Summary
N-Acetyltransferase 2 (NAT2) catalyzes the O-acetylation of N-hydroxy heterocyclic amines such as N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (N-OH-MeIQx) and N-hydroxy-2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (N-OH-PhIP) to DNA binding metabolites that initiate mutagenesis and carcinogenesis. NAT2 acetylator phenotype is associated with increased cancer risk. Single nucleotide polymorphisms (SNPs) have been identified in the NAT2 coding region. Although the effects of these SNPs on N-acetyltransferase activity have been reported, very little is known regarding their effects on O-acetylation activity. To investigate the functional consequences of SNPs in the NAT2 coding region on the O-acetylation of N-hydroxy heterocyclic amines, reference NAT2*4 and NAT2 variant alleles possessing one were cloned and expressed in yeast (Schizosaccaromyces pombe). T111C, C282T, C481T, C759T, and A803G (K268R) SNPs did not significantly (p>0.05) modify O-acetylation catalysis with N-OH-PhIP or N-OH-MeIQx. C190T (R64W), G191A (R64Q), T341C (I114T), A434C (E145P), G590A (R197Q) and A845C (K282T) significantly (p<0.01) reduced the O-acetylation of both N-OH-PhIP and N-OH-MeIQx whereas G857A (G286E) significantly (p<0.05) decreased catalytic activity towards the O-acetylation of N-OH-MeIQx but not N-OH-PhIP. These results have important implications towards the interpretation of molecular epidemiological studies of NAT2 genotype and cancer risk.
Keywords: N-acetyltransferase, NAT2, acetylator genotype, single nucleotide polymorphisms O-acetylation, PhIP, MeIQx
Introduction
Human epidemiological studies have investigated the role for N-acetyltransferase 2 (NAT2) polymorphisms in various cancers. 1 Heterocyclic amines such as 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) are present in the diet as protein pyrolysis products formed when meat is well-cooked.2 Although chemically distinct, both require metabolic activation in order to mutate DNA and initiate carcinogenesis. 3 Due to steric hindrance of the exocyclic amine group, both are poor substrates for N-acetylation.4 However, following N-oxidation by hepatic CYP1A2 and other extrahepatic P450 isozymes, 5 the N-hydroxy-heterocyclic amines are further activated (via O-acetylation) by N-acetyltransferases to acetoxy intermediates which react spontaneously with DNA to form DNA adducts.3,6 Previous studies have shown that N-OH-MeIQx and N-OH-PhIP undergo metabolic activation (O-acetylation) by human N-acetyltransferase 1 and NAT2, but predominantly by human NAT2.7,8 Based on positive evidence in animal bioassays and presumptive evidence in humans, MeIQx and PhIP are classified as reasonably expected to be carcinogens. 9
The reference NAT2*4 (associated with rapid acetylator phenotype) and over thirty NAT2 allelic variants have been identified in human populations. A listing is published by an international N-acetyltransferase nomenclature committee 10 at www.louisville.edu/medschool/pharmacology/NAT.html. These NAT2 variant alleles contain single nucleotide polymorphisms (SNPs), either alone or in combination, throughout the 870 base pair NAT2 coding region. Previous studies 11,12 have characterized the effects of these individual SNPs on the N-acetylation of arylamine drugs and carcinogens, as well as on protein and mRNA expression. However, no study to our knowledge has investigated the effects of each individual SNP on metabolic activation of N-hydroxy-heterocyclic amines via O-acetylation.
Several human epidemiological studies have reported associations between NAT2 acetylator genotype and cancer following exposures to heterocyclic amines. In particular, studies have shown that well-done meat intake and rapid NAT2 phenotype predispose to colorectal 13-16 and breast 17 cancers. One study reported that PhIP-DNA adduct levels in human breast tissue are higher in rapid than in slow acetylators.18 The biological basis for these findings requires experimental evidence that rapid NAT2 phenotype modifies the metabolic activation of heterocyclic amine carcinogens. Due to the high frequency and importance of the NAT2 acetylation polymorphism in susceptibility to various cancers, we tested the effects of each SNP on metabolic activation (via O-acetylation) of N-hydroxy-heterocyclic amines.
Methods
Cloning and recombinant expression of NAT2 allelic variants
The coding region of the reference NAT2*4 and NAT2 allelic variants possessing one SNP in the NAT2 coding region were amplified by polymerase chain reaction (PCR) using previously constructed plasmids containing the specific SNPs as previously described. 11,12 Each variant NAT2 allele possessed one of the following individual SNPs: T111C, C190T (R64W), G191A (R64Q), C282T, T341C (I114T), A434C (E145P), C481T, G590A (R197Q), C759T, A803G (K268R), A845C (K282T) or G857A (G286E). Briefly, the yeast vector pESP-3 (Stratagene, La Jolla, CA) was digested with NdeI and AscI at 37°C overnight and gel purified in a similar manner to the PCR products. Purified PCR products and 80 ng of plasmid were ligated overnight at 16°C with T4 DNA ligase (New England Biolabs, Inc.). Ligated plasmids were transformed into XL-10 Gold Ultracompetent Escherichia coli (Stratagene). Plasmids were isolated from cultures grown from selected colonies using the Qiagen Plasmid Midi kit (Qiagen, Valencia, CA) and sequenced using Thermosequenase (Amersham, Arlington Heights, IL). Constructs were then transformed into competent Schizosaccaromyces pombe and expressed following the manufacturer's instructions (Stratagene). Mock transformed yeast used pESP-3 vector with no NAT2 insert. Total cell lysates were prepared by vigorous agitation of yeast in a phosphate buffered saline containing acid-washed glass beads (Stratagene) for ten min at 4°C. Liquid fractions were collected from the lysed cells and centrifuged at 13,000 × g for twenty min. Supernatants were collected, aliquoted, and stored at −80°C.
O-acetyltransferase assays
N-OH-PhIP O-acetyltransferase assays were carried out using high performance liquid chromatography separation and quantitation as previously described.19 Briefly, reactions containing 300 μM N-OH-PhIP, 1 mM acetyl coenzyme A and yeast lysate were incubated at 37°C and reactions were terminated after 5 min. N-OH-MeIQx O-acetyltransferase assays were carried out in an identical manner except N-OH-MeIQx was substituted for N-OH-PhIP. Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA) and catalytic activities were normalized to total lysate protein.
Statistical analysis
Differences between NAT2 4 and variant NAT2 allozymes were tested for significance using one-way ANOVA followed by Dunnett's Multiple Comparison test.
Results
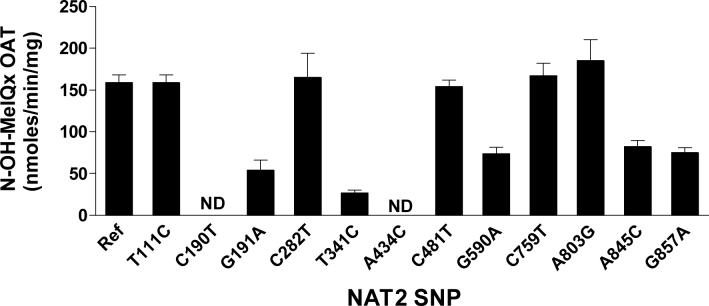
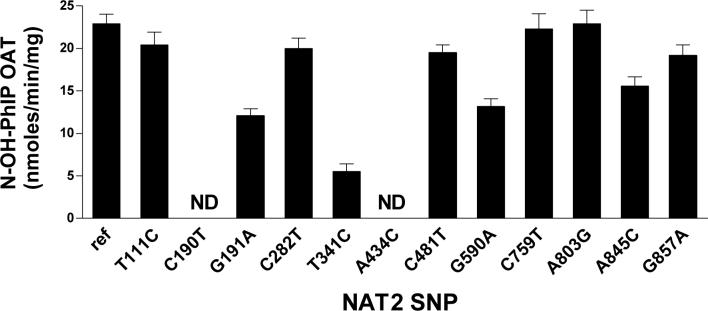
NAT2 4 and NAT2 allozymes encoded by alleles possessing one of the SNPs were tested for the metabolic activation (via O-acetylation) of N-OH-MeIQx and N-OH-PhIP. No activity towards either substrate was observed in mock transformed yeast. T111C, C282T, C481T, C759T, and A803G (K268R) did not modify O-acetylation catalysis with N-OH-MeIQx (Figure 1) or N-OH-PhIP (Figure 2). C190T (R64W) and A434C (E145P) each reduced metabolic activation of N-OH-MeIQx (Figure 1) and N-OH-PhIP (Figure 2) below the limit of assay detection (2.5 pmol/min/mg). G191A (R64Q), T341C (I114T), G590A (R197Q) and A845C (K282T) significantly (p<0.01) reduced the O-acetylation of both N-OH-MeIQx (Figure 1) and N-OH-PhIP (Figure 2) whereas G857A (G286E) significantly (p<0.01) decreased catalytic activity towards the O-acetylation of N-OH-MeIQx (Figure 1) but not N-OH-PhIP (Figure 2).
Figure 1.
Metabolic activation of N-OH-MeIQx by yeast expressing recombinant human NAT2 allozymes. NAT2 4 is the reference human NAT2 allozyme (Ref). Each of the other bars represents data for a human NAT2 allozyme possessing the single nucleotide polymorphism shown on the abscissa. Each bar represents Mean ± SEM for three determinations. C190T and A434C were below the limit of assay detection (2.5 pmol/min/mg). G191A, T341C, G590A, A845C, and G857A were each significantly (p<0.01) lower than NAT2 4.
Figure 2.
Metabolic activation of N-OH-PhIP by yeast expressing recombinant human NAT2 allozymes. NAT2 4 is the reference human NAT2 allozyme (Ref). Each of the other bars represents data for a human NAT2 allozyme possessing the single nucleotide polymorphism shown on the abscissa. Each bar represents Mean ± SEM for three determinations. C190T and A434C were below the limit of assay detection (2.5 pmol/min/mg). G191A, T341C, G590A, and A845C were each significantly (p<0.01) lower than NAT2 4.
Discussion
This is the first study to systematically test the effect of individual SNPs in the NAT2 gene on the metabolic activation (O-acetylation) of N-hydroxy heterocyclic amines. The importance of systematically investigating each SNP is because even synonymous SNPs may cause alterations in mRNA folding and translational efficiency, thereby causing phenotypic effects. 20 We previously reported the recombinant expression of reference NAT2*4 and novel NAT2 alleles in Schizosaccaromyces pombe 19 and subsequently investigated the effects of individual SNPs on N-acetyltransferase activity, protein and mRNA expression, and thermostability. 11,12 Human NAT2 protein levels following recombinant expression of these alleles in this yeast system was assessed by Western blot analysis. 11,12 As shown in Table 1, some of the SNPs reduced human NAT2 protein levels and some reduced NAT2 thermostability. Following transient expression in COS-1 cells, [T341C (I114T)] was shown to increase proteolytic degradation of NAT2 protein 21 It should be noted, however, that proteolytic degradation mechanisms may differ between yeast and COS-1 cells. In the present study, reference NAT2*4 and NAT2 alleles possessing one of twelve SNPs in the NAT2 coding region were cloned and expressed in yeast. The recombinant human NAT2 allozymes were tested for their capacity to activate (via O-acetylation) N-OH-MeIQx and N-OH-PhIP. Seven [C190T (R64W), G191A (R64Q), T341C (I114T), A434C (E145P), G590A (R197Q), A845C (K282T) and G857A (G286E)] non-synonymous SNPs reduced O-acetyltransferase activity towards N-OH-MeIQx whereas one [A803G (K268R)] did not. None of the synonymous SNPs [T111C, C282T, C481T, or C759T] modified O-acetyltransferase activity towards either substrate. The results were not normalized to NAT2-specific protein. Thus, the reductions in O-acetyltransferase activity caused by various SNPs are likely due to the previously reported reductions in NAT2 protein 11,12. Indeed, the differences observed between SNPs in the magnitude of the reductions in O-acetylation activity paralleled the differential effects of the SNPs on N-acetyltransferase activity reported previously (Table 1). This finding probably reflects the observation that the effects of these SNPs on reducing catalytic activity are often secondary to reducing NAT2 protein levels (Table 1).
Table 1.
Effects of NAT2 SNPs
| SNP | Amino Acid Change | N-acetyltransferase Activity | O-acetyltransferase Activity | mRNA Level | Protein Level | Thermostability | ||
|---|---|---|---|---|---|---|---|---|
| Sulfamethazine | 2-Aminofluorene | N-OH-MeIQx | N-OH-PhIP | |||||
| T111C | None | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| C282T | None | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| C481T | None | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| C759T | None | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| A803G |
K268R |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
| C190T | R64W | + | + | + | + | +++ | + | + |
| G191A |
R64Q |
+ |
+ |
+ |
++ |
+++ |
++ |
+ |
| T341C | I114T | + | + | + | + | +++ | + | +++ |
| A434C |
E145P |
+ |
+ |
+ |
+ |
+++ |
+ |
+++ |
| G590A |
R197Q |
++ |
++ |
++ |
++ |
+++ |
+ |
+ |
| A845C | K282T | +++ | ++ | ++ | ++ | +++ | +++ | + |
| G857A | G286E | +++ | ++ | ++ | +++ | +++ | +++ | + |
Differences between substrates were observed since G857A (G286E) significantly reduced activity for the O-acetylation of N-OH-MeIQx (53%) but not for N-OH-PhIP (16%). This is consistent with previous findings where G857A (G286E) reduced N-acetyltransferase activity towards 2-aminofluorene but not sulfamethazine.11 The NAT2 7B allozyme possesses two SNPs the G857A (G286E) in combination with C282T.1 Previous studies have shown that NAT2 7B has a higher affinity than other NAT2 allozymes for sulfamethazine and dapsone, but not for 2-aminofluorene, p-anisidine, or isoniazid. 22 Interestingly, recombinant expression of selected human NAT2 variant alleles in Escherichia coli showed that the NAT2 7B allozyme catalyzed N-OH-MeIQx at rates lower than NAT2 4 whereas the opposite was observed for N-OH-PhIP. 8 Taken together, these results suggest that the G857A (G286E) SNP may alter affinity for some but not all N-hydroxy-heterocyclic amines, but further investigation in different types of recombinant expression systems is needed.
Recently, some controversy has arisen regarding the assignment of NAT2*12 and NAT2*13 as rapid acetylator alleles.23 NAT2*12 alleles clusters possess the signature A803G (K268R) SNP whereas the NAT2*13 allele possesses the synonymous C282T SNP.1 Previous studies have clearly showed that the A803G (K268R) SNP characteristic of NAT2*12 alleles and the C282T SNP characteristic of NAT2*13 alleles do not alter N-acetyltransferase catalytic activity 11,24,25 which is consistent with our current findings for O-acetylation catalytic activity towards N-OH-MeIQx and N-OH-PhIP. Our findings are also consistent with in vivo studies that have shown that NAT2*12 and NAT2*13 are associated with rapid acetylator phenotype. 26-28
In summary, we showed that the effects of SNPs in the NAT2 coding region on the metabolic activation of N-hydroxy-heterocyclic amines paralleled effects on protein and N-acetyltransferase activities. Differential effects among the SNPs and between substrates were observed suggesting that some SNPs may alter protein conformation and access to binding and/or catalytic sites on the NAT2 protein. Experiments to further define such changes are in progress in our laboratory and others. Further functional studies are needed to reduce the likelihood of genotype/phenotype misclassifications in future human epidemiological studies investigating the role of the NAT2 acetylation polymorphism in human disease.
Acknowledgements
This work was partially supported by United States Public Health Service Grant CA-34627 from the National Cancer Institute and a grant from the Kentucky Lung Cancer Research Program.
References
- 1.Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH, Devanaboyina US, Nangju NA, Feng Y. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- 2.Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995;16:39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- 3.Turesky RJ. Heterocyclic aromatic amine metabolism, DNA adduct formation, mutagenesis, and carcinogenesis. Drug Metab Rev. 2002;34:625–50. doi: 10.1081/dmr-120005665. [DOI] [PubMed] [Google Scholar]
- 4.Hein DW, Doll MA, Rustan TD, Gray K, Feng Y, Ferguson RJ, Grant DM. Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis. 1993;14:1633–8. doi: 10.1093/carcin/14.8.1633. [DOI] [PubMed] [Google Scholar]
- 5.Turesky RJ, Guengerich FP, Guillouzo A, Langouet S. Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P4501A2. Mutat Res. 2002;506−507:187–95. doi: 10.1016/s0027-5107(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 6.Hein DW. Acetylator genotype and arylamine-induced carcinogenesis. Biochim Biophys Acta. 1988;948:37–66. doi: 10.1016/0304-419x(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 7.Minchin RF, Reeves PT, Teitel CH, McManus ME, Mojarrabi B, Ilett KF, Kadlubar FF. N-acetylation and O-acetylation of aromatic and heterocyclic amine carcinogens by human monomorphic and polymorphic acetyltransferases expressed in COS-1 cells. Biochemical and Biophysical Research Communications. 1992;185:839–44. doi: 10.1016/0006-291x(92)91703-s. [DOI] [PubMed] [Google Scholar]
- 8.Hein DW, Rustan TD, Ferguson RJ, Doll MA, Gray K. Metabolic activation of aromatic and heterocyclic N-hydroxyarylamines by wild-type and mutant recombinant human NAT1 and NAT2 acetyltransferases. Arch Toxicol. 1994;68:129–33. doi: 10.1007/s002040050045. [DOI] [PubMed] [Google Scholar]
- 9.National Toxicology Program . Report on Carcinogens. Eleventh Edition U.S. Department of Health and Human Services, Public Health Service; 2005. [Google Scholar]
- 10.Hein DW, Grant DM, Sim E. Update on consensus arylamine N-acetyltransferase gene nomenclature. Pharmacogenetics. 2000;10:291–2. doi: 10.1097/00008571-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Fretland AJ, Leff MA, Doll MA, Hein DW. Functional characterization of human N-acetyltransferase 2 (NAT2) single nucleotide polymorphisms. Pharmacogenetics. 2001;11:207–15. doi: 10.1097/00008571-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Doll MA, Hein DW. Functional genomics of C190T single nucleotide polymorphism in human N-acetyltransferase 2. Biol Chem. 2002;383:983–7. doi: 10.1515/BC.2002.105. [DOI] [PubMed] [Google Scholar]
- 13.Lang NP, Butler MA, Massengill J, Lawson M, Stotts RC, Hauer-Jensen M, Kadlubar FF. Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol Biomarkers Prev. 1994;3:675–82. [PubMed] [Google Scholar]
- 14.Welfare MR, Cooper J, Bassendine MF, Daly AK. Relationship between acetylator status, smoking, and diet and colorectal cancer risk in the north-east of England. Carcinogenesis. 1997;18:1351–4. doi: 10.1093/carcin/18.7.1351. [DOI] [PubMed] [Google Scholar]
- 15.Le Marchand L, Hankin JH, Wilkens LR, Pierce LM, Franke A, Kolonel LN, Seifried A, Custer LJ, Chang W, Lum-Jones A, Donlon T. Combined effects of well-done red meat, smoking, and rapid N-acetyltransferase 2 and CYP1A2 phenotypes in increasing colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1259–66. [PubMed] [Google Scholar]
- 16.Kidd LR, Martin RCG, Moore JH, Hein DW. Genetic polymorphism of N-acetyltransferase genes as risk modifiers of colorectal cancer from consumption of well-done meat. In: Choi S-W, Friso S, editors. Nutrition-gene interactions in cancer. CRC Taylor&Francis Group; Coca Raton: 2006. pp. 189–212. [Google Scholar]
- 17.Deitz AC, Zheng W, Leff MA, Gross M, Wen WQ, Doll MA, Xiao GH, Folsom AR, Hein DW. N-Acetyltransferase-2 genetic polymorphism, well-done meat intake, and breast cancer risk among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:905–10. [PubMed] [Google Scholar]
- 18.Zhu J, Chang P, Bondy ML, Sahin AA, Singletary SE, Takahashi S, Shirai T, Li D. Detection of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine-DNA adducts in normal breast tissues and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:830–7. [PubMed] [Google Scholar]
- 19.Leff MA, Fretland AJ, Doll MA, Hein DW. Novel human N-acetyltransferase 2 alleles that differ in mechanism for slow acetylator phenotype. J Biol Chem. 1999;274:34519–22. doi: 10.1074/jbc.274.49.34519. [DOI] [PubMed] [Google Scholar]
- 20.Shen LX, Basilion JP, Stanton VP., Jr. Single-nucleotide polymorphisms can cause different structural folds of mRNA. Proc Natl Acad Sci U S A. 1999;96:7871–6. doi: 10.1073/pnas.96.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zang Y, Zhao S, Doll MA, States JC, Hein DW. The T341C (Ile114Thr) polymorphism of N-acetyltransferase 2 yields slow acetylator phenotype by enhanced protein degradation. Pharmacogenetics. 2004;14:717–23. doi: 10.1097/00008571-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hickman D, Palamanda JR, Unadkat JD, Sim E. Enzyme kinetic properties of human recombinant arylamine N-acetyltransferase 2 allotypic variants expressed in Escherichia coli. Biochem Pharmacol. 1995;50:697–703. doi: 10.1016/0006-2952(95)00182-y. [DOI] [PubMed] [Google Scholar]
- 23.Bolt HM, Selinski S, Dannappel D, Blaszkewicz M, Golka K. Re-investigation of the concordance of human NAT2 phenotypes and genotypes. Arch Toxicol. 2005;79:196–200. doi: 10.1007/s00204-004-0622-8. [DOI] [PubMed] [Google Scholar]
- 24.Hein DW, Ferguson RJ, Doll MA, Rustan TD, Gray K. Molecular genetics of human polymorphic N-acetyltransferase: enzymatic analysis of 15 recombinant wild-type, mutant, and chimeric NAT2 allozymes. Hum Mol Genet. 1994;3:729–34. doi: 10.1093/hmg/3.5.729. [DOI] [PubMed] [Google Scholar]
- 25.Hein DW, Doll MA, Rustan TD, Ferguson RJ. Metabolic activation of N-hydroxyarylamines and N-hydroxyarylamides by 16 recombinant human NAT2 allozymes: effects of 7 specific NAT2 nucleic acid substitutions. Cancer Res. 1995;55:3531–6. [PubMed] [Google Scholar]
- 26.Cascorbi I, Brockmoller J, Bauer S, Reum T, Roots I. NAT2*12A (803A-->G) codes for rapid arylamine N-acetylation in humans. Pharmacogenetics. 1996;6:257–9. doi: 10.1097/00008571-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Parkin DP, Vandenplas S, Botha FJ, Vandenplas ML, Seifart HI, van Helden PD, van der Walt BJ, Donald PR, van Jaarsveld PP. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am J Respir Crit Care Med. 1997;155:1717–22. doi: 10.1164/ajrccm.155.5.9154882. [DOI] [PubMed] [Google Scholar]
- 28.Hein DW. N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene. 2006;25:1649–58. doi: 10.1038/sj.onc.1209374. [DOI] [PMC free article] [PubMed] [Google Scholar]