Abstract
TERMINAL FLOWER 1 (TFL1) encodes a protein with similarity to animal phosphatidylethanolamine-binding proteins and is required for normal trafficking to the protein storage vacuole. In Arabidopsis thaliana the tfl1 mutation produces severe developmental abnormalities. Here we show that most aspects of the tfl1 phenotype are lost in the cry1 cry2 double-mutant background lacking cryptochromes 1 and 2. The inhibition of hypocotyl growth by light is reduced in the tfl1 mutant but this effect is absent in the cry1 or cry2 mutant background. Although the promotion of flowering under long rather than short days is a key function of cryptochromes, in the tfl1 background, cryptochromes promoted flowering under short days. Thus, normal CRY control of photoperiod-dependent flowering and hypocotyl growth inhibition requires a functional TFL1 gene.
PLANT development is under the control of endogenous signals that provide cells with positional and temporal cues. Plants also adjust their growth and development in response to exogenous signals such as fluctuations of the environment that correlate with stress conditions (Casal et al. 2004). For instance, the control of flowering time by photoperiod is a clear example of a response to an environmental condition that correlates with the occurrence of a favorable season, where stress events are less likely. Arabidopsis plants detect long days (LD) by the coincidence between high levels of mRNA of CONSTANS (CO) and light (Suarez-Lopez et al. 2001; Yanovsky and Kay 2002). This light signal is perceived by cryptochrome 2 (cry2) (Guo et al. 1998), cryptochrome 1 (cry1) (Ahmad and Cashmore 1993), and phytochrome A. cry2 is a nuclear-localized photoreceptor (Guo et al. 1999), whereas cry1 has both nuclear and cytoplasmic functions (Wu and Spalding 2007). The expression of CO is controlled by the circadian clock and modulated by GIGANTEA (GI), FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1), and CYCLING DOF FACTOR 1 (CDF1) (Sawa et al. 2007). As a result of these controls, CO mRNA levels are low during the light period of short days (SD) but under LD, the rising phase of CO expression coincides with the presence of light, which stabilizes the CO protein (Valverde et al. 2004). In turn, CO promotes the expression of FLOWERING LOCUS T (FT) (Suarez-Lopez et al. 2001), which is a strong activator of flowering (Kardailsky et al. 1999; Kobayashi et al. 1999).
TERMINAL FLOWER 1 (TFL1) is a homolog of FT, but it acts as a repressor of flowering (Kardailsky et al. 1999; Kobayashi et al. 1999). It encodes a protein with similarity to animal phosphatidylethanolamine-binding proteins (Bradley et al. 1997). It is a small protein that accumulates in the apical and axillary meristems, through localized trafficking of the protein (Conti and Bradley 2007). TFL1 localizes to endomembrane compartments and is required for normal trafficking to the protein storage vacuole (Sohn et al. 2007). The tfl1 mutants have a severe phenotype characterized by early flowering, a terminal flower that limits the development of the indeterminate meristem that in the wild type (WT) simply senesces, and a reduced number of paraclades and basal ramifications (Shannon and Meeks-Wagner 1991). TFL1 delays the progression of vegetative and reproductive phases of shoot development and negatively regulates the expression of the meristem identity genes LEAFY (LFY) and APETALA1 (AP1), which in turn restrict the domain of expression of TFL1 (Ratcliffe et al. 1998, 1999). Ectopic expression of LFY1 or AP1 reproduces the early flowering and floral tfl1 phenotypes (Mandel and Yanofsky 1995; Weigel and Nilsson 1995) and the tfl1 phenotype is virtually absent in the lfy ap1 double-mutant background (Shannon and Meeks-Wagner 1993). Thus, to a large extent, the phenotype of the tfl1 can be accounted for by the increased LFY and AP1 levels.
Previous studies have suggested that the flowering phenotype of tfl1 is suppressed under SD (Shannon and Meeks-Wagner 1991). The dependency of the tfl1 phenotype on photoperiod could be the result of changes in sucrose levels that have been shown to alter TFL1 expression (Zimmermann et al. 2004) and the tfl1 phenotype (Ohto et al. 2001). Alternatively, since TFL1 expression is increased by CO (Simon et al. 1996), and LD stabilize CO (Valverde et al. 2004), LD perceived mainly by cry2 and cry1 could increase TFL1 expression, while under SD, TFL1 expression would be weaker and the tfl1 phenotype less pronounced. To test this hypothesis we investigated the effects of cry1 and cry2 on TFL1 expression under SD and LD. We also produced double and triple mutants of tfl1 with cry1 and cry2 and characterized the phenotype under SD and LD and under a range of fluence rates of photosynthetically active radiation.
MATERIALS AND METHODS
Plant material:
The tfl-1 (Shannon and Meeks-Wagner 1991), cry1-hy4-b104 (Bruggeman et al. 1996), cry2-1 (Guo et al. 1998, 1999) and their double- and triple-mutant combinations and the WT used in the analysis of growth and development were maintained in the Columbia background. The cry1 cry2 double mutant (Mazzella et al. 2001) and the WT used in expression experiments were in the Landsberg erecta background.
Growth conditions:
Seeds of each genotype were sown on 0.8% (w/v) agar in clear plastic boxes (40 mm × 33 mm × 15 mm height), incubated at 4° for 5 days and 1 day at 22° under either SD or LD to induce germination. Germinating seeds were transferred to pots (110 cm3) containing equal amounts of perlite (Perlome, Perfiltra, Rosario, Argentina), peat moss (Cuidad Floral, Escobar, Argentina), and vermiculite (Intersum, Aislater, Córdoba, Argentina) and watered as needed with a solution containing 1 g/liter of Hakaphos R (COMPO). In experiments where hypocotyl length was measured, the seedlings remained in the boxes with agar. White light was provided by high-pressure sodium lamps (400 W Philips SON) at the indicated fluence rates, in growth rooms at 22°.
Measurements:
The final number of leaves (rosette plus stem leaves) was used as a measurement of flowering time on a biological scale (Koornneef et al. 1991). The number of flowers on the main stem, the number of paraclades, and the length of the main stem were recorded at the end of the plant cycle. Hypocotyl length was measured to the nearest 0.5 mm with a ruler.
TFL1 and FT expression:
Samples were harvested in liquid nitrogen, and total RNA was extracted using the RNEasy plant mini kit (QIAGEN). In microarray experiments used to measure the expression of TLF1 in the cry1 cry2 mutant background, cDNA and cRNA synthesis and hybridization to ATH1 Affymetrix Arabidopsis gene chips were performed according to Affymetrix instructions. Expression data were normalized to the sum of each microarray (Clarke and Zhu 2006) and analyzed by ANOVA (using Excel). For RT–PCR experiments, 1 μg of RNA was reverse transcribed into cDNA using M-MLV reverse transcriptase (RT, Promega) according to the manufacturer's instructions. PCR was performed in 25 μl final volume. Primers, annealing temperatures, and the size of PCR products expected were as follows:
TFL1: 5′-ATCTCAGATCCTTCTTCACTTTGGTGATGA-3′, 5′-GGAGACCAAGATCATACTCGACCGCAAATT-3′; 55°; 289 bp.
UBQ10: 5′-GGTGTCAGAACTCTCCACCTCAAGAGTA-3′, 5′-TCAATTCTCTCTACCGTGATCAAGATGCA-3′; 63°; 318 bp.
FT: 5′-GCTACAACTGGAACAACCTTTGGCAAT-3′; 61.45°; 365 bp.
FT: 3′-TATAGGCATCATCACCGTTCGTTACTC-5′; 58.6°; 365 bp.
ACT2F: 5′-AGTGGTCGTACAACCGGTATTGTG-3′; 58.8°.
ACT2R: 5′-CCGATCCAGACACTGTACTTCCTT-3′; 58.1°; 593 bp.
In all cases, at least one of the primers used for PCR spanned intron–exon junctions, and amplification of genomic DNA was undetectable in RT controls. PCR products were resolved on 1.5% agarose gels in 1× TBE buffer containing 0.5 μg/ml ethidium bromide and quantified using Scion Image software. FT PCR products were detected by Southern blot (Cerdán and Chory 2003) and quantified using AlkPhos Direct labeling and detection system with CDP-Star, in the exponential range of amplification.
RESULTS
High expression of TFL1 does not overlap with high expression of CRY1 or CRY2:
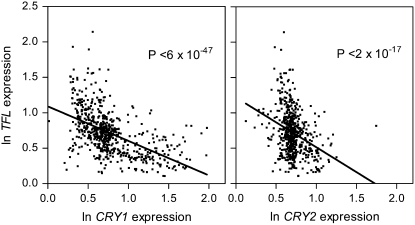
To investigate the relationship between the expression of TFL1, CRY1, and CRY2 we analyzed the correlation of expression between TFL1 and CRY1 and between TFL1 and CRY2 across 633 conditions representing different tissues, developmental stages, and differentially treated plants (http://www.arabidopsis.org). Publicly available data for each of the three genes were normalized to the median of each experiment and ln (x + 1) transformed to plot TFL1 expression against CRY1 or CRY2 expression (Figure 1). No condition shows simultaneously elevated expression of TFL1 and either CRY1 or CRY2 and there is a significant negative correlation between TFL1 and CRY1 and between TFL1 and CRY2 expression levels (Figure 1).
Figure 1.—
Negative correlation between the expression of CRY1 or CRY2 and the expression of TFL1. Shown are 633 data points corresponding to different developmental contexts and biotic or abiotic treatments (1–3 biological replicates per point) taken from 46 experiments (1388 microarrays, http://www.arabidopsis.org). Data were normalized to the median of each experiment and transformed as ln (x + 1). The lines show least-square linear fits of the 633 points and the significance is indicated.
Enhanced expression of TFL1 in the cry1 cry2 mutant background:
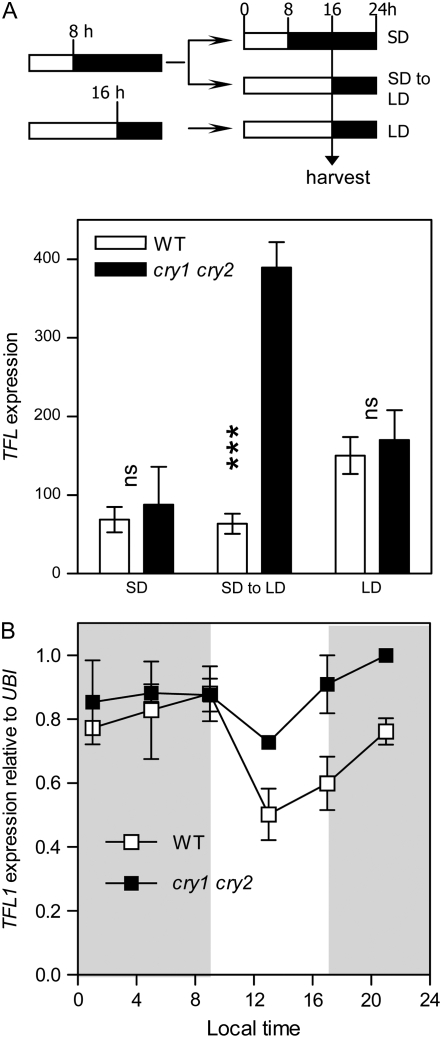
The negative correlation between TFL1 and CRY1 or CRY2 expression suggests that CRY1 and/or CRY2 could affect TFL1 expression or vice versa. No effects of the tfl1-1 mutation on CRY1 or CRY2 expression were observed (B. Strasser and P. D. Cerdán, unpublished results) and therefore we investigated TFL1 expression in the cry1 cry2 mutant background. Plants of the WT and of the cry1 cry2 double mutant of Arabidopsis were grown under SD (8 hr light, 16 hr darkness) for 24 days. At the end of the last photoperiod, half of the plants began their normal dark period (SD controls) and the rest were exposed to light (SD to LD) (Figure 2A). Eight hours later, i.e., in the middle of the night for SD controls and at the end of the first LD in the SD-to-LD condition, the plants were harvested. A third group of plants was grown under LD (16 hr light, 8 hr darkness) from sowing and harvested at the end of photoperiod 19, when the plants had nine leaves (Figure 2A). This time was chosen to have a comparable developmental stage because SD or SD-to-LD plants had nine leaves at harvest. These samples were used for microarray experiments and we observed that the expression of TFL1 was unaffected in the cry1 cry2 double mutant under either continuous SD or LD conditions. TFL1 expression in the cry1 cry2 double mutant was significantly higher than in WT by the end of the first LD (Figure 2A). We also investigated the expression of TFL1 throughout the daily cycle under SD. In accordance with microarray results, TFL1 expression was not significantly affected in cry1 cry2 seedlings harvested in the middle of the night (Figure 2B). However, in the cry1 cry2 double mutant, TFL1 transcripts accumulated to significantly higher levels during daytime and the first hours of the night relative to WT. These results suggest that cryptochromes are necessary to reduce the levels of TFL1 gene expression under SD. To explore the interactions between TFL1 and the cryptochromes, all possible mutant combinations between tfl1, cry1, and cry2 were generated and analyzed.
Figure 2.—
Enhanced expression of TFL1 in the cry1 cry2 mutant. (A) Seedlings were grown under SD, under SD followed by LD, or under LD and harvested 16 hr after the beginning of the last photoperiod, when they had nine leaves. Data are means and SE of two biological replicates. Factorial ANOVA indicates significant photoperiod by cry1 cry2 interaction (P < 0.005). ***P < 0.001 between CRY1 CRY2 and cry1 cry2 in a Bonferroni post test. NS, not significant. (B) Daily time course of TFL1 expression in seedlings of the WT and of the cry1 cry2 double mutant grown under SD. Data are means and SE of two biological replicates (data standardized to the maximum of each experiment). The slope of the relationship between expression and time is significantly affected by the cry1 cry2 mutation (P < 0.05).
Growth under SD does not suppress key features of the tfl1 phenotype:
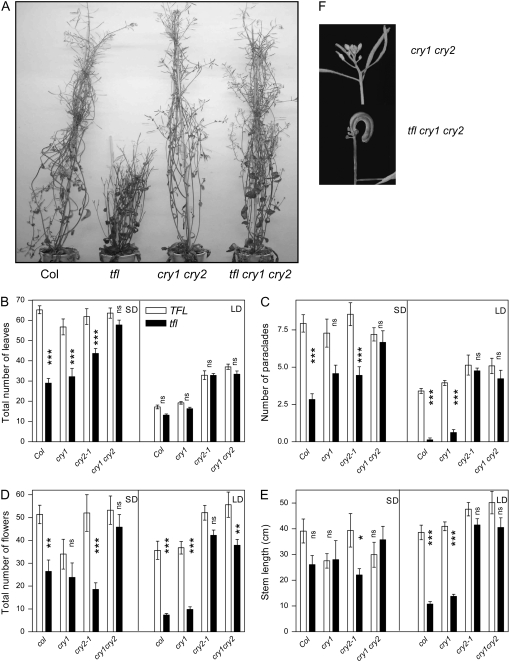
Plants of the WT and of the cry1, cry2, cry1 cry2, tfl1, tfl1 cry1, tfl1 cry2, and tfl1 cry1 cry2 mutants were grown under SD or LD. As reported previously (Shannon and Meeks-Wagner 1991), the tfl1 single mutant is early flowering, has a reduced stature, has a reduced number of paraclades, and has fewer flowers on the main shoot (Figure 3). Under LD growth, the cry1cry2 double mutant suppressed many of the defects associated with the tfl1 single mutant. Relative to tfl1 single mutant, the triple mutants flowered later, had a longer stem, produced more paraclades, and initiated more flowers (Figure 3). Very likely, the latter two responses are consequences of altering flowering time. Early flowering reduces the number of leaves and diminishes the number of buds where paraclades can emerge and the robustness of the plant supporting flower production. Compared to the WT, the cry1 cry2 mutant showed delayed flowering, an enhanced number of paraclades, and more flowers on the main shoot under LD, a phenotype consistent with the role of cry1 and cry2 as sensors of LD.
Figure 3.—
Strong suppression of the tfl1 phenotype by the cry1 cry2 mutant background. Plants of the WT and of the tfl1, cry1, and cry2 single, double, and triple mutants were grown under SD or LD (irradiance 200 μmol m−2 sec−1). (A) Representative plants of the WT, and of the tfl1, cry1 cry2, and tfl1 cry1 cry2 mutants grown under LD. (B) Flowering time on a biological scale (leaf number). (C) Number of paraclades. (D) Number of flowers on the main shoot. (E) Length of the main stem. (F) Detail of a terminal flower in tfl1 cry1 cry2. Data are means and SE of at least 10 replicate plants. Data were analyzed by factorial ANOVA followed by a Bonferroni post test. The effects of TFL1 vs. tfl1 are indicated: ***P < 0.001; **P < 0.01. NS, not significant.
We observed that tfl1 single mutants also had significant effects under SD growth; flowering time, stem length, paraclade number, and flower number were also significantly different from WT. As discussed previously (Shannon and Meeks-Wagner 1991), SD growth appears to alleviate some of the tfl1 phenotypes and this may be due to a longer vegetative phase of development. However, if strictly compared to the WT, the absolute impact of tfl1 on flowering time is stronger under SD than under LD; the impact of tfl1 on the number of paraclades and on the number of flowers is unaffected and only the impact on stem length is significantly reduced by SD compared to LD (Figure 3).
Significant suppression of the tfl1 phenotype by cry1 cry2:
Since cry1 and cry2 are key players in the perception of LD compared to SD (Lin 2002), we expected the cry1 cry2 background to mimic the effect of SD in plants grown under LD. In contrast, the cry1 cry2 mutant background significantly reduced or even eliminated the effects of tfl1 on flowering time, stem length, number of paraclades, and flower number (Figure 3). Noteworthy, these effects were observed both in plants grown under LD and in plants grown under SD, revealing a strong action of cryptochromes under SD.
The relative contribution of cry1 and cry2 to the suppression of the tfl1 phenotypes was complex. In effect, for flowering time and for the number of paraclades the cry2 mutation was fully epistatic to tfl1 under LD and only partially epistatic under SD where both cryptochromes showed redundancy (Figure 3). For stem length and flower number, cry1 was epistatic under SD and cry2 under LD. Although most aspects of the phenotype were strongly or fully suppressed by the cry1 cry2 background, the cry1 cry2 tfl1 triple mutant often retained a morphologically aberrant terminal flower (Figure 3F), which is typical of tfl1 (Shannon and Meeks-Wagner 1991).
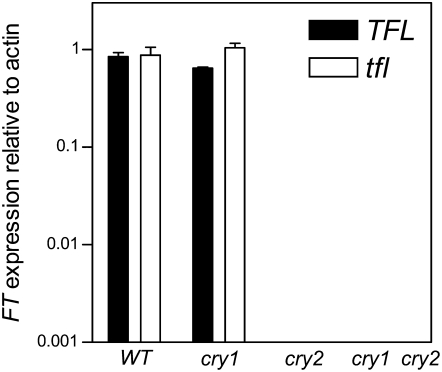
Expression of the flowering activator FT:
To further characterize the flowering time phenotypes we investigated the expression of the flowering activator FT, which acts downstream of cryptochromes. Plants were grown for 15 days under SD and harvested close to the end of the photoperiod. The expression of FT was unaffected in the tfl1 single mutant but it was significantly reduced in the cry2 mutant and cry1 cry2 double-mutant background (Figure 4).
Figure 4.—
Reduced expression of FT in the cry2 and cry1 cry2 double-mutant background under SD. Seedlings were grown under SD for 15 days and harvested close to the end of the photoperiod. Data are means and SE of two biological replicates. Factorial ANOVA indicates significant effects of CRY2 vs. cry2 (P < 0.0001) and no effects of TFL1 vs. tfl1 or interaction. FT expression is not detectable in the cry2 and cry1 cry2 mutant background under the nonsaturating conditions used here but it was detectable under saturating conditions.
Dependency of the tfl1 phenotype on the growth irradiance:
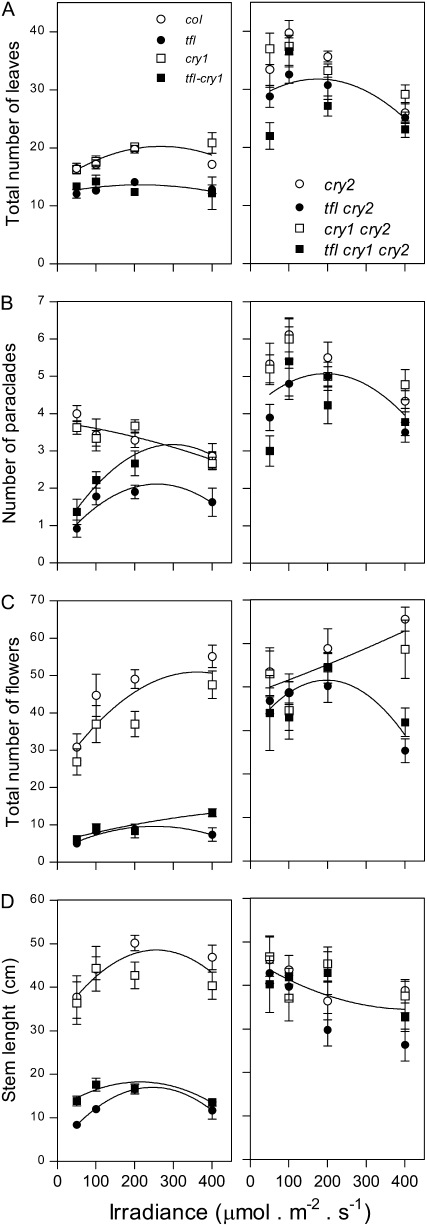
Despite the fact that cry2 and cry1 contribute to photoperiod perception, the cry1 cry2 background had a much more dramatic impact on the tfl1 phenotype than the growth under different photoperiods. Since cry1 and cry2 are also sensors of irradiance (Lin 2002), we decided to investigate the tfl1 phenotype under different irradiances. For this purpose the plants were grown under LD with a photosynthetic photon fluence rate of 50, 100, 200, or 400 μmol m−2 sec−1. In the WT, the number of leaves and stem length moderately increased with fluence rate reaching a maximum at 200 μmol m−2 sec−1, while the number of flowers on the main stem increased and the number of paraclades decreased with increasing irradiance (Figure 5). Compared to the WT, in the cry1 cry2 mutant the responses to irradiance showed some modifications in pattern but they were still present, indicating that although in dark-grown seedlings cry1 and cry2 are sensors of blue light irradiance the responses to irradiance reported here were not largely mediated by cry1 and cry2. The latter conclusion is particularly obvious when the cry1 mutant, affecting the main sensor of blue light irradiance in young seedlings, is compared to the WT. As described for the aforementioned experiments, the tfl1 mutant showed early flowering, reduced stature, reduced number of paraclades, and reduced number of flowers on the main shoot. The tfl1 mutation had little effect on the shape of flowering-time and stem-length responses to irradiance. However, the tfl1 mutant showed reduced response of the total number of flowers to irradiance and changed the response shape for the number of paraclades. The cry1 cry2 background largely suppressed the tfl1 phenotype in plants grown under 200 μmol m−2 sec−1, the irradiance used for SD–LD experiments, but a residual tfl1 phenotype in the cry1 cry2 background was evident for the total number of flowers at high irradiance and for flowering time and number of paraclades at the lowest fluence rates (Figure 5).
Figure 5.—
The tfl1 phenotype persists in plants grown under low irradiances. Plants of the WT and of the tfl1, cry1, and cry2 single, double, and triple mutants were grown under LD at the indicated irradiance. (A) Flowering time on a biological scale (leaf number). (B) Number of paraclades. (C) Number of flowers on the main shoot. (D) Length of the main stem. Data are means and SE of at least 10 replicate plants. Different lines indicate significantly different second-order polynomial fits (P < 0.01).
Long hypocotyl of the tfl1 mutant:
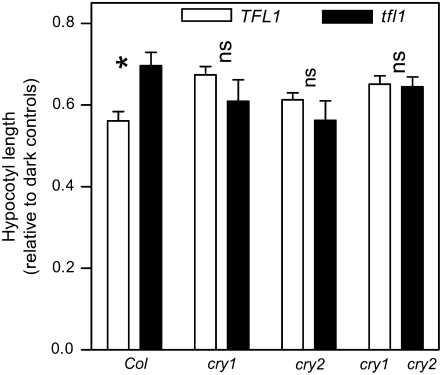
Since the tfl1 phenotype depends strongly on cry1 and cry2, we investigated whether the control of hypocotyl growth by light, which is also controlled by cry1 and cry2 (Lin 2002), was affected in tfl1. In seedlings grown under LD, tfl1 showed longer hypocotyls than the WT. The cry1 and/or cry2 mutations were epistatic to this novel tfl1 phenotype (Figure 6). A similar pattern was observed for seedlings grown under SD (data not shown).
Figure 6.—
Long hypocotyl in tfl1 seedlings grown under LD. Chilled seeds were transferred to LD (irradiance 5 μmol m−2 sec−1) or to continuous darkness and hypocotyl length was measured 4 days later. Data are means and SE of four replicate boxes (10 seedlings per box). Factorial ANOVA indicated significant interaction between TFL1 and CRY genes (P < 0.05). *P < 0.05 in Bonferroni post tests. NS, not significant.
DISCUSSION
In this report we have shown that many of the tfl1 mutant phenotypes are suppressed in the cry1 cry2 double mutant. Compared to the tfl1 single mutant, the tfl1 cry1 cry2 triple mutant flowered later, had a longer stem, produced more paraclades, and initiated more flowers (Figure 3). Noteworthy, this epistatic effect is equally important under LD and SD (Figure 3), despite the fact that cry1 and cry2 play a major role in the perception of LD, i.e., they are predicted to be active during a larger proportion of the 24-hr cycle under LD than under SD. These photoreceptors are also important for the perception of irradiance. However, the reduction of irradiance did not cause a general weakening of the tfl1 phenotype (Figure 5). In other words, the tfl1 mutant does not converge to the phenotype of the tfl1 cry1 cry2 triple mutant at low irradiances or under SD. Therefore, the tfl1 phenotype depends on the presence of functional CRY1 and CRY2 genes but it does not depend on cry1 and cry2 physiological activity within the (wide) range tested here. Subtle variations in the epistatic relationships between tfl1 and cry1/cry2 mutations (Figure 3) might reflect the involvement of different residues of the TFL1 protein in the control of flowering time and in the determinacy of the inflorescence meristem (Hanzawa et al. 2005) or different patterns of CRY1/CRY2 expression.
Previous reports have described the phenotype of mature plants of the tfl1 mutant (Shannon and Meeks-Wagner 1991). Seedling morphology is also affected, because the inhibition of hypocotyl growth by white light was reduced in the tfl1 single mutant (Figure 6). As described above for the classical aspects of the tfl1 phenotype, the cry1 cry2 mutation was also epistatic to tfl1 for hypocotyl growth. This suggests that the normal control of hypocotyl growth by cry1 and cry2 requires a functional TFL1 gene.
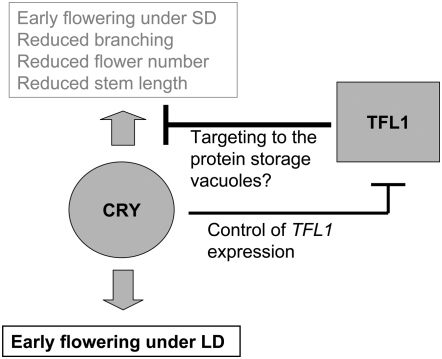
In the WT background, the cry1 and cry2 mutations cause little or no effects on flowering time under short days (Guo et al. 1998; Mockler et al. 1999) (Figure 3B). In the absence of a functional TFL1 allele, cry1 or cry2 cause very early flowering under SD (cf. tfl1 vs. tfl1 cry1 cry2 in Figure 3B). The Cvi allele of CRY2 also promotes flowering under SD but this allele represents an adaptation to low latitudes and shows no obvious role in photoperiodic responses (El-Assal et al. 2001). A key regulatory function of cryptochromes is to perceive the difference between SD and LD and induce flowering under the appropriate conditions for a LD plant like Arabidopsis (Lin 2002). TFL1 downregulates cry1- and cry2-mediated promotion of flowering under SD allowing adequate adjustment of development to photoperiodic conditions (Figure 3). We propose a model where a player acting downstream of cryptochromes is able to promote flowering if TLF1 is absent but not if TFL1 is present (Figure 7). TFL1 negatively regulates cryptochrome-mediated effects and cryptochromes downregulate TFL1 expression (Figure 2). This could provide a positive feedback mechanism on cryptochrome signaling (Figure 7).
Figure 7.—
Proposed model of the genetic interactions between TFL1 and CRY. According to this model, in the absence of TFL1, downstream products of cryptochrome signaling cause early flowering under SD and morphological distortions typical of the tfl1 phenotype.
Although cry2 enhances the expression of FT under LD compared to SD (Yanovsky and Kay 2002), a positive regulation of FT expression by cry2 occurs even under SD (Figure 4). In the presence of TFL1, the differences in FT expression caused by cry2 under SD do not translate into differences in flowering time (Figure 3B). TFL1 and FT are homologous genes with antagonistic action (Kobayashi et al. 1999; Hanzawa et al. 2005). TFL1 activity could set a minimum threshold of FT activity necessary to affect flowering and this threshold would not be reached by cry2-mediated promotion of FT expression under SD.
Pleiotropic effects of functional CRY1 or CRY2 alleles become evident in the tfl1 background. The tfl1 loss-of-function phenotype can therefore be interpreted in terms of cry1 and cry2 gain of function. In the tfl1 background cry1 and cry2 distort plant body form (cf. tfl1 and tfl1 cry1 cry2 in Figure 3A). A key function of cryptocrhomes is to promote flowering under LD compared to SD (Lin 2002) but in the tfl1 background cry1 and cry2 strongly promote flowering under SD (Figure 3B). In other words, TFL1 negatively regulates cry1- and cry2-mediated misregulation of flowering time and the pleiotropic distortions of plant body development (Figure 7). In genetic canalization, epistatic relationships permit a phenotype to remain relatively invariant in response to mutations (De Visser et al. 2003; Flatt 2005). Conversely, in the case reported here, TFL1 provides homeostasis not in the context of cry1 or cry2 mutations but in the context of functional CRY1 and CRY2 alleles.
On the basis of the recently discovered role of TFL1 in the control of trafficking to the protein storage vacuoles in vegetative tissues, Sohn et al. (2007) have proposed that these vacuoles could store factors involved in the control of flowering and meristem maintenance, until the proper combination of developmental and environmental cues trigger their secretion and activate the flowering pathway. An alternative, although not an exclusive proposal, would be that TFL1 could target selected downstream products of cry1 and cry2 signaling to the protein storage vacuoles (Figure 7). Therefore, these products would generate maladaptive misregulations in the absence of TFL1 but not in its presence.
Acknowledgments
We thank Todd Mockler (Oregon State University) for his kind provision of cry1 cry2 double-mutant seed. This work was financially supported by grants from Guggenheim Foundation to J.J.C., Agencia Nacional de Promoción Científica y Tecnológica (PICT 32492) to J.J.C. and (PICT 14287) to P.D.C., Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 5958) to J.J.C., and the International Centre for Genetic Engineering and Biotechnology (CRP/ARG05-02) to P.D.C. A.S.B. and B.S., respectively, were supported by fellowships from CONICET and YPF Foundation.
References
- Ahmad, M., and A. R. Cashmore, 1993. HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 162–166. [DOI] [PubMed] [Google Scholar]
- Bradley, D., O. Ratcliffe, C. Vincent, R. Carpenter and E. Coen, 1997. Inflorescence commitment and architecture in Arabidopsis. Science 275 80–83. [DOI] [PubMed] [Google Scholar]
- Bruggeman, E., K. Handwerger, C. Essex and G. Storz, 1996. Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J. 10 755–760. [DOI] [PubMed] [Google Scholar]
- Casal, J. J., C. Fankhauser, G. Coupland and M. A. Blázquez, 2004. Signalling for developmental plasticity. Trends Plant Sci. 9 309–314. [DOI] [PubMed] [Google Scholar]
- Cerdán, P., and J. Chory, 2003. Regulation of flowering time by light quality. Nature 423 881–885. [DOI] [PubMed] [Google Scholar]
- Clarke, J. D., and T. Zhu, 2006. Microarray analysis of the transcriptome as a stepping stone towards understanding biological systems: practical considerations and perspectives. Plant J. 45 630–650. [DOI] [PubMed] [Google Scholar]
- Conti, L., and D. Bradley, 2007. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Visser, J. A. G. M., J. Hermisson, G. P. Wagner, L. A. Meyers, H. Bagheri-Chaichian et al., 2003. Perspective: evolution and detection of genetic robustness. Evolution 57 1959–1972. [DOI] [PubMed] [Google Scholar]
- El-Assal, S.-D., C. Alonso Blanco, A. J. M. Peeters, V. Raz and M. Koornneef, 2001. A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet. 29 435–439. [DOI] [PubMed] [Google Scholar]
- Flatt, T., 2005. The evolutionary genetics of canalization. Q. Rev. Biol. 80 287–316. [DOI] [PubMed] [Google Scholar]
- Guo, H., H. Yang, T. C. Mockler and C. Lin, 1998. Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363. [DOI] [PubMed] [Google Scholar]
- Guo, H., H. Duong, N. Ma and C. Lin, 1999. The Arabidopsis blue-light receptor cryptochrome 2 is a nuclear protein regulated by a blue-light dependent post-transcriptional mechanism. Plant J. 19 279–289. [DOI] [PubMed] [Google Scholar]
- Hanzawa, Y., T. Money and D. Bradley, 2005. A single amino acid converts a repressor to an activator of flowering. Proc. Natl. Acad. Sci. USA 102 7748–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky, I., V. K. Shukla, J. H. Ahn, N. Dagenais, S. K. Christensen et al., 1999. Activation tagging of the floral inducer FT. Science 286 1962–1965. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y., H. Kaya, K. Goto, M. Iwabuchi and T. Araki, 1999. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286 1960–1962. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., C. J. Hanhart and J. H. van der Veen, 1991. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229 57–66. [DOI] [PubMed] [Google Scholar]
- Lin, C., 2002. Blue light receptors and signal transduction. Plant Cell 14 S207–S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel, M. A., and M. F. Yanofsky, 1995. A gene triggering flower formation in Arabidopsis. Nature 377 522–524. [DOI] [PubMed] [Google Scholar]
- Mazzella, M. A., P. D. Cerdán, R. Staneloni and J. J. Casal, 2001. Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsis development. Development 128 2291–2299. [DOI] [PubMed] [Google Scholar]
- Mockler, T., H. Guo, H. Yang, H. Duong and C. Lin, 1999. Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126 2073–2082. [DOI] [PubMed] [Google Scholar]
- Ohto, M.-A., K. Onai, Y. Furukawa, E. Aoki, T. Araki et al., 2001. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 127 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe, O. J., I. Amaya, C. A. Vincent, S. Rothstein, R. Carpenter et al., 1998. A common mechanism controls the life cycle and architecture of plants. Development 125 1609–1615. [DOI] [PubMed] [Google Scholar]
- Ratcliffe, O. J., D. J. Bradley and E. S. Coen, 1999. Separation of shoot and floral identity in Arabidopsis. Development 126 1109–1120. [DOI] [PubMed] [Google Scholar]
- Sawa, M., D. A. Nusinow, S. A. Kay and T. Imaizumi, 2007. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, S., and D. R. Meeks-Wagner, 1991. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, S., and D. R. Meeks-Wagner, 1993. Genetic interactions that regulate inflorescence development in Arabidopsis. Plant Cell 5 639–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, R., M. I. Igeno and G. Coupland, 1996. Activation of floral meristem identity genes in Arabidopsis. Nature 384 59–62. [DOI] [PubMed] [Google Scholar]
- Sohn, E. J., M. Rojas-Pierce, S. Pan, C. Carter, A. Serrano-Mislata et al., 2007. The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc. Natl. Acad. Sci. USA 104 18801–18806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez, P., K. Wheatley, F. Robson, H. Onouchi, F. Valverde et al., 2001. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 26 1116–1120. [DOI] [PubMed] [Google Scholar]
- Valverde, F., A. Mouradov, W. Soppe, D. Ravenscroft, A. Samach et al., 2004. Photoreceptor regulation of CONSTANS protein and the mechanism of photoperiodic flowering. Science 303 1003–1006. [DOI] [PubMed] [Google Scholar]
- Weigel, D., and O. Nilsson, 1995. A developmental switch sufficient for flower initiation in diverse plants. Nature 377 495–500. [DOI] [PubMed] [Google Scholar]
- Wu, G., and E. P. Spalding, 2007. Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 104 18813–18818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky, M. J., and S. A. Kay, 2002. Molecular basis of seasonal time measurement in Arabidopsis. Nature 419 308–312. [DOI] [PubMed] [Google Scholar]
- Zimmermann, P., M. Hirsch-Hoffmann, L. Hennig and W. Gruissem, 2004. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]