Abstract
The L1 family of single-pass transmembrane cell adhesion molecules (L1CAMs) is conserved from Caenorhabditis elegans and Drosophila to vertebrates and is required for axon guidance, neurite outgrowth, and maintenance of neuronal positions. The extracellular region of L1CAMs mediates cell adhesion via interactions with diverse cell-surface and extracellular matrix proteins. In contrast, less is known regarding the function of the intracellular domains in the L1CAM cytoplasmic tail. Previously, we identified a role of the C. elegans L1CAM homolog, SAX-7, in maintaining neuronal and axonal positioning. Here, we demonstrate that this function is dependent on three conserved motifs that reside in the SAX-7 cytoplasmic tail: (1) the FERM-binding motif, (2) the ankyrin-binding domain, and (3) the PDZ-binding motif. Furthermore, we provide molecular and genetic evidence that UNC-44 ankyrin and STN-2 γ-syntrophin bind SAX-7 via the respective ankyrin-binding and PDZ-binding motifs to regulate SAX-7 function in maintaining neuronal positioning.
L1CAMs are single-pass transmembrane proteins that play critical roles in the development and function of the nervous system. In mammals, there are four L1CAMs: L1, NrCAM, neurofascin, and CHL1 (reviewed in Hortsch 2000). Mutations in the human L1 gene confer a variety of neurological symptoms that are collectively termed CRASH, an acronym for corpus callosum agenesis, mental retardation, adducted thumbs, spastic paraplegia, and hydrocephalus (Fransen et al. 1995). Pathological mutations have been mapped throughout the L1 protein, thus revealing the importance of both the extracellular region and the cytoplasmic tail for L1 function (reviewed in Weller and Gartner 2001).
The extracellular region of L1CAMs contains six immunoglobulin-like and five fibronectin type III repeats that bind a diverse range of proteins that include L1CAMs themselves, other cell surface proteins such as axonin1 and integrins, as well as extracellular matrix (ECM) proteins such as laminin and neurocan (reviewed in Haspel and Grumet 2003). These interactions reveal that the L1CAM ectodomains are crucial in mediating both cell–cell and cell–ECM adhesion.
In contrast to the extracellular region, the function of the L1CAM intracellular region is less clear. The L1CAM cytoplasmic tail contains several highly conserved motifs that include a protein 4.1, ezrin, radixin, moesin (FERM)-binding domain, an ankyrin-binding domain, and a PSD 95, disc-large, ZO1 (PDZ)-binding motif (Davis and Bennett 1994; Koroll et al. 2001; Dickson et al. 2002; Gunn-Moore et al. 2006). Of these domains, the ankyrin-binding domain is well characterized and has been shown to directly bind ankyrin, an adaptor protein that links diverse membrane proteins to the spectrin–actin cytoskeleton (Davis and Bennett 1994). Phosphorylation of a tyrosine residue in the ankyrin-binding motif prevents ankyrin binding to L1CAM, suggesting that interaction with ankryin is regulated (Garver et al. 1997). Cell-culture studies have shown that when L1CAMs are unable to bind ankyrin, because of either tyrosine phosphorylation or mutation of the ankyrin-binding motif, a reduction in cell–cell adhesion is observed, presumably as a result of a loss of cytoskeletal linkage (Tuvia et al. 1997; Zhang et al. 1998). While these studies reveal important insights into the mechanisms of L1CAM activity, it is not known how, on an organismal level, the L1CAM intracellular motifs and protein interactions, such as ankyrin, coordinate to regulate L1CAM activity.
Caenorhabditis elegans has two L1CAM genes: lad-2 and sax-7. lad-2 encodes an L1CAM with conserved ectodomains but a divergent cytoplasmic tail that does not share any sequence homology with vertebrate L1CAMs (Wang et al. 2008). In contrast, the sax-7 gene encodes a canonical L1CAM that more closely resembles vertebrate L1CAMs; both the ectodomains and intracellular motifs present in vertebrate L1CAMs are conserved in the SAX-7 protein (Chen et al. 2001; Sasakura et al. 2005; Wang et al. 2005). sax-7 is required to maintain proper neuronal and axon positioning (Zallen et al. 1999; Sasakura et al. 2005; Wang et al. 2005; Pocock et al. 2008). For example, GABAergic neurons are displaced in 95% of sax-7(eq1) adult animals (Wang et al. 2005). This highly penetrant phenotype provides the opportunity to assess in an organismal context how the intracellular motifs of SAX-7 function in maintaining neuronal positioning. Collectively, our results provide evidence that integration of SAX-7 with cytoskeletal components is required for proper neuronal positioning maintenance.
MATERIALS AND METHODS
Strains:
C. elegans strains were grown on nematode growth medium plates at 20° as described by Brenner (1974). N2 bristol served as the wild-type strain. The alleles used are listed by linkage groups as follows:
LGI: stn-1(ok292) (Grisoni et al. 2003).
LGIII: unc-119 (e2498) (Maduro and Pilgrim 1995).
LGIV: unc-44(e362) and unc-44(e1260) (Boontrakulpoontawee and Otsuka 2002), sax-7(eq1) and sax-7(eq2) (Wang et al. 2005), sax-7(nj13) and sax-7(nj48) (Sasakura et al. 2005), sax-7(ky146) (Zallen et al. 1999), sax-7(tm1448) (Japanese National Bioresource Project), sax-7(ok1244) (C. elegans Knockout Consortium).
LGX: stn-2(tm1869), stn-2(ok2417).
Both stn-2 strains were outcrossed eight times to remove background mutations. The sax-7(eq1) animals used in this study were subjected to additional outcrossing of the right arm of LGIV to remove linked background mutations; the resulting sax-7(eq1) strain is LH81. The oxIs12 (Punc-47∷gfp) (McIntire et al. 1997) and evIs111(Punc129∷gfp) (Colavita et al. 1998) integrated transgenes were crossed into respective strains to visualize GABA and cholinergic neurons, respectively.
Plasmid constructions for injecting in C. elegans and cell culture assay:
Psax-7∷sax-7 (sax-7 genomic sequence, pLC 262):
sax-7 promoter (7.0 kb upstream of the start codon) and sax-7 genomic DNA (14 kb) were cloned into pWKS30 between SacI and KpnI.
Psur-5∷sax-7L (pLC 449) and Psur-5∷sax-7S (pLC468):
Both sax-7L and sax-7S cDNAs encoding the SAX-7 extracellular domain were cloned together with a 2.1-kb genomic sequence encoding the remaining SAX-7 transmembrane domain, cytoplasmic tail, and 3′-UTR into the pBluescript vector between BamHI and KpnI. The 2.7-kb sur-5 promoter was cloned from PTG96 (Yochem et al. 1998) and inserted upstream of the sax-7 sequences into pBluescript II KS− between SacII and SacI sites.
Mutant sax-7 constructs:
All mutant sax-7 constructs were generated via site-directed mutagenesis of wild-type sax-7L using the Quickchange site-directed mutagenesis kit (Stratagene). These constructs are as follows:
sax-7∷ΔCT∷gfp (pLC542), sax-7∷ΔCT∷lad-2CT (pLC520), sax-7∷ΔFB (pLC550), sax-7∷ΔSFIGQY, sax-7∷SFIGQA (pLC472), sax-7∷SFIGQE (pLC480), sax-7∷SFIGQF (pLC475), sax-7∷ΔSTFV (pLC478), sax-7∷YSLA (pLC471).
stn-2∷gfp (pLC538): This construct contains the stn-2 promoter (3.82 kb sequences upstream of start codon) and 3.97 kb of the gene that were fused with gfp coding sequences and the unc-54 3′-UTR, all subcloned into the pBluescript II KS− vector between the NotI and ApaI sites.
Neurofascin∷SAX-7CT (pLC101) (Chen et al. 2001).
Neurofascin∷SAX-7CTΔAB (pLC580): The ankyrin-binding motif, SFIGQY, was deleted from pLC101.
UNC-44∷GFP (pLC111): unc-44 cDNA encoding the conventional ankyrin isoform was subcloned in frame with GFP and downstream of a CMV promoter.
Transgenic strains:
All sax-7 constructs were injected into both sax-7(eq1) and wild-type background at 50 ng/μl with 50 ng/μl of Pceh-22∷gfp (Okkema and Fire 1994) as a co-injection marker. This concentration was used because it gave the most consistent sax-7 expression as indicated by whole-mount antibody staining of SAX-7 as well as the most consistent rescue levels. Other concentrations tested included 10–100 ng/μl. The stn-2∷gfp construct was injected at 50 ng/μl with 50 ng/μl of Psur-5∷rfp into stn-2(tm1869) animals. The stn-2∷gfp construct rescues the hyperactivity and head-bending phenotype exhibited by stn-2(tm1869) animals.
Recruitment/binding assay in HEK293 cells:
The assay was performed as described (Zhang et al. 1998). Briefly, cells were transfected with respective constructs. To visualize the expressed protein, cells were fixed with formaldehyde, washed, and incubated with anti-GFP antibodies to visualize UNC-44∷GFP and anti-SAX-7 antibody to detect neurofascin∷SAX-7CT or neurofascin∷SAX-7CTΔAB.
Immunofluorescence:
Animals were fixed in methanol and stained for indirect immunofluorescence using the freeze-crack methanol fixation method (Miller and Shakes 1995). The primary antibodies that were used—rabbit polyclonal 6991 antibodies against the SAX-7 cytoplasmic tail (Chen et al. 2001), rabbit antisera AO280 against the spectrin-binding domain in UNC-44 (Chen et al. 2001; a gift from A. Otsuka), and monoclonal anti-GFP antibodies (3E6 from Q-BIOgene)—were used at concentration 1:500 while guinea pig polyclonal antibody 1622 against the LAD-2 cytoplasmic tail was used at concentration 1:250. Secondary antibodies (Alexa 488 and 568, Molecular Probes) were used at concentration 1:500. Fixed animals were viewed using the Zeiss Axioplan 2 IE microscope and images were acquired using the Axiocam MRM and Axiovision software. Animals of different sax7 backgrounds as well as sax-7(eq1) animals expressing mutant sax-7 constructs were stained with the 6991 antibody. Proper localization of SAX-7∷ΔCT∷GFP in sax-7(eq1) animals expressing sax-7∷ΔCT∷gfp was confirmed by visualizing the GFP epifluorescence as well as immunostaining using anti-GFP antibodies. sax-7(eq1) animals expressing sax-7∷ΔCT∷lad-2CT were stained with the 1622 antibody to confirm proper localization of the transgenic SAX-7. stn-2(tm1869) animals expressing stn-2∷gfp were stained with anti-GFP and 6991 antibodies to examine for colocalization of STN-2∷GFP and SAX-7.
Live animal microscopy—scoring for displaced neurons:
Displaced GABA and cholinergic neurons were scored in animals of different genetic background or expressing variant sax-7 constructs using the oxIs12 or evIs111 GFP markers. Synchronized young adult animals were mounted on 2% agarose pads and scored for defects using fluorescence microscopy.
Yeast two-hybrid interaction screen and assays—constructs:
All bait and and prey constructs were generated by subcloning coding sequences into the respective Clontech's Matchmaker GAL4 two hybrid system 3 vectors. The bait (pLC208) contains the SAX-7 cytoplasmic tail (CT) subcloned into the pGBKT7 vector (Trp selective marker) in frame with the GAL4 DNA-binding domain. We also generated alternative baits (pLC557 and pLC228), which contain the SAX-7 cytoplasmic tail that lacks either the ankyrin-binding (AB) motif (SFIGQY) or the PDZ-binding (PB) motif (STFV), respectively. The preys were generated by subcloning cDNAs of 14 genes encoding PDZ proteins and UNC-44 ankyrin (obtained from Yuji Kohara, Mishima, Japan) into the pGADT7 vector (Leu selective marker) in frame with the GAL4 DNA-activating domain.
Yeast two-hybrid screen/assay:
All yeast assays were performed in the AH109 yeast strain, which were cultured on SD minimal media lacking specified amino acids, following the protocol provided by Clontech: (1) SD/−Leu, (2) SD/−Trp, (3) SD/−Leu/−Trp, (4) SD/−Leu/−Trp/−His/+2.5 mm 3-AT, (5) SD/−Leu/−Trp/−His/−Ade/+2.5 mm 3-AT, (6) SD/−Leu/−His/+2.5 mm 3-AT, and (7) SD/−Trp/His/+2.5 mm 3-AT. 3-AT (2.5 mm) was added to reduce number of background colonies due to leaky His expression.
We confirmed that the SAX-7CT bait construct (1) did not activate readout reporters on its own, e.g., grow on media lacking His or His/Ade, and (2) did not interact nonspecifically with the provided negative control, pGADT7–T, which encodes for SV40 large T-antigen. The screen was performed by cotransforming each prey construct with the SAX-7CT bait. A positive interaction was considered robust if there were numerous large non-red (white) colonies growing on media lacking Leu/Trp/His/Ade. Four clones showed robust interaction (large white colonies), four clones showed weak interaction (small red colonies), and the remaining five clones showed no interaction (no growth). Only the clones that showed robust interaction with SAX-7 were further tested to confirm that the prey construct (1) did not grow on media lacking His or His/Ade in the absence of the bait and (2) did not interact nonspecifically with the provided negative control vector, pGBKT7-lam, which encodes for human lamin-C. Clones that passed these tests were further analyzed to determine if the interaction required the SAX-7 PB motif or the PDZ domain(s) in the prey. X-α- and β-galactosidase assays, additional tests for a positive interaction between bait and prey, were performed on SAX-7 + STN-2 cotransformed yeast colonies, which turned strongly blue, confirming a robust interaction. In contrast to STN-2, STN-1 did not interact with SAX-7CT as indicated by no growth on media lacking Leu/Trp/His or Leu/Trp/His.
RESULTS
The two SAX-7 isoforms, SAX-7S and SAX-7L, rescue sax-7(eq1) phenotypes:
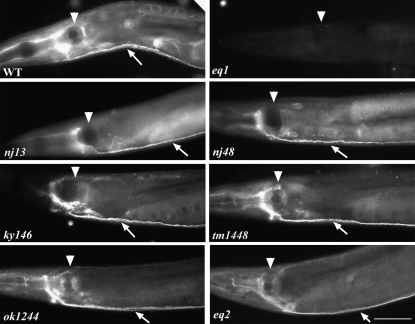
To assess the functional importance of the conserved SAX-7 intracellular domains, we first established a transgenic rescue system using sax-7(eq1). Molecular and genetic data indicate that eq1 is a molecular and genetic null allele (Wang et al. 2005). In contrast, the other sax-7 alleles still show residual SAX-7 protein (Figure 1), as revealed by whole-mount immunostaining using an anti-SAX-7 antibody (Wang et al. 2005). Thus, unlike eq1, the other sax-7 alleles are not molecular null alleles.
Figure 1.—
Immunofluorescence analysis shows positive SAX-7 immunostaining in the different sax-7 mutant backgrounds, except for sax-7(eq1). SAX-7 staining is strong in the nervous system; arrow points to the ventral nerve cord while arrowhead points to the pharyngeal posterior bulb. Bar, 50 μm.
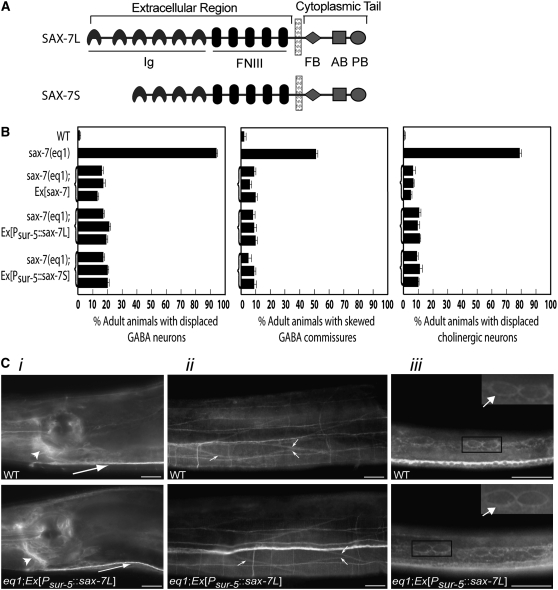
sax-7(eq1) adult animals exhibit highly penetrant displacement of the ventral nerve cord GABAergic and cholinergic neurons and skewed commissural axons, a phenotype caused by defective maintenance of neuron positioning. This phenotype can be rescued by a genomic sax-7 construct (Wang et al. 2005; Figure 2B). Optimal positional maintenance of these neurons requires sax-7 activity in the neurons as well as in the adjacent hypodermal and body-wall muscle tissues, suggesting that sax-7 mediates adhesion of neurons to the adjacent tissues (Wang et al. 2005). We generated two constructs, Psur-5∷sax-7L and Psur-5∷sax-7S, which are composed of sax-7 cDNA corresponding to either of the two SAX-7 isoforms, SAX-7L and SAX-7S (Figure 2A), driven by the broadly expressing sur-5 promoter (Yochem et al. 1998). Both Psur-5∷sax-7L and Psur-5∷sax-7S rescue the sax-7(eq1) neuronal displacement phenotype just as well as a genomic sax-7 construct does (Figure 2B). Immunostaining of these transgenic sax-7(eq1) animals revealed that the accumulation and localization of transgenic SAX-7L and SAX-7S are similar to that of endogenous SAX-7 in wild-type animals (Figure 2C). Our transgene rescue system allows us to test the ability of SAX-7 constructs containing engineered mutations to rescue sax-7(eq1) neuronal displacement, thereby providing us a means for assessing functional contributions of SAX-7 intracellular domains in a whole-animal context.
Figure 2.—
Psur-5∷sax-7 is similarly expressed as endogenous sax-7 in wild-type animals and rescues the positional defects in the GABA neuronal soma and commissural axons in sax-7(eq1) animals just as well as genomic sax-7 does. (A) A schematic of the two SAX-7 protein isoforms, SAX-7L and SAX-7S. The extracellular region contains immunoglobulin (Ig) and fibronectin type III (FNIII) repeats while the cytoplasmic tail contains the FB, AB, and PB motifs. (B) Quantitation of displaced cholinergic and GABA neurons and commissural axons in wild-type, sax-7(eq1), and transgenic sax-7(eq1) animals. Three independent transgenic lines for each construct were analyzed. Error bar shows standard error of the proportions of three sample sets where in each set n = 100. (C) Immunofluorescence analyses of transgenic sax-7(eq1) animals show that transgenic SAX-7 is similarly expressed and localized as endogenous SAX-7 in wild-type animals in multiple tissues, including the nervous system (i), body-wall muscle (ii), and hypodermis (iii), the three tissues where sax-7 activity is required for optimal positioning maintenance of GABA neurons. Respective images of wild-type and transgenic sax-7(eq1) animals are taken with the same exposure times. SAX-7 is present in (i) the nerve ring (large arrow) and ventral nerve cord (small arrow), (ii) the cell membrane of the elliptical-shaped body-wall muscle cells (arrows), and (iii) the cell membrane of the hypodermal seam cells (the arrows in the enlarged insets show SAX-7 localization on the membrane of two seam cells). Bar, 20 μm.
We selected SAX-7L for use in our assays for two reasons: (1) the rescue abilities of SAX-7L and SAX-7S are comparable and (2) SAX-7L is more similar to vertebrate L1CAMs and there is no comparable SAX-7S isoform in vertebrates. For each sax-7L (referred to as sax-7 henceforth) construct assayed, we immunostained the respective transgenic animals to confirm that the expression and localization of the transgenic SAX-7 is similar to endogenous SAX-7 in wild-type animals (data not shown; see materials and methods). Moreover, we did not detect abnormalities when these constructs were expressed in wild-type animals, ruling out the possibility that these transgenic SAX-7 proteins may act in a dominant fashion (Figure 3).
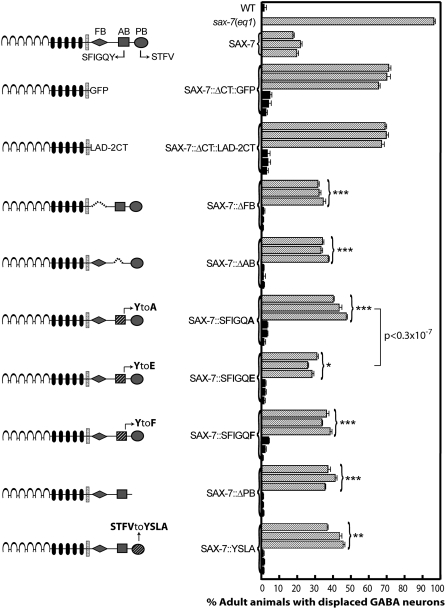
Figure 3.—
Quantitation of displaced GABA neurons in sax-7(eq1) transgenic animals expressing SAX-7 variants with targeted mutations in the FB, AB, and PB motifs in the SAX-7 cytoplasmic tail reveals the functional importance of these conserved domains. Each sax-7 construct is introduced into sax-7(eq1) animals (stippled bars) to test the ability of these constructs to rescue neuronal displacement. Each sax-7 construct is introduced also in wild-type animals (solid bars) to confirm that these constructs do not function in a dominant fashion. Next to the rescue data for each variant SAX-7 construct is a schematic of the SAX-7 protein containing the corresponding mutation. Dashed lines indicate deletion of the respective motif. Three independent transgenic lines for each construct in both sax-7 and wild-type backgrounds were analyzed. Error bar shows the standard error of the proportions of three sample sets where in each set n = 100. Statistical significance was assessed by t-test where ***P < 0.5 × 10−8, **P < 0.1 × 10−4, and *P < 0.5 × 10−4, as compared to sax-7(eq1); Ex[Psur-5∷sax-7L] animals. The statistical significance between the rescue ability of SAX-7:;SFIGQA and SFIGQE was assessed by the Student's t-test and is as stated in the figure.
The SAX-7 cytoplasmic tail is required to maintain neuronal positioning:
To investigate the role of the SAX-7 cytoplasmic tail (SAX-7CT) in neuronal positioning, we generated two constructs: (1) SAX-7ΔCT∷GFP, where the cytoplasmic tail is removed and GFP is fused to the remaining SAX-7 sequence and (2) SAX-7ΔCT∷LAD-2CT where the SAX-7 cytoplasmic tail is deleted and replaced by the divergent LAD-2 cytoplasmic tail, which lacks the intracellular motifs found in SAX-7 (Wang et al. 2008). Transgenic sax-7(eq1) animals expressing either construct exhibited displaced GABAergic neurons, revealing that specific sequences in the SAX-7 cytoplasmic tail are required to mediate neuronal positioning.
The FERM-binding motif contributes to SAX-7 function:
The SAX-7 cytoplasmic tail contains consensus sites for a FERM-binding (FB), an AB, and a PB motif (Figure 2A; Chen et al. 2001; Pocock et al. 2008). To determine which of these motifs contribute to SAX-7 function, we engineered deletion and point mutations within each domain and assessed the ability of these altered forms of SAX-7 to rescue the displaced GABAergic neurons. The FB domain (RGQNYPVSQR) is localized in the juxtamembrane region of the SAX-7 cytoplasmic tail. Deletion of this domain (SAX-7ΔFB) reduced but did not eliminate the rescue activity of the displaced GABA neurons in sax-7(eq1) animals (Figure 3). This incomplete rescue reveals a functional contribution by the FB domain to SAX-7 function.
The ankyrin-binding domain contributes to SAX-7 function:
The AB motif (composed of the amino acids SFIGQY) is localized downstream of the FB domain. Deletion of the AB motif (SAX-7ΔAB) also showed reduced rescue of the displaced GABA neurons in sax-7(eq1) animals (Figure 3), indicating that the AB motif contributes to SAX-7 function. The AB motif in vertebrate L1CAMs directly binds ankyrin (Davis and Bennett 1994). The importance of the SAX-7 AB motif in proper neuronal positioning suggests that SAX-7 function may require interaction with ankyrin.
unc-44 genetically interacts with sax-7 to maintain neuronal positioning:
C. elegans contains a single ankyrin gene, unc-44 (Otsuka et al. 1995). To test the hypothesis that ankyrin interaction is important for SAX-7 function, we examined unc-44 mutant animals for displaced neurons. Animals homozygous for different unc-44 alleles exhibit pleiotropic phenotypes, which is not surprising as unc-44 is broadly expressed in multiple tissues (Chen et al. 2001). These phenotypes include a Dpy (Dumpy, short and fat) body that is uncoordinated and barely moves as well as multiple neuronal defects such as neuron and axon migration defects (Hedgecock et al. 1985; Otsuka et al. 1995), thus making it a challenge to ascertain if unc-44 phenotypes include defects in maintaining neuronal positioning. To circumvent this difficulty, we performed genetic experiments using sensitized sax-7 and unc-44 mutant backgrounds. unc-44(e362) was used in our experiments because e362 is the most severe unc-44 allele available on the basis of overall phenotypes (Boontrakulpoontawee and Otsuka 2002).
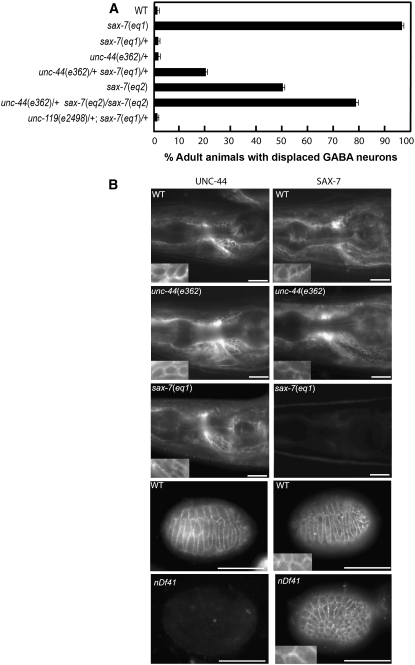
unc-44/+ or sax-7/+ heterozygous animals appear wild type and do not show displaced neurons. In contrast, 20% of unc-44(e362)/+ sax-7(eq1)/+ double heterozygous animals exhibit GABA neuronal displacement (Figure 4). Moreover, the neuronal displacement defect in animals homozygous for a hypomorphic sax-7 allele, eq2 (Wang et al. 2005), is enhanced by the introduction of a single unc-44 allele. Indeed, 80% of unc-44(e362)/+ sax-7(eq2)/sax-7(eq2) animals exhibit displaced GABA neurons as opposed to 50% of sax-7(eq2)/sax-7(eq2) single-mutant animals (Figure 4). These data show a genetic interaction between unc-44 and sax-7, revealing a role for unc-44 in maintaining neuron positions.
Figure 4.—
sax-7 and unc-44 genetically interact in neuronal positioning maintenance. (A) Quantitation of animals exhibiting displaced GABA neurons in sax-7, unc-44, and unc-119 mutant backgrounds. Error bar shows the standard error of the proportions of three sample sets where in each set n = 100. (B) UNC-44 and SAX-7 localizations are not interdependent. (Left) UNC-44 immunostaining. (Right) SAX-7 immunostaining in wild-type, unc-44(e362), and sax-7 (eq1) adult animals as well as arrested embryos homozygous for the nDf41 chromosomal deficiency, which removes the unc-44 gene; as expected, these embryos do not show UNC-44 staining. Images of UNC-44 and SAX-7 immunostaining in respective animals of different genetic backgrounds were taken with the same exposure times. Insets show corresponding UNC-44 and SAX-7 localization to the membrane boundaries of cells. Bar, 20 μm.
To confirm the specificity of this unc-44 genetic interaction with sax-7, we assayed whether another gene that also functions in nervous system patterning would similarly interact with sax-7. unc-119 animals, like unc-44 animals, are Dpy, severely uncoordinated, and barely move and have multiple neuronal defects (Maduro and Pilgrim 1995; Knobel et al. 2001). However, in contrast to unc-44, sax-7 does not genetically interact with unc-119. We did not detect displaced neurons in unc-119(e2498)/+; sax-7(eq1)/+ double heterozygous animals (Figure 4). The lack of genetic interaction between sax-7 and unc-119 underscores the specificity of the unc-44 interaction with sax-7, supporting the hypothesis that UNC-44 ankyrin functions together with SAX-7 to maintain neuronal positioning.
Yeast and protein recruitment assays reveal an interaction between SAX-7 and UNC-44:
Ankyrin directly binds the AB motif in vertebrate L1CAM (Davis and Bennett 1994). Although it has not been formally shown whether UNC-44 binds SAX-7, the complete conservation of the AB motif in SAX-7 suggests that ankyrin is likely to bind SAX-7. Consistent with this hypothesis, both SAX-7 and UNC-44 show similar ubiquitous expression and overlapping localization at cell membranes in C. elegans (Chen et al. 2001; also Figure 4B). Moreover, it was previously shown that the SAX-7 cytoplasmic tail can interact with murine ankyrin (Chen et al. 2001).
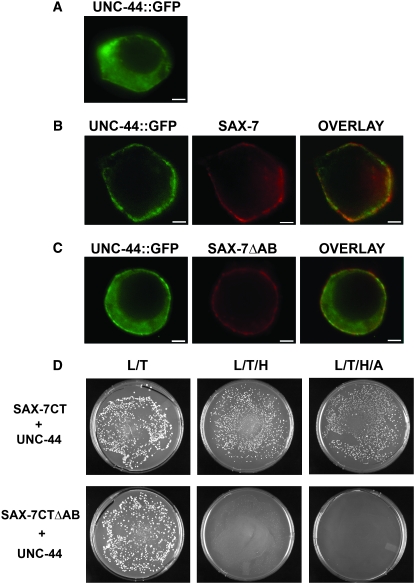
To test if SAX-7 molecularly interacts with UNC-44, we performed a similar assay that was previously developed to evaluate neurofascin–ankyrin interactions in cultured cells (Zhang et al. 1998). In transfected HEK293 cells, UNC-44∷GFP is primarily localized in the cytoplasm (Figure 5A). Upon cotransfection with a neurofascin∷SAX-7CT construct, which is composed of rat neurofascin extracellular and transmembrane domains fused to SAX-7CT, UNC-44∷GFP is redistributed to the plasma membrane, colocalizing with the neurofascin∷SAX-7CT chimera protein (Figure 5B). In cells cotransfected with neurofascin∷SAX-7CTΔAB, which lacks the AB motif, UNC-44∷GFP recruitment to the plasma membrane is reduced so that UNC-44∷GFP largely remains cytoplasmic, despite membrane localization of neurofascin∷SAX-7CTΔAB (Figure 5C). Together, these results suggest that the SAX-7CT can interact with UNC-44; moreover, this interaction requires the AB motif.
Figure 5.—
SAX-7 and UNC-44 molecularly interact as determined in a recruitment/binding assay in HEK293 cells and in yeast. (A) UNC-44∷GFP is primarily localized in the cytoplasm of transformed HEK293 cells. (B) Cotransfection with neurofascin∷SAX-7CT (labeled SAX-7) causes UNC-44∷GFP to be redistributed to the plasma membrane, colocalizing with neurofascin∷SAX-7CT. (C) However, loss of the AB motif in neurofascin∷SAX-7CT (labeled SAX-7ΔAB) dramatically reduces this redistribution of UNC-44∷GFP so that UNC-44∷GFP largely remains in the cytoplasm. Bar, 5 μm. (D) Cell growth on selective media (L/T) shows yeast cells that are successfully transformed with the SAX-7 and UNC-44 constructs. Cell growth on selective media (L/T/H or L/T/H/A) reveals positive interaction between UNC-44 and SAX-7CT in yeast. In contrast, no cell growth is seen on selective media in cells transformed with UNC-44 and SAX-7CTΔAB, revealing the requirement for the AB motif for the UNC-44/SAX-7 interaction.
We also tested for an interaction between SAX-7 and UNC-44 via the yeast two-hybrid assay (see materials and methods). Yeast transformed with SAX-7CT as bait and UNC-44 as prey were cultured on media lacking leucine, tryptophan, and histidine. Robust cell growth on this selective media revealed a positive interaction between SAX-7 and UNC-44. Moreover, we saw cell growth of transformed yeast on media with more stringent growth requirements, i.e., lacking leucine (L), tryptophan (T), histidine (H), and adenine (A), confirming an interaction between SAX-7 and UNC-44 (Figure 5D). In contrast, deletion of the AB motif in the SAX-7CT bait abolished interaction with UNC-44; cells transformed with SAX-7CTΔAB bait and UNC-44 prey did not grow on media lacking L/T/H or L/T/H/A (Figure 5D). The SAX-7CT bait and the UNC-44 prey behaved as expected with control vectors (data unshown). Taken together, these results indicate that the UNC-44 interaction with SAX-7 is specific and dependent on the AB motif.
Mutation analysis underscores the importance of UNC-44 interaction for SAX-7 function in maintaining neuronal positions:
The functional importance of the SAX-7 AB motif, together with the demonstrated physical association of UNC-44 with SAX-7 and genetic data showing unc-44 functions with sax-7 in neuronal positioning, suggest that UNC-44 binding is required for SAX-7 to mediate proper neuronal positioning. To test this hypothesis, we assayed the rescue ability of SAX-7 constructs containing engineered point mutations that were previously shown to affect ankyrin binding to rat neurofascin.
A Y-to-A substitution in the AB motif of rat neurofascin prevents ankyrin binding (Zhang et al. 1998). SAX-7 containing this mutation (SAX-7∷SFIGQA) showed incomplete rescue of the displaced GABA neurons in sax-7(eq1) animals (Figure 3). These data are consistent with the hypothesis that UNC-44 ankyrin binds and regulates SAX-7.
Phosphorylation of the tyrosine residue in the neurofascin AB motif abolishes ankyrin binding (Garver et al. 1997). The phospho-mimic Y-to-E mutation in the AB motif reduces but does not completely abolish ankyrin binding to rat neurofascin (Zhang et al. 1998). In C. elegans, SAX-7 is also phosphorylated at the tyrosine residue in the AB motif. Importantly, phosphorylated SAX-7 accumulates at sites that are free of UNC-44 (Chen et al. 2001), in agreement with data indicating tyrosine phosphorylation of the AB motif prevents ankyrin binding. SAX-7 containing the phospho-mimic mutation, SAX-7∷SFIGQE, can rescue eq1 phenotypes better than SAX-7∷SFIGQA, which is predicted to be unable to bind ankyrin (Figure 3), consistent with SFIGQE having residual ankyrin-binding activity and revealing the importance of ankyrin interaction.
A Y-to-H mutation in the AB motif of L1 results in the CRASH disorder (Fransen et al. 1995). Similar to the Y-to-A substitution described above, this Y-to-H mutation in rat neurofascin prevents ankyrin binding and tyrosine phosphorylation (Zhang et al. 1998). However, it is not clear if it is the absence of ankyrin binding or the loss of phosphorylation that is the underlying cause of the disease. To analyze the functional significance of the tyrosine phosphorylation without inhibiting ankyrin binding, we generated a SAX-7 construct containing a Y-to-F substitution (SFIGQF); rat neurofascin containing this mutation showed ankyrin-binding activity similar to wild-type neurofascin but it could not be phosphorylated (Garver et al. 1997; Zhang et al. 1998). SAX-7∷SFIGQF showed reduced rescue activity of the eq1 defects, revealing that tyrosine phosphorylation of the AB motif is important for SAX-7 function (Figure 3).
UNC-44 and SAX-7 are not interdependent for protein localization:
The reduced rescue ability of SAX-7 containing mutations that affect ankyrin binding together with the genetic interaction of sax-7 with unc-44 suggest that UNC-44 binds and regulates SAX-7 function. We hypothesized that UNC-44 might regulate SAX-7 function by influencing the localization of SAX-7 protein. However, SAX-7 accumulation appeared wild type in unc-44(e362) animals and in embryos that are homozygous for the nDf41 deficiency (Figure 4B; Chen et al. 2001), which completely removes the unc-44 gene (Bowerman et al. 1992). These results suggest that UNC-44 ankyrin is not likely to contribute to SAX-7 function by regulating SAX-7 localization.
We also considered that SAX-7 might regulate UNC-44 localization. To test this hypothesis, we examined UNC-44 accumulation in sax-7(eq1) animals using an anti-UNC-44 antibody (Otsuka et al. 1995). No detectable difference from wild-type animals was seen (Figure 4), thus suggesting that SAX-7 is not likely to regulate UNC-44 localization. Taken together, these results suggest that the SAX-7 and UNC-44 are not interdependent for protein localization.
The SAX-7 PDZ-binding domain contributes to SAX-7 function:
SAX-7 contains a PB motif at its C terminus (Chen et al. 2001). PDZ-binding motifs are classified into at least three different classes, depending on the class of PDZ proteins that bind the motif (reviewed in Nourry et al. 2003). The SAX-7 PB motif (composed of the amino acids STFV) is a consensus class I site. To determine if the PB motif contributes to SAX-7 function, we generated two SAX-7 constructs: (1) SAX-7ΔSTFV, where the PB motif is deleted, and (2) SAX-7∷YSLA, where the PB motif has been substituted by the rat neurofascin class II PB motif. Both SAX-7 constructs show similar but incomplete rescue of the eq1 neuronal displacement phenotype, revealing the contribution by the PB motif to SAX-7 function and that a type II PB motif cannot fully substitute for a type I PB motif (Figure 3).
stn-2 γ-syntrophin binds SAX-7:
The functional importance of the PB motif suggests that a PDZ protein might bind and regulate SAX-7. PDZ proteins generally function as adaptors that link structural proteins, receptors, and signaling molecules in large supramolecular complexes (reviewed in Nourry et al. 2003). Because the SAX-7 PB motif is a consensus binding site for class I PDZ proteins, we performed a focused yeast two hybrid (Y2H) specifically for class I PDZ proteins that bind SAX-7, using the SAX-7 cytoplasmic tail as bait. There are at least 16 genes encoding for class I PDZ proteins (K. Mach and S. Kim, personal communication), of which we screened 13 for interaction with SAX-7; 4 of them showed robust interaction with SAX-7. These four candidate SAX-7 interactors behaved as expected with control vectors, suggesting that their interactions with SAX-7 are specific (data not shown; see materials and methods). One of these interactors was γ-syntrophin encoded by F27D9.8 (Grisoni et al. 2003). Syntrophins are a family of adaptor molecules that associate ion channels and signaling proteins to the dystrophin family of cytoskeletal proteins (Albrecht and Froehner 2002). We have named the F27D9.8 gene stn-2 because it is the second syntrophin in C. elegans. The only other C. elegans syntrophin is α/β-syntrophin, encoded by the stn-1 gene (Grisoni et al. 2003). STN-1 is also a type I PDZ protein, but it does not interact with SAX-7 in yeast, suggesting that the interaction between STN-2 and SAX-7 is specific (data not shown; see materials and methods). Deletion of the PB motif in SAX-7 or the PDZ domain in STN-2 abolished the interaction between both proteins in yeast (Table 1). Thus the interaction between SAX-7 and STN-2 γ-syntrophin is mediated by the SAX-7 PB motif and the STN-2 PDZ domain.
TABLE 1.
The SAX-7 cytoplasmic tail and STN-2 interact by yeast two-hybrid assays
| Growth media | SAX-7 | SAX-7 + pGADT7-53 | SAX-7 + STN-2 | SAX-7ΔPB + STN-2 | SAX-7 + STN-2ΔPDZ | STN-2 + pGBKT7-T | STN-2 |
|---|---|---|---|---|---|---|---|
| L− | ND | ND | ND | ND | ND | ND | + |
| T− | + | ND | ND | ND | ND | ND | − |
| L−/T− | − | + | + | + | + | + | − |
| L−/T−/H− | − | − | + | − | − | − | − |
| L−/T−/H−/A− | − | − | + | − | − | − | − |
The Y2H interaction between the SAX-7 cytoplasmic tail (SAX-7) and STN-2 requires the SAX-7 PB motif and the STN-2 PDZ domain. ND, not determined. +, growth; −, no growth on minimal media lacking the following specified amino acids: L, leucine; T, tryptophan; H, histidine; and A, adenine. Growth on L−/T−/H− and L−/T−/H−/A− media indicates robust positive interaction. pGADT7-53 and pGBKT7 are negative control vectors that do not interact with SAX-7 and STN-2, respectively.
stn-2 genetically interacts with sax-7 in neuronal positioning:
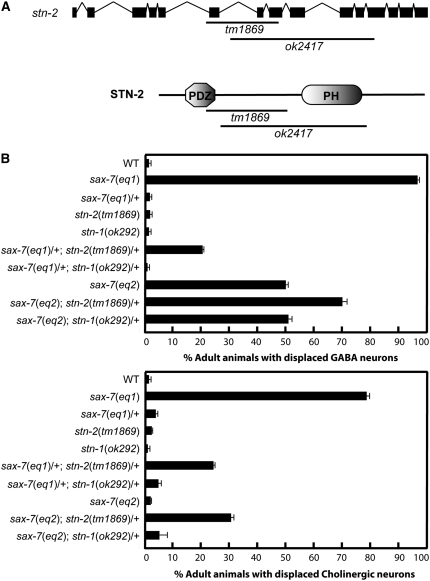
There are two available stn-2 alleles, tm1869 and ok2417, which were isolated independently from the Japanese National Bioresource Project and the C. elegans Knockout Consortium, respectively. We confirmed that tm1869 is an 811-bp deletion that removes part of the PDZ domain located in the N-terminal part of the protein. ok2417 is a 1569-bp deletion with an 11-bp insertion that removes sequences just downstream of the PDZ domain (Figure 6A). Both alleles are out-of-frame deletions that are predicted to result in a premature stop. stn-2(tm1869) and stn-2(ok2417) animals, which are indistinguishable from each other, exhibit hyperactive movements and extended bending of heads, phenotypes that also are associated with mutants of the dystrophin protein complex (DPC). This large protein complex includes stn-1 α/β-syntrophin, dys-1 dystrophin, and dyb-1 dystrobrevin (Bessou et al. 1998; Gieseler et al. 2001; Grisoni et al. 2003).
Figure 6.—
sax-7 and stn-2 interact genetically in neuronal positioning maintenance. (A) A schematic of the genomic structure of the stn-2 gene (solid boxes represent coding sequence and lines represent introns) and the predicted protein structure. The position of the stn-2 deletion alleles, tm1869 and ok2417, are shown relative to the gene and protein structure. (B) Quantitation of animals exhibiting displaced GABA and cholinergic neurons in sax-7, stn-2, and stn-1 mutant backgrounds. Error bar shows the standard error of the proportions of three sample sets where in each set n = 100.
The functional importance of the SAX-7 PB motif, together with the demonstrated physical association of STN-2 with SAX-7 in yeast, suggests that STN-2 binds and regulates SAX-7 function in maintaining neuronal positioning. To test this hypothesis, we examined stn-2 animals but did not detect displaced neurons (Figure 6B). We also assayed for a genetic interaction between stn-2 and sax-7 using sensitized sax-7 mutant backgrounds. stn-2/+ or sax-7/+ heterozygous animals appear wild type and do not display neuronal displacement. In contrast, 20% of sax-7(eq1)/+; stn-2(tm1869)/+ double heterozygous animals exhibit displaced GABA neurons (Figure 6B). This genetic interaction between sax-7 and stn-2 is further illustrated by an enhancement of the neuronal displacement phenotype by a single stn-2 allele in animals homozygous for the hypomorphic sax-7 allele, eq2 (Wang et al. 2005). Indeed, 70% of sax-7(eq2); stn-2(tm1869)/+ double-mutant animals exhibit displaced GABA neurons, as compared to ∼50% of sax-7(eq2) single-mutant animals. This enhancement is also seen in cholinergic neurons where ∼30% of sax-7(eq2); stn-2(tm1869)/+ double-mutant animals exhibit displaced cholinergic neurons, as compared to ∼5% of sax-7(eq2) single-mutant animals. Taken together with the physical association of SAX-7 to STN-2, the genetic interaction between sax-7 and stn-2 strongly supports the hypothesis that STN-2 binds and regulates SAX-7 function in maintaining neuronal positioning.
stn-2 and stn-1 animals are indistinguishable, exhibiting hyperactive movements and extended bending of the head. But unlike stn-2, stn-1 does not genetically interact with sax-7. stn-1(ok292)/+; sax-7(eq1)/+ double heterozygous animals do not exhibit displaced neurons, in contrast to stn-2/+; sax-7/+ animals. Moreover, a single allele of stn-1 cannot enhance displacement of GABAergic or cholinergic neurons in sax-7(eq2)/sax-7(eq2) homozygous animals, unlike stn-2 (Figure 6B). The lack of genetic interaction between stn-1 and sax-7 is consistent with the Y2H data indicating that STN-1 protein does not bind SAX-7 in yeast and further demonstrates the specificity of the stn-2 genetic interaction with sax-7.
We were deliberate in using stn-2/+ or stn-1/+ heterozygosity in our experiments to assay for a sax-7 interaction with stn-2 or stn-1. stn-2/+ or stn-1/+ heterozygous animals exhibit wild-type movements unlike stn-2 and stn-1 homozygous animals, which display hyperactive movements. The increased body movements resulting from hyperactivity may nonspecifically enhance neuronal displacement in sax-7 animals. It was demonstrated previously that the neuronal displacement phenotype in sax-7 animals can be suppressed by reducing body movements (Sasakura et al. 2005).
STN-2 and SAX-7 are localized to the sarcomeres of striated muscles:
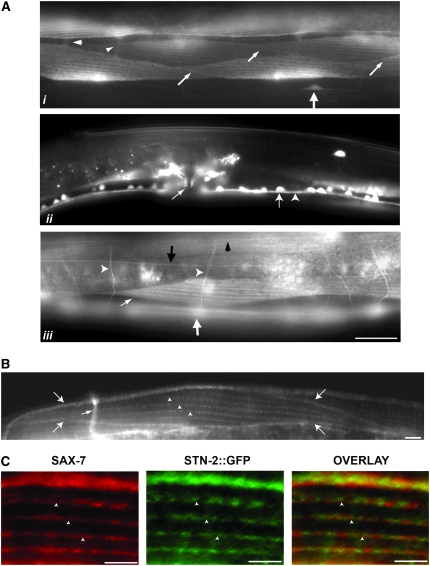
To determine stn-2 expression, we generated a stn-2∷gfp construct using genomic stn-2. stn-2∷gfp can rescue the stn-2 hyperactive and head-bending phenotypes, suggesting that STN-2∷GFP is functional and properly localized. STN-2∷GFP is expressed in the nervous system and in body-wall, vulval, and enteric muscles (Figure 7A). In neurons, STN-2∷GFP is localized to the cell bodies and their processes (Figure 7A). While it is apparent that STN-2∷GFP is localized in the cytoplasm, the robust expression of STN-2∷GFP makes it difficult to rule out membrane localization of the protein. Thus, it is possible that a subpopulation of STN-2∷GFP could partially colocalize with SAX-7, which is present in the plasma membrane of neuronal cell bodies and processes (Chen et al. 2001).
Figure 7.—
stn-2 is expressed in neurons and striated muscles where STN-2 partially colocalizes with SAX-7. (A) On the basis of a GFP epifluorescence of a rescuing stn-2∷gfp reporter, STN-2∷GFP, is detected in neurons (large arrow in i), including the ventral nerve cord motor neurons (arrow in ii), and axonal processes (ventral nerve cord, arrowhead in ii; commissural axons, open arrowheads in iii; dorsal nerve cord, solid arrowheads in iii; lateral nerve cord, solid arrow in iii). Bar, 20 μm. (B) Immunostaining of SAX-7 in wild-type animals reveals that SAX-7 is localized to muscle membrane boundaries (arrows) and sarcomeres (arrowheads) in body-wall muscles. The small arrow is pointing to a commissural axon that also shows high SAX-7 expression. Bar, 5 μm. (C) In sarcomeres, SAX-7 and STN-2 partially colocalize, as shown by co-immunostaining of SAX-7 and GFP in stn-2(tm1839) animals expressing stn-2∷gfp. Bar, 5 μm.
Similar to neurons, STN-2∷GFP is largely cytoplasmic in body-wall muscles. However, we detect a subpopulation of STN-2∷GFP localized to distinct muscle structures that include sarcomeres (Figure 7A) as well as in muscle membrane boundaries (small arrows, Figure 7Ai). SAX-7 is also expressed in muscle, as revealed by immunostaining of SAX-7 in wild-type animals. Like STN-2∷GFP, SAX-7 is localized to sarcomeres (Figure 7B, arrowheads) and membrane boundaries (Figure 7B, larger arrows); both signals are absent in sax-7(eq1) animals (data not shown). The similar localization of both proteins in body-wall muscle is consistent with the demonstrated STN-2 binding with SAX-7 in yeast. Furthermore, we detected partial colocalization of SAX-7 and STN-2∷GFP in the sarcomeres, as revealed via co-immunostaining of SAX-7 and GFP in stn-2 animals expressing a rescuing stn-2∷gfp construct.
DISCUSSION
While it has been well documented that the extracellular region of L1CAMs is essential for their functions, this study shows that the cytoplasmic tail is important as well. We present results of a systematic analysis of three conserved L1CAM intracellular domains and their contributions to SAX-7 function, revealing the functional importance of the FB, AB, and PB domains in SAX-7 in maintaining neuronal positioning of GABAergic neurons in the ventral nerve cord.
A recent study of SAX-7 by Pocock et al. (2008) also revealed that the AB motif is required for SAX-7 function. However, there are notable differences in how the two studies were carried out. A comparison of both studies provides additional insights into SAX-7 functions. First, in the Pocock et al. (2008) study, SAX-7 function was assessed by observing adhesion between two adjacent interneurons, AIY and AVK. In contrast, our study analyzed SAX-7 function in the ventral nerve cord GABA and in cholinergic motorneurons, which require SAX-7 function in neurons, body-wall muscle, and hypodermal tissues. Second, the Pocock et al. (2008) study characterized the SAX-7S isoform while our study analyzed the SAX-7L isoform. Despite these differences, both studies revealed the requirement of the AB motif, suggesting its importance to both SAX-7S and SAX-7L function.
Pocock et al. (2008) also suggested that the AB motif is the only intracellular motif required for SAX-7 function in AIY/AVK neurons. In contrast, our studies found that all three motifs (the FB, AB, and PB motifs) are important for SAX-7 function in maintaining ventral nerve cord neuron positions. This difference may reflect cell-type specificity requirements or differential requirements for these motifs in SAX-7S vs. SAX-7L. Another possibility is that the Pocock et al. (2008) study used sax-7(nj48), an allele that we showed is not a molecular null and still accumulates a form of SAX-7 that contains some, if not all, of the cytoplasmic tail, thus possibly adding complexity to their assays.
Additionally, our study also provides molecular and genetic evidence for how these motifs regulate SAX-7 function. In particular, we identified a physical interaction between UNC-44 ankyrin and STN-2 γ-syntrophin with the SAX-7 AB and PB motifs, respectively. Furthermore, we showed that unc-44 ankyrin and stn-2 γ-syntrophin genetically interact with SAX-7 to maintain neuronal positioning, thus revealing functional mechanisms of L1CAMs in an organismal context.
Regulation by UNC-44 ankyrin:
Our genetic studies have revealed a role for unc-44 in maintaining neuron positions that is similar to that of sax-7. Previous analyses in the mouse and Drosophila revealed that ankyrin mutants exhibit phenotypes similar to the respective mouse L1 and Drosophila neuroglian mutants, suggesting that both ankyrin and L1CAM function in the same process (Scotland et al. 1998; Yamamoto et al. 2006). Together with data showing that UNC-44 binds SAX-7, we hypothesize that a mechanism by which UNC-44 regulates SAX-7 function may be in providing SAX-7 anchorage to the spectrin–actin cytoskeleton, thereby increasing SAX-7-mediated adhesion. Previous cell-culture studies show increased lateral mobility of neurofascin that is defective in ankyrin binding, a likely result of weak cytoskeletal linkage; the cells expressing this mutant neurofascin show decreased cell–cell adhesion (Garver et al. 1997; Tuvia et al. 1997). Consistent with this hypothesis, SAX-7-carrying mutations that prevent ankyrin binding show impaired ability to maintain neuronal positions. An alternative mechanism by which UNC-44 regulates SAX-7 function may be in controlling SAX-7 localization. Previous studies show that ankyrin is required to maintain L1 in some axon tracts in the mouse and, more recently, in Drosophila (Scotland et al. 1998; Yamamoto et al. 2006). However, our data do not support this idea. One explanation for this disparity may be that the diameter of the C. elegans axons is much smaller than those of the mammalian and Drosophila counterparts so that differences in subcellular localization (e.g., cytoplasmic vs. plasma membrane) may not be as easily detected in C. elegans.
Biochemical studies show that tyrosine phosphorylation of the L1CAM AB motif abolishes ankyrin binding. Although tyrosine phosphorylation of the L1CAM AB motif has been reported in C. elegans, Drosophila, and mammals (Garver et al. 1997; Chen et al. 2001; Jenkins et al. 2001), the significance of this phosphorylation event has not been determined, particularly on an organismal level. We have now shown that this tyrosine phosphorylation is indeed important for L1CAM function in vivo as the loss of this phosphorylation from a Y-to-F mutation, which does not impair ankyrin binding, impairs SAX-7 function in maintaining neuronal positioning.
Loss of phosphorylation is detrimental to sax-7 function possibly because of constitutive ankyrin binding. Alternatively or additionally, phosphorylation may confer on SAX-7 the ability to interact with other proteins because either SAX-7 is no longer binding to ankyrin and is thus accessible to other interacting proteins or SAX-7 can now bind phospho-tyrosine-binding proteins. Previously, doublecortin, a microtubule-binding protein associated with the X-linked lissencephaly and doublecortex syndrome brain disorders, was shown to bind phospho-FIGQY tyrosine neurofascin but not unphosphorylated neurofascin (Kizhatil et al. 2002). Thus loss of phosphorylation could abolish additional SAX-7 interactions. Association with doublecortin, a protein that binds the microtubule cytoskeleton rather than the actin cytoskeleton, raises the possibility that phosphorylation may confer functions that are distinct from those of unphosphorylated L1CAMs.
STN-2 γ-syntrophin and the dystrophin protein complex:
In addition to UNC-44, we identified a novel regulator of SAX-7 function, STN-2 γ-syntrophin, which can bind SAX-7 via the PB motif and regulate SAX-7 function in maintaining neuronal position. On the basis of a rescuing STN-2∷GFP construct, we detected STN-2∷GFP in neurons and striated muscles, tissues that also express SAX-7. Thus STN-2 may regulate SAX-7 function in the nervous system and/or muscle.
In addition to a neuronal requirement for SAX-7, it is clear that positional maintenance of ventral cord neurons also requires SAX-7 in body-wall muscles and the hypodermis (Wang et al. 2005), suggesting that SAX-7 facilitates adhesion of the neurons to the adjacent tissues. It is not known if SAX-7 mediates this adhesion via trans-homophilic interactions and/or by binding to other membrane-associated and ECM proteins. SAX-7 in body-wall muscle is localized at muscle cell boundaries as well as the sarcomeres where there is overlap with dense bodies. We speculate that SAX-7 in muscle may bind either directly to ECM proteins that in turn bind neuronal SAX-7 or indirectly to ECM proteins via ECM receptors like integrins. Integrins, which have been shown to bind vertebrate L1CAMs (reviewed in Hortsch 2000), are localized to the muscle cell boundaries and dense bodies in C. elegans (Gettner et al. 1995) and thus could interact with SAX-7. The importance of SAX-7 in body-wall muscles in maintaining neuronal positions is underscored by a functional interaction between SAX-7 and STN-2 γ-syntrophin, which colocalizes with SAX-7 in muscle cell boundaries and in the sarcomeres.
It is not unexpected that STN-2 and SAX-7 localizations do not completely overlap. As a PDZ protein, STN-2 is likely to function as an adaptor molecule, binding multiple diverse proteins that do not necessarily function in the SAX-7 pathway (reviewed in Nourry et al. 2003). Indeed, the vertebrate syntrophin family, which is composed of α, β1, β2, γ1, and γ2 isoforms, has been shown to bind multiple proteins. The α-, β1-, and β2-syntrophins have been shown to link ion channels and signaling molecules to the DPC, which acts as a mechanical link between the actin cytoskeleton and the extracellular matrix (Albrecht and Froehner 2002). Loss of dystrophin, a cytoskeletal protein and also a central component of the DPC, causes Duchenne muscular dystrophy, a progressive muscle disease where the integrity of muscle cells is defective. The γ-syntrophins are less well characterized but have been shown to interact with signaling molecules, ion channels, adhesion molecules, and components of the DPC such as dystrophin and dystrobrevin (Piluso et al. 2000; Hogan et al. 2001; Ou et al. 2003).
Consistent with γ-syntrophins being linked to the DPC, stn-2 mutant animals share phenotypes with animals mutant for DPC proteins, including stn-1 α/β-syntrophin, dys-1 dystrophin, and dyb-1 dystrobrevin (Bessou et al. 1998; Gieseler et al. 2001; Grisoni et al. 2003). Moreover, like DPC components, we show that stn-2 is similarly expressed in neurons and body-wall muscles. It is intriguing to speculate that the DPC might provide SAX-7 the cytoskeletal linkage and a plethora of signaling molecules that may regulate L1CAM function. While our studies suggest that stn-1 does not function with sax-7, it is not clear whether other DPC components, such as dystrophin, contribute to SAX-7 function. The availability of genetic mutants in the DPC components will allow us to directly assess if SAX-7 function in neuronal positioning requires the DPC. Alternatively, stn-2 may regulate SAX-7 function independently of its putative DPC-associated roles.
stn-2 mutant animals surprisingly do not exhibit neuronal displacement, thus suggesting that additional PDZ proteins function redundantly with STN-2. Indeed, our Y2H screen identified additional SAX-7-interacting PDZ proteins that may regulate SAX-7 function. These PDZ proteins may function in neurons and/or the hypodermis or redundantly with STN-2 in muscle.
FERM proteins:
The functional importance of the SAX-7 FB domain suggests that one or more FERM proteins bind and regulate SAX-7. FERM proteins generally function as linkers of transmembrane proteins to the actin cytoskeleton and signaling pathways (reviewed in Bretscher et al. 2002). Thus the SAX-7 FB domain may provide an additional or alternative means to link SAX-7 to the actin cytoskeleton and signaling pathways. L1 and neurofascin have been shown to bind ezrin (Dickson et al. 2002; Gunn-Moore et al. 2006). Among the multiple FERM proteins present in C. elegans is a single ezrin homolog that is encoded by the erm-1 gene (Göbel et al. 2004; Van Fürden et al. 2004). erm-1 has been shown to be essential for intestinal lumen morphogenesis, but a role for erm-1 in neuronal positioning is currently unknown.
The L1CAM cytoplasmic tail provides linkage to the actin cytoskeleton and signaling pathways:
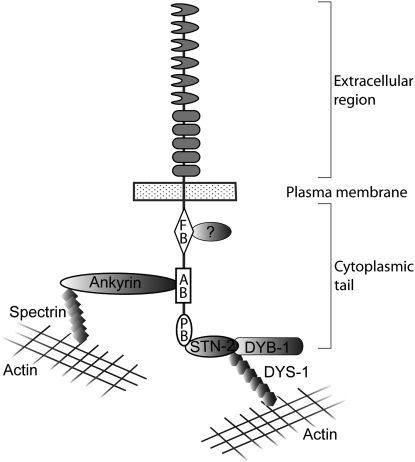
The three intracellular motifs that contribute to SAX-7 function are consensus sites for interaction with proteins that function as linkers of membrane proteins to the actin cytoskeleton and signaling molecules, underscoring the importance of cytoskeletal anchorage and signaling pathways to L1CAM function (Figure 8). We have identified two SAX-7-interacting proteins that function in the described capacity: UNC-44 ankyrin and STN-2 γ-syntrophin.
Figure 8.—
A model of intracellular interactions with the SAX-7 cytoplasmic tail. The model speculates that UNC-44 ankyrin and STN-2 γ-syntrophin link SAX-7 to the actin cytoskeleton. It is not known if UNC-44 and STN-2 bind simultaneously to SAX-7 as depicted in the model. Studies in both vertebrates and C. elegans reveal that γ-syntrophins are likely components of the DPC, thus suggesting that other DPC proteins, such as DYB-1 dystrobrevin and DYS-1 dystrophin, may also function together with SAX-7 to maintain neuronal positioning.
The identification of these interacting proteins raises several questions concerning coordination of these interactions and the tissues in which these interactions occur. For example, can more than one protein bind SAX-7 simultaneously? The collective loss of the SAX-7 intracellular domains negatively affects SAX-7 function more dramatically than the loss of a single intracellular domain, thus suggesting that the contribution of each domain is cumulative and thus SAX-7 is likely to bind multiple proteins simultaneously. The FB site is far apart from the AB and PB motifs, making it possible that FERM proteins can bind SAX-7 simultaneously with UNC-44 or STN-2. In contrast, the closeness of the AB and PB motifs (17 amino acids apart) and the large size of the conventional ankyrin isoform (220–240 kDa) (Otsuka et al. 1995) makes it more unlikely that both proteins bind SAX-7 simultaneously. If this hypothesis holds, then phosphorylation of the AB motif may be important for STN-2 to interact with SAX-7. Further dissection of these interactions and their coordination will provide insight into L1CAM mechanistic roles and the physiological basis of the CRASH syndrome.
Acknowledgments
We thank Kathleen Mach and Stuart Kim for sharing unpublished data on C. elegans PDZ proteins and Lisa Timmons, Ann Rougvie, and David Greenstein for constructive comments on the manuscript. This study was supported by grant NS045873 from the National Institute of Neurological Disorders and Stroke.
References
- Albrecht, D. E., and S. C. Froehner, 2002. Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals 11 123–129. [DOI] [PubMed] [Google Scholar]
- Bessou, C., J. B. Giugia, C. J. Franks, L. Holden-Dye and L. Ségalat, 1998. Mutations in the Caenorhabditis elegans dystrophin-like gene dys-1 lead to hyperactivity and suggest a link with cholinergic transmission. Neurogenetics 2 61–72. [DOI] [PubMed] [Google Scholar]
- Boontrakulpoontawee, P., and A. J. Otsuka, 2002. Mutational analysis of the Caenorhabditis elegans ankyrin gene unc-44 demonstrates that the large spliceoform is critical for neural development. Mol. Genet. Genomics 267 291–302. [DOI] [PubMed] [Google Scholar]
- Bowerman, B., B. A. Eaton and J. R. Priess, 1992. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell 68 1061–1075. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, A., K. Edwards and R. G. Fehon, 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3 586–599. [DOI] [PubMed] [Google Scholar]
- Chen, L., B. Ong and V. Bennett, 2001. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J. Cell Biol. 154 841–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita, A., S. Krishna, H. Zheng, R. W. Padgett and J. G. Culotti, 1998. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science 281 706–709. [DOI] [PubMed] [Google Scholar]
- Davis, J. Q., and V. Bennett, 1994. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J. Biol. Chem. 269 27163–27166. [PubMed] [Google Scholar]
- Dickson, T. C., C. D. Mintz, D. L. Benson and S. R. Salton, 2002. Functional binding interaction identified between the axonal CAM L1 and members of the ERM family. J. Cell Biol. 157 1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen, E., V. Lemmon, G. Van Camp, L. Vits, P. Coucke et al., 1995. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur. J. Hum. Genet. 3 273–284. [DOI] [PubMed] [Google Scholar]
- Garver, T. D., Q. Ren, S. Tuvia and V. Bennett, 1997. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J. Cell Biol. 137 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettner, S. N., C. Kenyon and L. F. Reichardt, 1995. Characterization of beta pat-3 heterodimers, a family of essential integrin receptors in C. elegans. J. Cell Biol. 129 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseler, K., M. C. Mariol, C. Bessou, M. Migaud, C. J. Franks et al., 2001. Molecular, genetic and physiological characterisation of dystrobrevin-like (dyb-1) mutants of Caenorhabditis elegans. J. Mol. Biol. 307 107–117. [DOI] [PubMed] [Google Scholar]
- Göbel, V., P. L. Barrett, D. H. Hall and J. T. Fleming, 2004. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell 6 865–873. [DOI] [PubMed] [Google Scholar]
- Grisoni, K., K. Gieseler, M. C. Mariol, E. Martin, M. Carre-Pierrat et al., 2003. The stn-1 syntrophin gene of C. elegans is functionally related to dystrophin and dystrobrevin. J. Mol. Biol. 332 1037–1046. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore, F. J., M. Hill, F. Davey, L. R. Herron, S. Tait et al., 2006. A functional FERM domain binding motif in neurofascin. Mol. Cell. Neurosci. 33 441–446. [DOI] [PubMed] [Google Scholar]
- Haspel, J., and M. Grumet, 2003. The L1CAM extracellular region: a multi-domain protein with modular and cooperative binding modes. Front. Biosci. 8 1210–1225. [DOI] [PubMed] [Google Scholar]
- Hedgecock, E. M., J. G. Culotti, J. N. Thomson and L. A. Perkins, 1985. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111 158–170. [DOI] [PubMed] [Google Scholar]
- Hogan, A., L. Shepherd, J. Chabot, S. Quenneville, S. M. Prescott et al., 2001. Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. J. Biol. Chem. 276 26526–26533. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., 2000. Structural and functional evolution of the L1 family: Are four adhesion molecules better than one? Mol. Cell. Neurosci. 15 1–10. [DOI] [PubMed] [Google Scholar]
- Jenkins, S. M., K. Kizhatil, N. R. Kramarcy, A. Sen, R. Sealock et al., 2001. FIGQY phosphorylation defines discrete population of L1 cell adhesion molecules at sites of cell-cell contact and in migrating neurons. J. Cell Sci. 114 3823–3835. [DOI] [PubMed] [Google Scholar]
- Kizhatil, K., Y. X. Wu, A. Sen and V. Bennett, 2002. A new activity of doublecortin in recognition of the phospho-FIGQY tyrosine in the cytoplasmic domain of neurofascin. J. Neurosci. 22 7948–7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobel, K. M., W. S. Davis, E. M. Jorgensen and M. J. Bastiani, 2001. UNC-119 suppresses axon branching in C. elegans. Development 128 4079–4092. [DOI] [PubMed] [Google Scholar]
- Koroll, M., F. G. Rathjen and H. Volkmer, 2001. The neural cell recognition molecule neurofascin interacts with syntenin-1 but not with syntenin-2, both of which reveal self-associating activity. J. Biol. Chem. 276 10646–10654. [DOI] [PubMed] [Google Scholar]
- Maduro, M., and D. Pilgrim, 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire, S. L., R. J. Reimer, K. Schuske, R. H. Edwards and E. M. Jorgensen, 1997. Identification and characterization of the vesicular GABA transporter. Nature 389 870–876. [DOI] [PubMed] [Google Scholar]
- Miller, D. M., and D. C. Shakes, 1995. Immunofluorescence microscopy, pp. 365–388 in Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by H. Epstein and D. Shakes, Acadmic Press, San Diego.
- Nourry, C., S. G. Grant and J. P. Borg, 2003. PDZ domain proteins: Plug and play! Sci. STKE 179 RE7. [DOI] [PubMed] [Google Scholar]
- Okkema, P., and A. Fire, 1994. The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development 120 2175–2186. [DOI] [PubMed] [Google Scholar]
- Otsuka, A. J., R. Franco, B. Yang, K. H. Shim, L. Z. Tang et al., 1995. An ankyrin-related gene (unc-44) is necessary for proper axonal guidance in Caenorhabditis elegans. J. Cell Biol. 129 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, Y., P. Strege, S. M. Miller, J. Makielski, M. Ackerman et al., 2003. Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J. Biol. Chem. 278 1915–1923. [DOI] [PubMed] [Google Scholar]
- Piluso, G., M. Mirabella, E. Ricci, A. Belsito, C. Abbondanza et al., 2000. Gamma1- and gamma2-syntrophins, two novel dystrophin-binding proteins localized in neuronal cells. J. Biol. Chem. 275 15851–15860. [DOI] [PubMed] [Google Scholar]
- Pocock, R., C. Y. Bénard, L. Shapiro and O. Hobert, 2008. Functional dissection of the C. elegans cell adhesion molecule SAX-7, a homologue of human L1. Mol. Cell. Neurosci. 37 56–68. [DOI] [PubMed] [Google Scholar]
- Sasakura, H., H. Inada, A. Kuhara, E. Fusaoka, D. Takemoto et al., 2005. Maintenance of neuronal positions in organized ganglia by SAX-7, a Caenorhabditis elegans homologue of L1. EMBO J. 24 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland, P., D. Zhou, H. Benveniste and V. Bennett, 1998. Nervous system defects of ankyrinB (−/−) mice suggest functional overlap between the cell adhesion molecule L1 and 440-kD ankyrinB in premyelinated axons. J. Cell Biol. 143 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvia, S., T. D. Garver and V. Bennett, 1997. The phosphorylation state of the FIGQY tyrosine of neurofascin determines ankyrin-binding activity and patterns of cell segregation. Proc. Natl. Acad. Sci. USA 94 12957–12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Fürden, D., K. Johnson, C. Segbert and O. Bossinger, 2004. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev. Biol. 272 262–276. [DOI] [PubMed] [Google Scholar]
- Wang, X., J. Kweon, S. Larson and L. Chen, 2005. A role for the C. elegans L1CAM homologue lad-1/sax-7 in maintaining tissue attachment. Dev. Biol. 284 273–291. [DOI] [PubMed] [Google Scholar]
- Wang, X., W. Zhang, T. Cheever, V. Schwarz, K. Opperman et al., 2008. The C. elegans L1CAM homologue LAD-2 functions as a co-receptor in MAB-20/Sema2 mediated axon guidance. J. Cell Biol. 180 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, S, and J. Gartner, 2001. Genetic and clinical aspects of X-linked hydrocephalus (L1 disease): mutations in the L1CAM gene. Hum. Mutat. 18 1–12. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M., R. Ueda, K. Takahashi, K. Saigo and T. Uemura, 2006. Control of axonal sprouting and dendrite branching by the Nrg-Ank complex at the neuron-glia interface. Curr. Biol. 16 1678–1683. [DOI] [PubMed] [Google Scholar]
- Yochem, J., T. Gu and M. Han, 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen, J. A., S. A. Kirch and C. I. Bargmann, 1999. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development 126 3679–3692. [DOI] [PubMed] [Google Scholar]
- Zhang, X., J. Q. Davis, S. Carpenter and V. Bennett, 1998. Structural requirements for association of neurofascin with ankyrin. J. Biol. Chem. 273 30785–30794. [DOI] [PubMed] [Google Scholar]