgenic mice, indicating that the transgene did not increase prenatal survival. Four of the five transgenic homozygotes died by postnatal day 4, as did 5 of the 6 nontransgenic homozygotes, and the 2 survivors did not live beyond weaning, indicating that the presence of the transgene did not contribute to postnatal survival. Thus, impaired splicing by SCNM1R187X is not responsible for the lethality of the frequent flyer mutation of Brunol4 on strain C57BL/6J.
TABLE 5.
The wild-type SCNM1 transgene Tg580 does not rescue the lethality of B6.Brunol4Ff/Ff (null) mice or B6.Imfa−/− null mice
| Genotype at mutation
|
|||||||
|---|---|---|---|---|---|---|---|
| −/−
| |||||||
| Total | Tg+ | +/+ | +/− | Tg+ | Tg− | P-value | |
| Cross 1: Brunol4Ff/+ × Brunol4Ff/+,Tg+ | 41 | 20 | 10 | 20 | 5 | 6 | 0.95 |
| Cross 2: Imfa+/− × Imfa+/−,Tg+ | 156 | 80 | 52 | 102 | 2 | 0 | 0.65 |
Cross 1 was genotyped on postnatal day 1. None of the homozygous mice survived beyond weaning, and most did not survive beyond P4. Cross 2 was genotyped on postnatal day 14 (P14). In both crosses, there was no significant difference between the number of null offspring that inherited the Scnm1 transgene (Tg) and those that did not (5 vs. 6 and 2 vs. 0, P-values indicated). The Scnm1 wild-type transgene failed to rescue the postnatal lethality of Bruno14Ff/Ff homozygotes and the prenatal lethality of Imfa−/− homozygotes.
SCNM1 interacts with I-MFA, a transcriptional repressor:
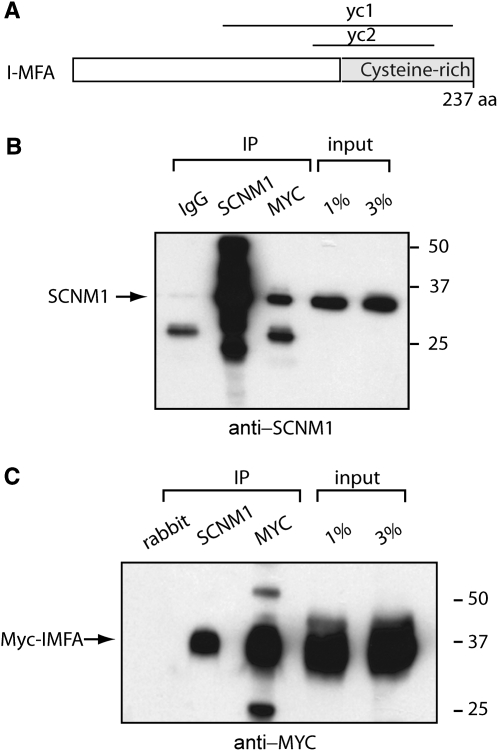
I-MFA (Inhibitor of MyoD Family isoform A) is a transcriptional regulator active during myogenesis. In the course of screening a mouse embryo yeast two-hybrid cDNA library with SCNM1 as bait (Howell et al. 2007), we recovered two overlapping cDNA clones containing the cysteine-rich region of I-MFA (Figure 3A). Cysteine-rich domains are known to interact with C2H2 zinc-finger domains (Chen et al. 1996; Mizugishi et al. 2004). To verify the interaction between SCNM1 and I-MFA, we cotransfected COS7 cells with full-length cDNAs for myc-tagged I-MFA and SCNM1. The two proteins were coprecipitated from the COS7 cell extracts by anti-SCNM1 and by anti-myc antisera (Figure 3, B and C). The interaction with I-MFA suggests that SCNM1 might have a second role in transcriptional regulation.
Figure 3.—
I-MFA interacts with SCNM1. (A) Two overlapping clones from the cysteine-rich region of I-MFA, yc1 and yc2, were recovered in a yeast two-hybrid screen with SCNM1 as bait, indicating that the two proteins can interact. (B and C) Confirmation of protein interaction by co-immunoprecipitation from lysates of transfected COS7 cells. (B) The MYC/I-MFA immunoprecipitate contains SCNM1, but the IgG negative control does not. SCNM1 was detected on the Western blot with anti-SCNM1 antiserum. (C) The anti-SCNM1 immunoprecipitate contains MYC/I-MFA, but the preimmune rabbit serum does not.
To test the biological significance of this interaction, we tested the ability of wild-type Scnm1 to rescue a targeted null allele of I-MFA, which exhibits embryonic lethality on strain C57BL/6J but not on strain 129/Sv (Kraut et al. 1998). As described above, we crossed the I-MFA (Mdfi) null mice with C57BL/6J mice carrying the wild-type Scnm1 transgene Tg580 (Buchner et al. 2003). Among 156 offspring of an F2 cross, only two homozygous null mice were recovered, both of which were transgenic (Table 5). Since complete rescue of lethality by the transgene is predicted to generate 12% rescued mice (18/156), it appears that Scnm1R187X is not responsible for lethality of the I-MFA null genotype on the C57BL/6J background.
An ENU-induced allele of Scnm1:
We screened stored, mutagenized genomic DNA from the Cryopreserved Mutant Mouse Bank at the Oak Ridge National Laboratory to identify new variants of SCNM1. DNA was analyzed by heteroduplex analysis as previously described (Michaud et al. 2005). The nonsynonymous variants I112T, S167S, and L228S and the intron substitution IVS5 + 62T > C were identified in genomic DNA from mutagenized mice. We selected I112T for in vivo analysis because of the evolutionary conservation of residue isoleucine 112. Sperm carrying this mutation were thawed and used for in vitro fertilization by intracytoplasmic sperm injection. Heterozygous Scnm1I112T mice were recovered and intercrossed to generate I112T homozygotes, which were viable and fertile. We then generated Scnm1I112T/I112T, Scn8amedJ/medJ double homozygotes. These mice did not survive beyond weaning, but they did not exhibit the hind-limb paralysis described above for Scnm1Δ3-5. Thus, I112T does not reduce SCNM1 function as severely as Scnm1Δ3-5.
DISCUSSION
Analysis of an allele series can often provide additional insights into gene function beyond that available from a single allele (Oliver et al. 2007). We previously described a naturally occurring variant of the splice factor SCNM1 in C57BL/6J and related inbred strains of mice. It was not clear from previous work whether the C57BL/6J allele, Scnm1R187X, causes partial or complete loss of function. In this work, we sought to generate a null allele of Scnm1 by targeted deletion of exons 3–5. This deletion removes a further 92 residues, including the zinc-finger domain, from the 187-residue protein characteristic of C57BL/6J mice. The deleted protein reduced the correct Scn8amedJ splicing from ∼4% of normal in Scnm1R187X homozygotes to <2% in Scnm1Δ3-5 homozygotes. This result demonstrates that SCNM1R187X retains partial function and that SCNM1Δ3-5 is a more severe allele. Because so much of the protein has been deleted, it seems likely that SCNM1Δ3-5 is a complete null. The residual 1.4% of correctly spliced Scn8amedJ transcript in Scnm1Δ3-5 homozygotes would then be produced by other proteins with overlapping activity toward nonconsensus splice donor sites.
RNA from Scnm1Δ3-5 homozygotes was analyzed to search for additional transcripts dependent on splicing by SCNM1. Expression arrays have been successfully used to identify substrates of splice factors such as NOVA-1 and NOVA-2 (Ule et al. 2005). However, hybridization of several different splicing arrays did not uncover additional splicing events that depend heavily on the function of SCNM1. To date, the Scn8amedJ mutant splice site is the only site at which SCNM1 function can be measured.
Another approach that we applied to investigate SCNM1 function was transgene rescue of mutant phenotypes in vivo. We tested the ability of wild-type Scnm1 to rescue C57BL/6J-specific phenotypes associated with null alleles of I-MFA, a binding partner of SCNM1, and BRUNOL4, an RNA-binding protein. Phenotypic rescue was not observed in either case, indicating that the C57BL/6J-specific lethality of these mutants cannot be attributed to Scnm1R187X.
SCNM1 interacts directly with the recently identified splice factor LUC7L2 (Howell et al. 2007). To evaluate their functional interaction in vivo, we generated two lines of mice carrying gene trap alleles of LUC7L2. Unfortunately, both alleles retained ∼25% of wild-type expression (data not shown). Array analysis of RNA from double mutants homozygous for a LUC7L2 gene trap allele and for Scnm1Δ3-5 did not detect altered transcripts (our unpublished observations). For future studies, we plan to generate a true null allele of Luc7l2 by gene targeting and to examine transcripts in double homozygotes with Scnm1Δ3-5.
Splice-site mutations compose ∼15% of all disease-causing mutations, and a similar number occur in splice enhancer and suppressor sequences (Nissim-Rafinia and Kerem 2005). Splice-site mutations are often associated with clinical heterogeneity within families, as described for Duchenne and Becker muscular dystrophy, cardiac sodium channelopathy (SCN5A), familial adenomatous polyposis (APC), severe combined immunodeficiency (JAK3), and neurofibromatosis type 2 (NF2) (Kluwe et al. 1998; Frucht et al. 2001; Mohamed et al. 2003; Rossenbacker et al. 2005; Gurvich et al. 2008). There is evidence that the clinical severity of these disorders is influenced by splicing efficiency. Therapeutic strategies that target the correction of splicing defects are under development for spinal muscular atrophy, familial dysautonomia, and cystic fibrosis (Nissim-Rafinia et al. 2004; Hims et al. 2007; Hua et al. 2008). The molecular basis for individual variation in splicing efficiency is largely unknown, but identification of the responsible factors could facilitate the development of therapies. The Scn8amedJ mutant utilized in our work is a sensitive reporter for splicing of nonconsensus splice donor sites, since a twofold decrease in splicing results in a visible change in phenotype. Scn8amedJ could be used for in vivo discovery of splicing modifier genes or for testing small-molecule therapeutics for splicing deficiencies.
In conclusion, we have generated a new, severe allele for dissection of the splicing function of SCNM1 and the mechanism of splice donor site choice. Future work will focus on combining this allele with mutations of other accessory splice factors. Cultured cells from Scnm1Δ3-5 mice will also be useful for defining the structural requirements of SCNM1 splice-site substrates and the effects of knockdown of other factors on minigene splicing. From the clinical variability for Scn8amedJ mice caused by variants of Scnm1, we predict that genetic variants of human SCNM1 and other trans-acting splice factors may be responsible for some of the variability of splicing-related disorders.
Acknowledgments
We are grateful to Susannah Cheek, Darcy Butts, Kian Preston-Suni, and Connie Mahaffey for technical assistance. We thank Christi Preston, Lily Shiue, and Manny Ares for analysis using their printed oligonucleotide alternative splicing arrays (supported by R24 GM070857 to M. Ares, D. Black, and X.-D. Fu). We acknowledge the contributions of Elizabeth Hughes and Virginia Zawistowski of the University of Michigan Transgenic Animal Model Core for ES cell targeting, with Core support from the Center for Organogenesis. We thank Lauren Snider and Stephen Tapscott (Fred Hutchinson Cancer Research Center) for the gift of I-MFA knockout mice. We thank Carmen Foster for the rederivation of Scnm1I112T mice. Affymetrix gene expression array analysis was carried out at the University of Michigan Microarray Core with support from the University of Michigan Cancer Center Support grant 5 P30 CA46592. The production and screening of the Cryopreserved Mutant Mouse Bank and rederivation of ENU-induced mutant mice from cryopreserved sperm were supported by the U. S. Department of Energy at Oak Ridge National Laboratory, managed by UT-Battelle under contract DE-AC05-00OR22725. This work was supported by National Institutes of Health grants R01 GM24872 to M.H.M. and R01 NS31348 to W.N.F. V.M.H. acknowledges the National Health and Medical Research Council of Australia for the award of CJ Martin Research Fellowship 358774.
References
- Buchner, D. A., M. Trudeau and M. H. Meisler, 2003. SCNM1, a putative RNA splicing factor that modifies disease severity in mice. Science 301 967–969. [DOI] [PubMed] [Google Scholar]
- Chen, C. M., N. Kraut, M. Groudine and H. Weintraub, 1996. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell 86 731–741. [DOI] [PubMed] [Google Scholar]
- Fortes, P., D. Bilbao-Cortes, M. Fornerod, G. Rigaut, W. Raymond et al., 1999. Luc7p, a novel yeast U1 snRNP protein with a role in 5′ splice site recognition. Genes Dev. 13 2425–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frucht, D. M., M. Gadina, G. J. Jagadeesh, I. Aksentijevich, K. Takada et al., 2001. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun. 2 422–432. [DOI] [PubMed] [Google Scholar]
- Gurvich, O. L., T. M. Tuohy, M. T. Howard, R. S. Finkel, L. Medne et al., 2008. DMD pseudoexon mutations: splicing efficiency, phenotype, and potential therapy. Ann. Neurol. 63 81–89. [DOI] [PubMed] [Google Scholar]
- Hims, M. M., E. C. Ibrahim, M. Leyne, J. Mull, L. Liu et al., 2007. Therapeutic potential and mechanism of kinetin as a treatment for the human splicing disease familial dysautonomia. J. Mol. Med. 85 149–161. [DOI] [PubMed] [Google Scholar]
- Howell, V. M., J. M. Jones, S. K. Bergren, L. Li, A. C. Billi et al., 2007. Evidence for a direct role of the disease modifier SCNM1 in splicing. Hum. Mol. Genet. 16 3506–3516. [DOI] [PubMed] [Google Scholar]
- Hua, Y., T. A. Vickers, H. L. Okunola, C. F. Bennett and A. R. Krainer, 2008. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am. J. Hum. Genet. 82 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. S., S. H. Shi, J. Ule, M. Ruggiu, L. A. Barker et al., 2005. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell 123 105–118. [DOI] [PubMed] [Google Scholar]
- Kearney, J. A., D. A. Buchner, G. De Haan, M. Adamska, S. I. Levin et al., 2002. Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6). Hum. Mol. Genet. 11 2765–2775. [DOI] [PubMed] [Google Scholar]
- Kluwe, L., M. MacCollin, M. Tatagiba, S. Thomas, W. Hazim et al., 1998. Phenotypic variability associated with 14 splice-site mutations in the NF2 gene. Am. J. Med. Genet. 77 228–233. [PubMed] [Google Scholar]
- Kohrman, D. C., J. B. Harris and M. H. Meisler, 1996. Mutation detection in the med and medJ alleles of the sodium channel Scn8a. Unusual splicing due to a minor class AT-AC intron. J. Biol. Chem. 271 17576–17581. [DOI] [PubMed] [Google Scholar]
- Kraut, N., L. Snider, C. M. Chen, S. J. Tapscott and M. Groudine, 1998. Requirement of the mouse I-mfa gene for placental development and skeletal patterning. EMBO J. 17 6276–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso, M., J. G. Pichel, J. R. Gorman, B. Sauer, Y. Okamoto et al., 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler, M. H., N. W. Plummer, D. L. Burgess, D. A. Buchner and L. K. Sprunger, 2004. Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions. Genetica 122 37–45. [DOI] [PubMed] [Google Scholar]
- Michaud, E. J., C. T. Culiat, M. L. Klebig, P. E. Barker, K. T. Cain et al., 2005. Efficient gene-driven germ-line point mutagenesis of C57BL/6J mice. BMC Genomics 6: 164. [DOI] [PMC free article] [PubMed]
- Mizugishi, K., M. Hatayama, T. Tohmonda, M. Ogawa, T. Inoue et al., 2004. Myogenic repressor I-mfa interferes with the function of Zic family proteins. Biochem. Biophys. Res. Commun. 320 233–240. [DOI] [PubMed] [Google Scholar]
- Mohamed, Z., R. Ahmad, N. S. Yoke, Z. Zakaria, H. Ahmad et al., 2003. A nonsense mutation in exon 8 of the APC gene (Arg283Ter) causes clinically variable FAP in a Malaysian Chinese family. Cancer Sci. 94 725–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche, L. A., and V. E. Papaioannou, 2007. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis 45 768–775. [DOI] [PubMed] [Google Scholar]
- Nissim-Rafinia, M., and B. Kerem, 2005. The splicing machinery is a genetic modifier of disease severity. Trends Genet. 21 480–483. [DOI] [PubMed] [Google Scholar]
- Nissim-Rafinia, M., M. Aviram, S. H. Randell, L. Shushi, E. Ozeri et al., 2004. Restoration of the cystic fibrosis transmembrane conductance regulator function by splicing modulation. EMBO Rep. 5 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, P. L., E. Bitoun and K. E. Davies, 2007. Comparative genetic analysis: the utility of mouse genetic systems for studying human monogenic disease. Mamm. Genome 18 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacione, L. R., M. J. Szego, S. Ikeda, P. M. Nishina and R. R. McInnes, 2003. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu. Rev. Neurosci. 26 657–700. [DOI] [PubMed] [Google Scholar]
- Roca, X., R. Sachidanandam and A. R. Krainer, 2005. Determinants of the inherent strength of human 5′ splice sites. RNA 11 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossenbacker, T., E. Schollen, C. Kuiperi, T. J. de Ravel, K. Devriendt et al., 2005. Unconventional intronic splice site mutation in SCN5A associates with cardiac sodium channelopathy. J. Med. Genet. 42 e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster-Gossler, K., A. W. Lee, C. P. Lerner, H. J. Parker, V. W. Dyer et al., 2001. Use of coisogenic host blastocysts for efficient establishment of germline chimeras with C57BL/6J ES cell lines. Biotechniques 31 1022–1024, 1026. [DOI] [PubMed] [Google Scholar]
- Srinivasan, K., L. Shiue, J. D. Hayes, R. Centers, S. Fitzwater et al., 2005. Detection and measurement of alternative splicing using splicing-sensitive microarrays. Methods 37 345–359. [DOI] [PubMed] [Google Scholar]
- Ule, J., A. Ule, J. Spencer, A. Williams, J. S. Hu et al., 2005. Nova regulates brain-specific splicing to shape the synapse. Nat. Genet. 37 844–852. [DOI] [PubMed] [Google Scholar]
- Ule, J., G. Stefani, A. Mele, M. Ruggiu, X. Wang et al., 2006. An RNA map predicting Nova-dependent splicing regulation. Nature 444 580–586. [DOI] [PubMed] [Google Scholar]
- Xu, X., D. Yang, J. H. Ding, W. Wang, P. H. Chu et al., 2005. ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell 120 59–72. [DOI] [PubMed] [Google Scholar]
- Yang, Y., C. L. Mahaffey, N. Berube, T. P. Maddatu, G. A. Cox et al., 2007. Complex seizure disorder caused by Brunol4 deficiency in mice. PLoS Genet. 3 e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, L., M. Kawada, N. Havlioglu, H. Tang and J. Y. Wu, 2005. Mutations in PRPF31 inhibit pre-mRNA splicing of rhodopsin gene and cause apoptosis of retinal cells. J. Neurosci. 25 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]