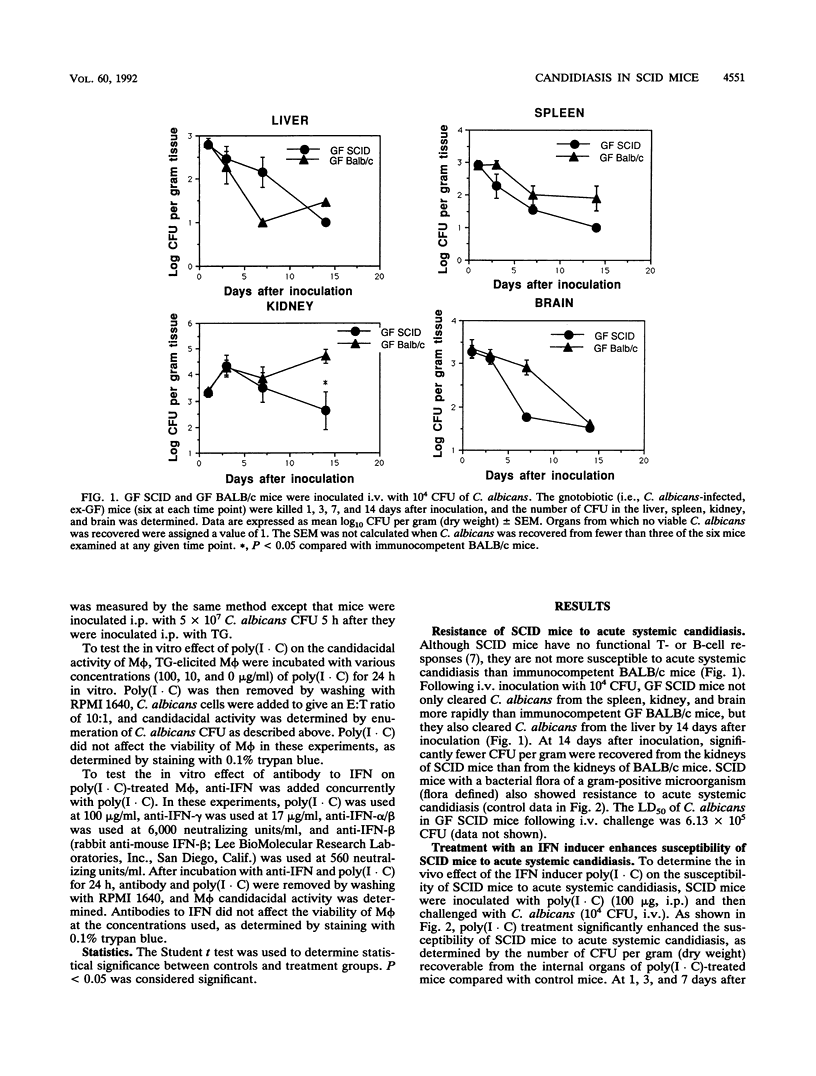
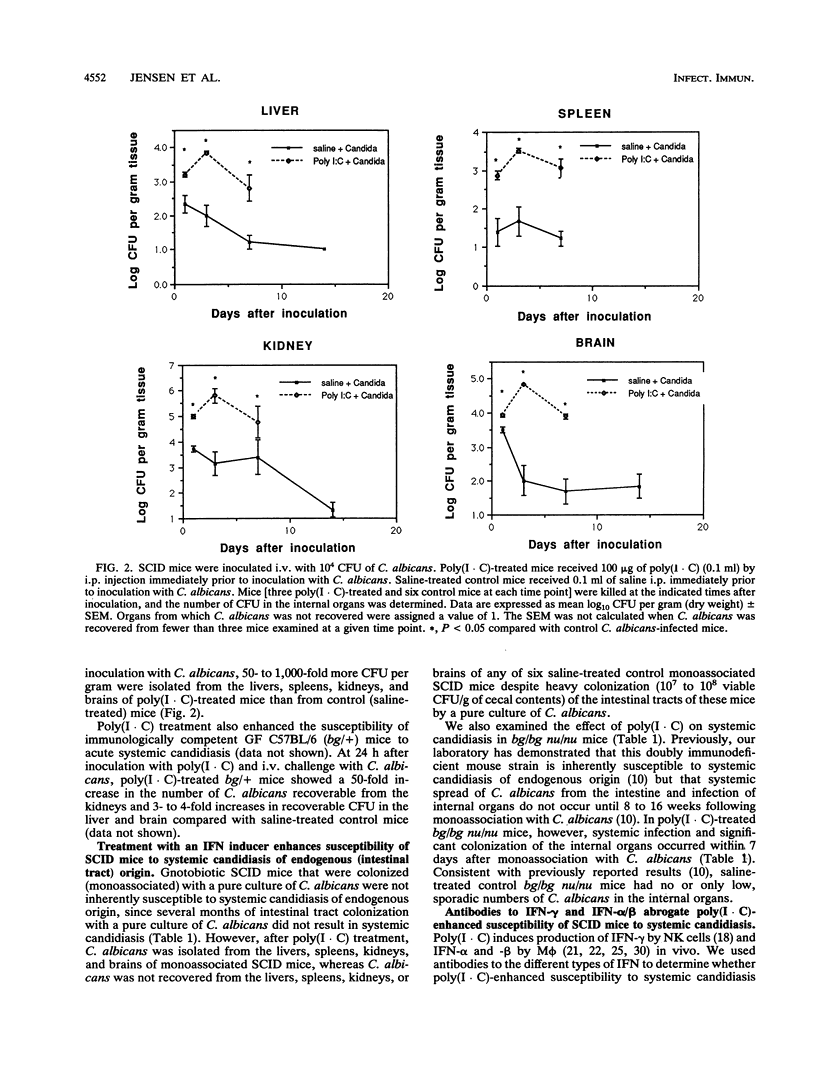
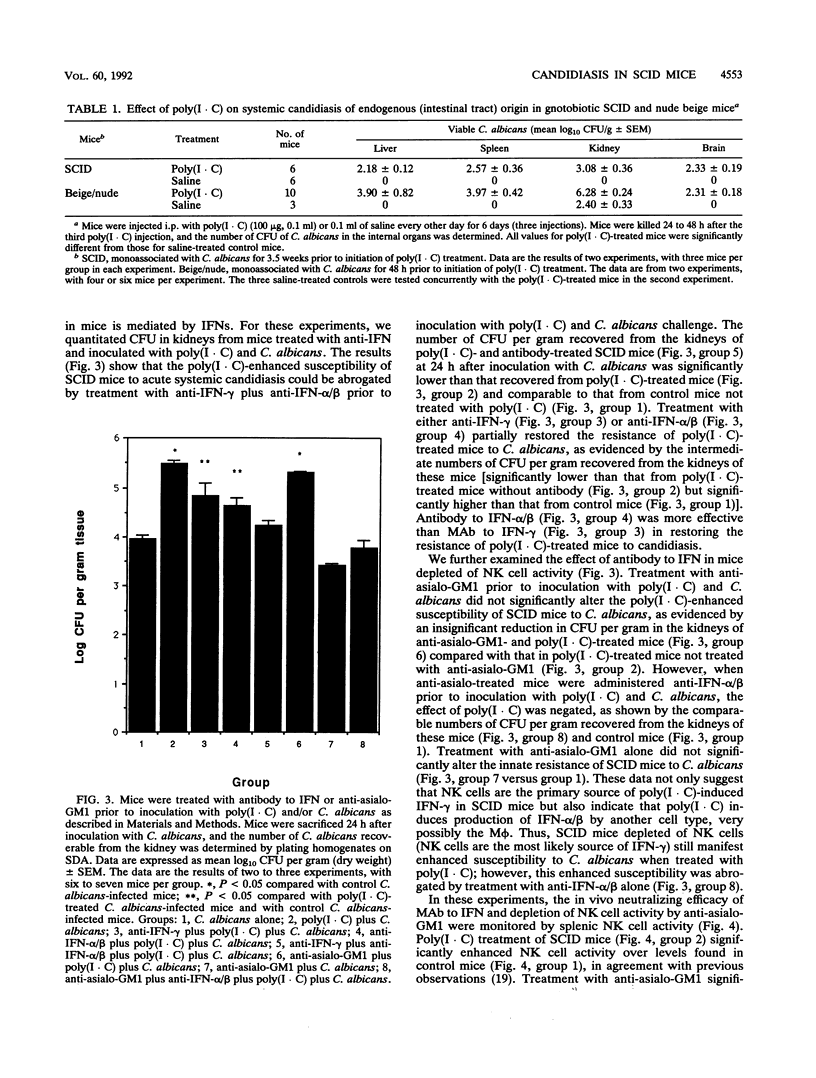
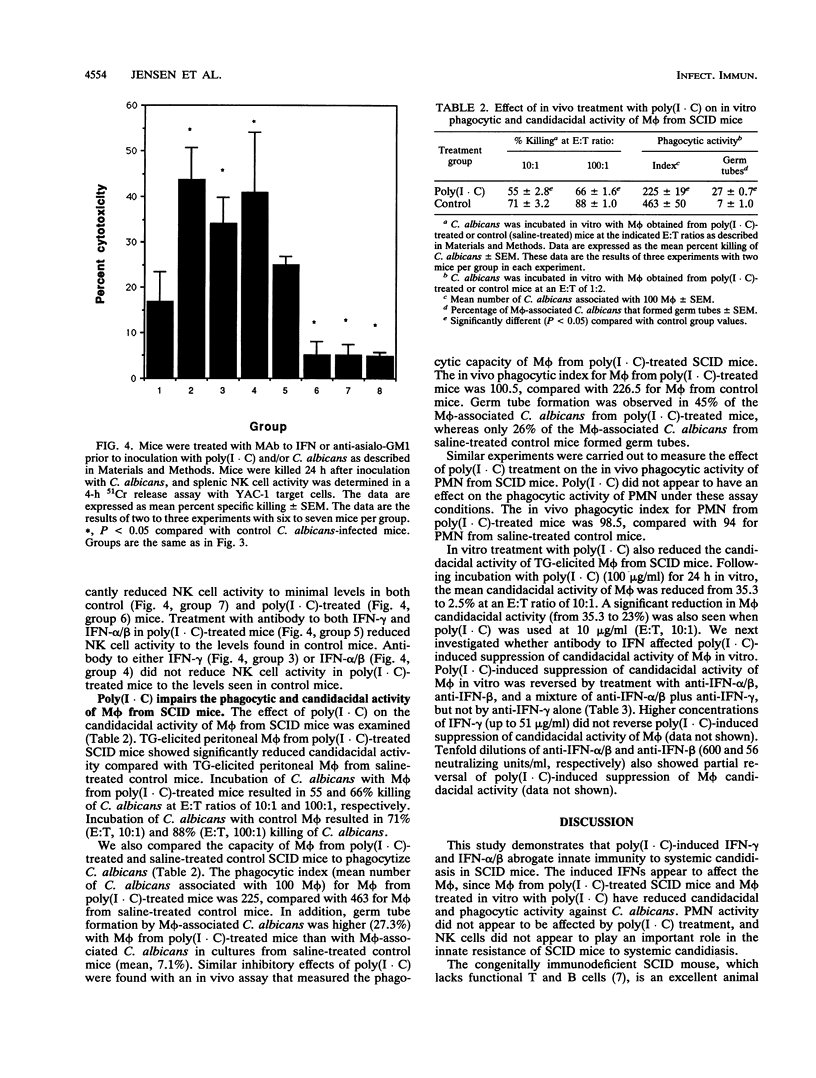
Abstract
In the absence of any demonstrable T- or B-cell responses, gnotobiotic CB-17 SCID (severe combined immunodeficient) mice not only show innate resistance to acute systemic (intravenous challenge) candidiasis but also manifest innate resistance to systemic candidiasis of endogenous (gastrointestinal tract) origin. Poly(I. C), a potent inducer of interferons (IFNs) in vivo, enhanced the susceptibility of CB-17 SCID mice to acute systemic candidiasis and to systemic candidiasis of endogenous origin, as demonstrated by increased numbers of viable Candida albicans in internal organs after poly(I. C) treatment. The poly(I. C)-enhanced susceptibility of mice to candidiasis was abrogated by in vivo treatment with antibodies to IFN-alpha, -beta, and -gamma. In vivo depletion of natural killer cells from SCID mice did not significantly enhance their susceptibility to systemic candidiasis or abrogate poly(I. C)-enhanced susceptibility. In vivo and in vitro, treatment with poly(I. C) impaired the candidacidal and phagocytic activity of thioglycollate-elicited macrophages from SCID mice. Antibody to IFN-alpha/beta or IFN-beta alone interfered with the ability of poly(I. C) to impair the candidacidal activity of macrophages from SCID mice in vitro. These data suggest that poly(I. C)-induced interferons can impair the candidacidal activity of macrophages in SCID mice and decrease their innate resistance to acute systemic candidiasis and to systemic candidiasis of endogenous origin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B., Papadimitriou J. M. Susceptibility of beige mutant mice to candidiasis may be linked to a defect in granulocyte production by bone marrow stem cells. Infect Immun. 1991 Jun;59(6):2140–2146. doi: 10.1128/iai.59.6.2140-2146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Balish M. J., Salkowski C. A., Lee K. W., Bartizal K. F. Colonization of congenitally athymic, gnotobiotic mice by Candida albicans. Appl Environ Microbiol. 1984 Apr;47(4):647–652. doi: 10.1128/aem.47.4.647-652.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Filutowicz H., Oberley T. D. Correlates of cell-mediated immunity in Candida albicans-colonized gnotobiotic mice. Infect Immun. 1990 Jan;58(1):107–113. doi: 10.1128/iai.58.1.107-113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Filutowicz H. Serum antibody response of gnotobiotic athymic and euthymic mice following alimentary tract colonization and infection with Candida albicans. Can J Microbiol. 1991 Mar;37(3):204–210. doi: 10.1139/m91-031. [DOI] [PubMed] [Google Scholar]
- Balish E., Phillips A. W. Growth and virulence of Candida albicans after oral inoculation in the chick with a monoflora of either Escherichia coli or Streptococcus faecalis. J Bacteriol. 1966 May;91(5):1744–1749. doi: 10.1128/jb.91.5.1744-1749.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Acquired immunity to systemic candidiasis in immunodeficient mice. J Infect Dis. 1991 Nov;164(5):936–943. doi: 10.1093/infdis/164.5.936. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990 Apr;58(4):1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect Immun. 1991 Jul;59(7):2447–2455. doi: 10.1128/iai.59.7.2447-2455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M., Mook D., Balish E. Resistance of congenitally immunodeficient gnotobiotic mice to vaginal candidiasis. Infect Immun. 1990 Nov;58(11):3813–3815. doi: 10.1128/iai.58.11.3813-3815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Bartocci A., Puccetti P., Mocci S., Stanley E. R., Bistoni F. Macrophage colony-stimulating factor in murine candidiasis: serum and tissue levels during infection and protective effect of exogenous administration. Infect Immun. 1991 Mar;59(3):868–872. doi: 10.1128/iai.59.3.868-872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Isturiz R. E., Malech H. L., Root R. K., Wilfert C. M., Gutman L., Buckley R. H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981 Jul;71(1):59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Epstein J. B., Truelove E. L., Izutzu K. T. Oral candidiasis: pathogenesis and host defense. Rev Infect Dis. 1984 Jan-Feb;6(1):96–106. doi: 10.1093/clinids/6.1.96. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Rabinovitch M. Production of interferon by in vitro derived bone marrow macrophages. Cell Immunol. 1981 Jan 15;57(2):495–504. doi: 10.1016/0008-8749(81)90107-6. [DOI] [PubMed] [Google Scholar]
- Garner R. E., Kuruganti U., Czarniecki C. W., Chiu H. H., Domer J. E. In vivo immune responses to Candida albicans modified by treatment with recombinant murine gamma interferon. Infect Immun. 1989 Jun;57(6):1800–1808. doi: 10.1128/iai.57.6.1800-1808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- Jullien P., De Maeyer-Guignard J., De Maeyer E. Interferon synthesis in x-irradiated animals v. Origin of mouse serum interferon induced by polyinosinic-polycytidylic Acid and encephalomyocarditis virus. Infect Immun. 1974 Nov;10(5):1023–1028. doi: 10.1128/iai.10.5.1023-1028.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbassi A., Becker J. M., Foster J. S., Moore R. N. Enhanced killing of Candida albicans by murine macrophages treated with macrophage colony-stimulating factor: evidence for augmented expression of mannose receptors. J Immunol. 1987 Jul 15;139(2):417–421. [PubMed] [Google Scholar]
- Klein R. S., Harris C. A., Small C. B., Moll B., Lesser M., Friedland G. H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 9;311(6):354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Lee K. W., Balish E. Systemic candidosis in silica-treated athymic and euthymic mice. Infect Immun. 1983 Sep;41(3):902–907. doi: 10.1128/iai.41.3.902-907.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S., Greenfield R. A., Joyce W. A., Kincade P. W. Inoculation candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1988 Dec;56(12):3162–3166. doi: 10.1128/iai.56.12.3162-3166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D., Lynch R. J., Meeker J. B., Field A. K. Macrophage dependence of polyriboinosinic acid-polyribocytidylic acid-induced resistance to herpes simplex virus infection in mice. Infect Immun. 1980 Apr;28(1):147–153. doi: 10.1128/iai.28.1.147-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier-Carpentier F., Kiehn T. E., Armstrong D. Fungemia in the immunocompromised host. Changing patterns, antigenemia, high mortality. Am J Med. 1981 Sep;71(3):363–370. doi: 10.1016/0002-9343(81)90162-5. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Pitruzzello F. J., Larsen H. S., Rouse B. T. Feedback regulation of colony-stimulating factor (CSF-1)-induced macrophage proliferation by endogenous E prostaglandins and interferon-alpha/beta. J Immunol. 1984 Aug;133(2):541–543. [PubMed] [Google Scholar]
- Morrison C. J., Brummer E., Isenberg R. A., Stevens D. A. Activation of murine polymorphonuclear neutrophils for fungicidal activity by recombinant gamma interferon. J Leukoc Biol. 1987 May;41(5):434–440. doi: 10.1002/jlb.41.5.434. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Joyce W. A., Greenfield R. A. Gastrointestinal candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1991 Jun;59(6):2116–2119. doi: 10.1128/iai.59.6.2116-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Granger D. L., Durack D. T. Effects of antifungal agents and gamma interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987 Aug;156(2):316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- Phillips A. W., Balish E. Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol. 1966 Sep;14(5):737–741. doi: 10.1128/am.14.5.737-741.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo S., Gangemi J. D., Ghaffar A., Mayer E. P. Poly I:C-induced anti-herpes simplex virus type 1 activity in inflammatory macrophages is mediated by induction of interferon-beta. J Leukoc Biol. 1991 Nov;50(5):479–487. doi: 10.1002/jlb.50.5.479. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Two levels of regulation of beta-interferon gene expression in human cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3923–3927. doi: 10.1073/pnas.80.13.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. The role of activated macrophages in resistance to experimental renal candidiasis. J Reticuloendothel Soc. 1977 Oct;22(4):309–318. [PubMed] [Google Scholar]
- Salkowski C. A., Balish E. Role of natural killer cells in resistance to systemic cryptococcosis. J Leukoc Biol. 1991 Aug;50(2):151–159. doi: 10.1002/jlb.50.2.151. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Weber H. The interferon genes. Prog Nucleic Acid Res Mol Biol. 1986;33:251–300. doi: 10.1016/s0079-6603(08)60026-4. [DOI] [PubMed] [Google Scholar]
- Worthington M., Hasenclever H. F. Effect of an interferon stimulator, polyinosinic: polycytidylic acid, on experimental fungus infections. Infect Immun. 1972 Feb;5(2):199–202. doi: 10.1128/iai.5.2.199-202.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino S. J., Hudig D. Interactions between human natural killer (NK) lymphocytes and yeast cells: human NK cells do not kill Candida albicans, although C. albicans blocks NK lysis of K562 cells. Infect Immun. 1988 Mar;56(3):564–569. doi: 10.1128/iai.56.3.564-569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



