Abstract
Background
Season of birth has been associated with the development of atopy and asthma. Relationships among a particular birth season, maternal allergen exposure during the birth season, and childhood development of allergies to allergens in higher concentration during the birth season may be important.
Objective
To investigate the effects of winter birth (January 1 to March 31) and prenatal cockroach and mouse allergens in settled dust on indoor allergen–specific cord blood mononuclear cell (CBMC) proliferation, TH2 production, and cord blood IgE concentration.
Methods
As part of an ongoing prospective study, 350 cord blood samples were collected. The CBMCs were cultured with cockroach, dust mite, and mouse protein extracts, and proliferation was measured. Interleukin 5, interferon-γ, and total IgE levels were measured. Home dust samples were analyzed for cockroach and mouse allergens.
Results
An isolated association was observed between winter birth and a greater mean (SD) cockroach interleukin 5 ratio (winter vs nonwinter birth: 26,043 [11,403] vs 11,344 [3,701]; P = .02). Other associations between winter birth and increased CBMC proliferation, T-helper cytokines, or cord blood IgE levels were not detected. Higher mouse allergen levels were associated with decreased mouse-induced proliferation (winter vs nonwinter birth: mean [SD] stimulation index, 1.72 [0.12] vs 2.02 [0.11]; P = 04).
Conclusions
Winter birth and increased cockroach or mouse allergen levels during pregnancy were not consistently abssociated with greater CBMC proliferation, T-helper cytokine production, or cord blood IgE levels. Greater indoor allergen exposure during pregnancy does not seem to affect the development of cockroach or mouse immune responses in utero.
INTRODUCTION
The prenatal period may represent a time when the impact of inhaled environmental agents on respiratory outcomes in the offspring is heightened.1–3 Season of birth also has been associated with subsequent development of atopy and asthma in multiple studies.4–7 In particular, the autumn and winter birth seasons have been associated with atopic dermatitis, food allergy, and asthma in children followed up from birth to the age of 15 years.4,5 However, studies examining the relationship between a specific season of birth and childhood sensitization to allergens found in higher concentrations during later pregnancy are limited. One study7 found that winter birth was associated with positive cockroach skin test results in asthmatic children at the age of 5 years. The authors suggested that pregnant mothers spend more time indoors during the winter, leading to increased prenatal cockroach exposure and heightened risk for subsequent cockroach-specific allergic immune responses.
One of the difficulties with determining whether season of birth may be causally related to an increased subsequent risk of seasonal allergies is that mechanistic and biomarker data linking birth month to later atopy are limited. However, one study8 found that gestation beyond 22 weeks during the spring was associated with increased birch-specific cord blood mononuclear cell (CBMC) proliferation compared with later pregnancy. The authors suggested that inhalation of greater levels of spring birch pollen during later pregnancy was associated with induction of birch-specific T-cell proliferative immune responses at birth. In addition, a German study9 found that cord blood obtained from mothers who were in their first 6 months of pregnancy during the birch pollen season were more likely to exhibit positive birch allergen–specific CBMC proliferation responses. They also found that cord blood obtained from mothers who were in the first 6 months of pregnancy during the summer grass pollen season were more likely to exhibit positive grass-specific CBMC proliferation responses.9 Studies focusing on the association of prenatal exposure to indoor allergens with potentially related biomarkers have been more limited. One such study10 found no association between measured levels of dust mite allergen in the mother’s bed during pregnancy and dust mite–induced CBMC proliferation.
Growing evidence suggests that exposure and sensitization to cockroach and mouse allergens are important to the pathogenesis of inner-city asthma.11,12 Hence, in evaluating the relationships among birth during a specific season, measured prenatal residential indoor allergen levels, and allergen-specific cord blood immune responses in an inner-city cohort, we hypothesized that (1) being born in winter is associated with indoor allergen–specific CBMC proliferation and proallergic TH2 cytokine production and (2) increased cockroach or mouse prenatal allergen exposures are associated with cockroach- or mouse-specific CBMC proliferation and TH2 cytokine production. Preliminary analysis of a smaller subset of this cohort did not find a correlation between prenatal levels of cockroach and mouse allergens in home dust and cockroach- or mouse-induced CBMC proliferation.2 The present analysis involves a larger sample and a more extensive statistical model.
MATERIALS AND METHODS
Women were recruited during pregnancy from clinics affiliated with New York Presbyterian Hospital (Columbia campus) as part of an ongoing longitudinal birth cohort study conducted in Northern Manhattan and the South Bronx, where rates of urban asthma are high,13 under the auspices of the Columbia Center for Children’s Environmental Health, as described elsewhere.2,3,14 Nonsmoking pregnant women aged 18 to 35 years who self-identified as African American or Dominican were enrolled between January 15, 1998, and July 28, 2006. The exclusion criteria included a diagnosis of diabetes mellitus or human immunodeficiency virus infection, secondary cigarette exposure during pregnancy, and residence in New York City for less than 1 year before pregnancy.
A prenatal questionnaire was administered during recruitment to evaluate maternal history of asthma, demographics, environmental exposures, and activities during pregnancy. The sample for the analyses consisted of cord blood samples from 350 women. A few women (n = 7) reported moving during pregnancy. Their data were included in the analyses except when examining seasonal variation in allergen levels. This study was approved by the Columbia University institutional review board, and written informed consent was obtained from all the participants.
Measurement of Indoor Allergens
Most residential dust samples (n = 273) were collected during pregnancy, and a few (n = 28) were collected postpartum. Previous work from our group14 has shown high correlations between levels measured at both time points (prenatally and postpartum) in participants who did not move. Dust samples were vacuumed separately from the kitchen and the mother’s bed as described elsewhere.14,15 Mouse urinary protein was assayed using a competitive enzyme-linked immunosorbent assay (ELISA).14 Dust mite (Der f 1 and Der p 1) and German cockroach (Bla g 2) antigens were assayed by means of ELISA (Indoor Biotechnologies, Charlottesville, Virginia).16
Blood Collection, CBMC Proliferation, and Cytokine Assays
Maternal and cord blood samples were collected after delivery. The CBMCs were separated by means of density centrifugation and were plated in triplicate for proliferation assays and in duplicate for cytokine assays, as described elsewhere.2 Increased proliferation in response to antigen was defined as (1) a stimulation index (averaged counts per minute in the presence of antigen divided by averaged counts per minute without antigen) greater than 2 and (2) antigen-induced averaged counts per minute greater than 1,000 above background. Cytokines were analyzed in duplicate using ELISA kits specific for interleukin 5 (IL-5) and interferon-γ (IFN-γ) (Immunotech, Marseille, France). Cytokine responses were measured using the following cytokine ratio: (measured response to antigen)/(measured response to background conditions). Maternal and cord blood total serum IgE levels were measured in duplicate by means of immunoradiometric assay (n = 275) (Total IgE IRMA; Diagnostics Products Corp, Los Angeles, California) except for 32 cord blood samples that were measured by means of ImmunoCAP (Phadia, Uppsala, Sweden). Cord blood serum was not obtained in 43 cases.
Statistical Analysis
Winter births were defined as those occurring between January 1 and March 31. The dates were chosen to include women who were in their third trimester of pregnancy during the winter. Allergen exposures in the bed and kitchen were analyzed in separate models as continuous or categorical variables. The data were natural log transformed when necessary to more closely approximate a normal distribution. Nonparametric tests (Wilcoxon rank sum, Kruskal-Wallis, and Spearman correlation coefficient) also were run to ensure that the results were not distorted by the failure of a variable to fulfill the distribution requirements of the parametric test. Statistical significance was defined as a 2-tailed P < .05.
For comparing 2 categorical variables, the Pearson χ2 statistic was used. When 1 or more cells in a table had an expected cell count of less than 5, the Fisher exact test was used. To compare the mean of a continuous variable across categories of a second variable, the t test or analysis of variance (ANOVA) was used (eg, to examine the seasonal variation of cockroach and mouse dust levels in the home). To examine the association of 2 or more continuous variables, linear regression and the Pearson correlation coefficient were used. Multiple linear regression and interaction terms were used to examine the possible synergistic effect of winter birth and allergen levels after controlling for the possible confounders of maternal asthma, maternal IgE level (elevated defined as total IgE level ≥100 IU/mL), and ethnicity.
RESULTS
This cohort of mothers is predominantly Dominican (64.3%). The participants are primarily of lower socioeconomic status, with only 15.4% having a high school education and most receiving Medicaid health insurance. Fifteen percent of the cohort mothers report a previous physician diagnosis of asthma (Table 1).
Table 1.
Demographic Characteristics of a Cohort of 350 Mothers
| Characteristic | Mothers, No. (%) |
|---|---|
| Ethnicity | |
| Dominican | 225 (64.3) |
| African American | 125 (35.7) |
| History of asthma | 52 (14.9) |
| Child’s sex | |
| Male | 166 (47.4) |
| Female | 184 (52.6) |
| High school education | 54 (15.4) |
| Currently receiving public assistance | 142 (40.6) |
| Currently receiving Medicaid | 312 (89.1) |
Winter Birth and CBMC Proliferation and Cytokine Production
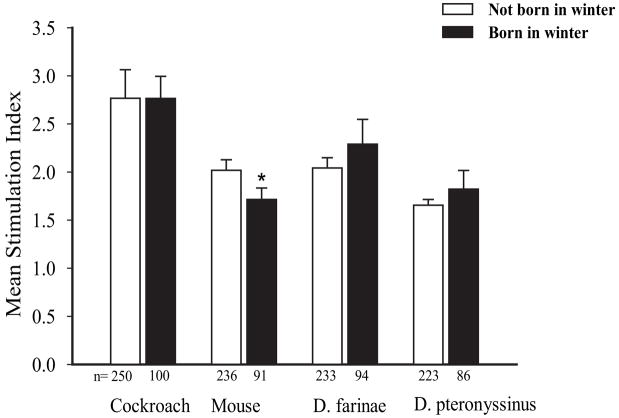
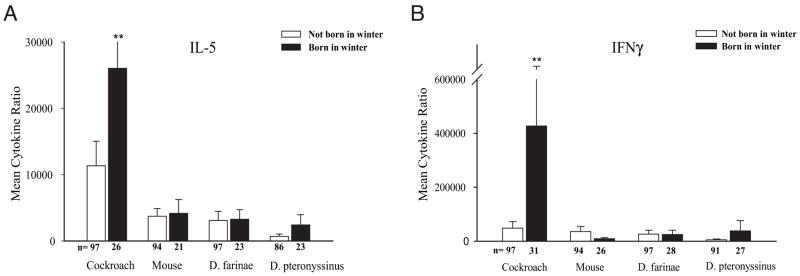
To determine whether winter birth is associated with indoor allergen–specific CBMC proliferation, the stimulation index in response to indoor allergens was compared between cord blood collected during the winter vs other times of the year. A small but significant negative association between winter birth and mouse-specific CBMC proliferation was observed (winter vs nonwinter birth: mean [SD] stimulation index, 1.72 [0.12] vs 2.02 [0.11]; P = .04, t test on log-transformed data) (Fig 1). The finding was confirmed using a multiple linear regression model that controlled for maternal asthma, maternal IgE level, and ethnicity (β = .160, P = .03). However, winter birth was not associated with any other changes in cockroach-, mouse-, or dust mite–specific CBMC proliferation. In addition, a single significant association between winter birth and greater cockroach-specific (but not mouse-or dust mite–specific) IL-5 production was seen by analyzing the log-transformed data (winter vs nonwinter birth: IL-5 mean [SD] cockroach ratio, 26,043 [11,403] vs 11,344 [3,701]; P = .02) (Fig 2A). Winter birth was not significantly associated with altered cockroach-, mouse-, or dust mite–induced IFN-γ production (Fig 2B). Also, we found no association between winter birth and total cord blood IgE levels (data not shown). Again, stratification by sex, maternal IgE level greater than 100 IU/mL or 100 IU/mL or less, ethnicity, and maternal history of asthma did not alter the significance of any of the findings.
Figure 1.
Winter birth and indoor allergen–specific cord blood mononuclear cell proliferation. Sample sizes per group are provided below the bars. Error bars represent SE. *P < .05, t test on log-transformed data.
Figure 2.
Winter birth and indoor allergen–specific interleukin 5 (A) and interferon-γ (B) production. The cytokine ratio is defined as (measured response to antigen)/(measured response to background conditions). Sample sizes per group are provided below the bars. Error bars represent SE. **The SE for the cytokine ratio is larger than the scale provided (SE = 427,327 [285,187]).
Cockroach and Mouse Allergen Exposure, CBMC Proliferation, and Cytokine Production
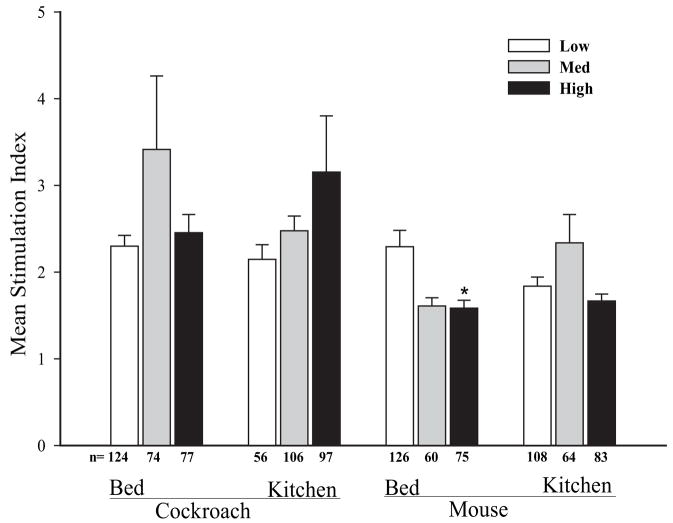
In light of seasonal variations in residential cockroach levels reported in the Boston area,17 this study replicated the earlier findings from Chew et al14 using a more complete subset of samples. Significant seasonal differences in cockroach and mouse allergen dust levels in participants’ homes were not detected by means of ANOVA (data not shown). We observed an association between high prenatal levels of mouse allergen in the bed (>1.85 μg/g of dust) and decreased mouse proliferation using ANOVA (low vs medium vs high: mean [SD] stimulation index, 2.29 [2.12] vs 1.61 [0.74] vs 1.58 [0.79]; P = .002) (Fig 3). However, higher levels of cockroach allergen levels in bed and kitchen dust during pregnancy were not associated with altered cockroach-induced CBMC proliferation (Fig 3). Stratification by sex, maternal IgE level greater than 100 IU/mL or 100 IU/mL or less, ethnicity, and maternal history of asthma did not alter the main results.
Figure 3.
Prenatal allergen levels in the bed and kitchen and allergen-specific cord blood mononuclear cell proliferation. Tertile levels of allergen exposure in the bed and kitchen are classified as follows: bed—cockroach: low, <0.03 μg/g (n = 124); medium, 0.03 to 0.14 μg/g (n = 74); and high, >0.14 μg/g (n = 77); mouse: low, <0.5 μg/g (n = 126); medium, 0.5 to 1.85 μg/g (n = 60); and high, >1.85 μg/g (n = 75); kitchen—cockroach: low, <0.04 μg/g (n = 56); medium, 0.04 to 1.48 μg/g (n = 106); and high, >1.48 μg/g (n = 97); mouse: low, <1 μg/g (n = 108); medium, 1 to 12 μg/g (n = 64); and high, >12 μg/g (n = 83). Sample sizes per group are provided below the bars. Error bars represent SE. *P < .05 (analysis of variance).
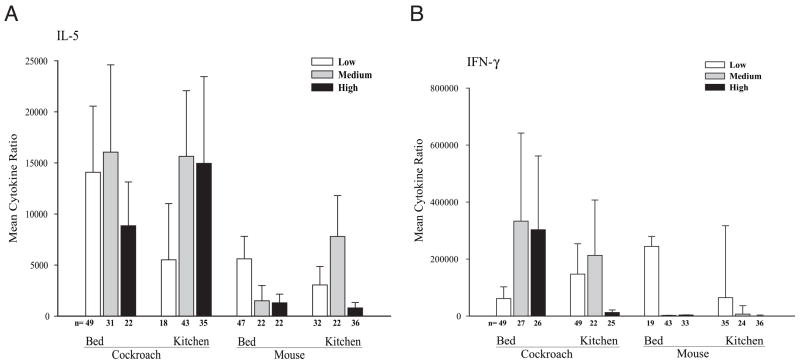
Associations between prenatal mouse allergen exposure in the kitchen or bed and between prenatal cockroach levels in the kitchen or bed and cord blood IgE levels were not found. Multivariate testing did not reveal any additional significant associations, even after controlling for maternal asthma, maternal IgE levels, and ethnicity. Furthermore, cockroach and mouse allergen levels (kitchen or bed) measured during pregnancy were not associated with altered cockroach and mouse IL-5 and IFN-γ production as evaluated by means of ANOVA (Fig 4). Again, stratification by sex, maternal IgE level greater than 100 IU/mL or 100 IU/mL or less, ethnicity, and maternal history of asthma and multivariate analyses did not alter these findings.
Figure 4.
Prenatal allergen dust levels and indoor allergen–specific interleukin 5 (A) and interferon γ (B) production. Tertile levels of allergen exposure in the bed and kitchen for interleukin 5 are classified as follows: bed—cockroach: low, <0.03 μg/g (n = 49); medium, 0.03 to 0.14 μg/g (n = 31); and high, >0.14 μg/g (n = 22); mouse: low, <0.5 μg/g (n = 47); medium, 0.5 to 1.85 μg/g (n = 22); and high, >1.85 μg/g (n = 22); kitchen—cockroach: low, <0.04 μg/g (n = 18); medium, 0.04 to 1.48 μg/g (n = 43); and high, >1.48; μg/g (n = 35); mouse: low, <1 μg/g (n = 32); medium, 1 to 12 μg/g (n = 22); and high, >12 μg/g (n = 36). Tertile levels of allergen exposure in the bed and kitchen for interferon γ are classified as follows: bed—cockroach: low, <0.03 μg/g (n = 49); medium, 0.03 to 0.14 μg/g (n = 27); and high, >0.14 μg/g (n = 26); mouse: low, <0.5 μg/g (n = 49), medium, 0.5 to 1.85 μg/g (n = 22); and high, >1.85 μg/g (n = 25); kitchen—cockroach: low, <0.04 μg/g (n = 19); medium, 0.04 to 1.48 μg/g (n = 43); and high, >1.48 μg/g (n = 33); mouse: low, <1 μg/g (n = 35); medium, 1 to 12 μg/g (n = 24); and high, >12 μg/g (n = 36). Error bars represent SE.
Finally, in exploratory models assessing interactions between winter birth and indoor allergen levels, a borderline negative interaction between high prenatal mouse allergen exposure in the bed and winter birth on mouse-specific CBMC proliferation was observed (β = −.101, P = .05), even when controlling for maternal asthma, maternal IgE level, and ethnicity in the models. Additional interactions between winter birth and indoor allergen levels on CBMC proliferation, cytokine production, and IgE levels were not found.
DISCUSSION
There is increasing evidence that development of the fetal immune system can be affected by environmental exposures in utero.1,2,18 Since the 1970s, studies19,20 have addressed the question of whether birth season, and, hence, prenatal allergen exposure, can affect the risk of subsequent atopy. Later studies6,9,21 focused on exposure to specific seasonal allergens (eg, birch pollen), house dust mite, animal dander, and food allergens (eg, cow’s milk) during infancy and compared the month of birth with the development of allergies later in life. Few studies have prospectively measured prenatal indoor allergen levels or intermediate biomarkers, such as allergen-specific CBMC proliferation, and compared these with atopy risk later in life.
In the present inner-city cohort study, several findings reached statistical significance, including the associations between winter birth and higher cockroach antigen–induced IL-5 production, between winter birth and decreased mouse-induced CBMC proliferation, and between prenatal mouse allergen levels in the bed and decreased mouse-induced CBMC proliferation. A possible interaction effect between winter birth and mouse allergen levels on decreased mouse-induced CBMC proliferation was also identified. The first finding raises the possibility that winter birth may predispose the fetal immune system to cockroach-induced allergic immune responses specifically. The latter findings raise the possibility that winter birth or prenatal indoor mouse allergen exposure may predispose the fetal immune system to protection from the development of mouse-specific memory T-cell immune responses. Associations between early pet allergen exposure and protection from allergic sensitization have been reported elsewhere.22,23
However, neither winter birth nor higher levels of cockroach and mouse allergens in home dust during pregnancy were associated consistently with altered cockroach- or mouse-specific cord blood immune responses. There are several possible explanations for the absence of strong associations between either winter birth or prenatal cockroach or mouse exposure (evaluated by settled dust measurements) and the cord blood immune responses reported herein. These findings may suggest that the intensity of prenatal allergen exposure does not affect the degree of allergen-specific CBMC proliferation or cytokine production. For example, a Manchester study10 showed no association between CBMC responses in high-risk infants after stimulation with dust mite and measured levels of dust mite in the mother’s bed during pregnancy. Alternatively, allergen-specific CBMC proliferation, cytokine production, and IgE levels may not predict the effect of birth season on atopy. The present results complement work by Scirica and colleagues24 in which season of birth in a Boston cohort did not correlate with cord blood IgE levels, even after correcting for other potential predictors (type of delivery, maternal parity, and gestational age).
Another explanation may involve the effect of seasonal variation on indoor allergen levels. For example, cockroach allergen levels in the home were twice as high in the summer (June and August) than in the winter in Boston,17 raising the possibility that summer birth would be more likely to predispose the fetal immune system to cockroach-related allergic immune responses. However, indoor allergen levels did not vary significantly by season in the present mothers’ homes, thereby supporting the premise that actual indoor allergen exposure experienced by the mother is likely to be greater during colder months, when individuals tend to spend more time indoors. Also, the mother may spend more time at home during the later stages of pregnancy, hence reducing the variability in seasonal exposure to indoor allergens.
A large body of work has examined the utility of cord blood biomarkers, including IgE levels and CBMC proliferation assays, for predicting subsequent atopy. The predictive value of cord blood IgE levels for later atopy has been suggested in some,25,26 but not other,27–29 research studies. Proliferation of CBMCs after in vitro stimulation with allergens has been associated with increased risk of food allergy, asthma, and atopic dermatitis.2,30–32 For example, children with higher levels of cockroach (Bla g 2)-induced IFN-γ produced by CBMCs at birth had a significantly lower risk of acute lower respiratory illness.33 In contrast, another recent prospective study34 found that dust mite–specific CBMC responses were not associated with the later development of atopy (positive skin prick test and IgE responses) at the age of 2 years. Rather, the postnatal dust mite specific IgE levels were associated with TH2 cytokine responses at the age of 2 years.34 Other studies6,35 also suggest that early postnatal exposure during infancy, rather than in utero exposure, may play a more critical role in later sensitization to allergens.
We acknowledge several limitations of this study. A single determination of antigen content in settled dust is only a surrogate for the actual amount of antigen inhaled into the pregnant mother’s lungs. It could be an unreliable measure of the biologically effective dose that reaches the fetus. Also, actual exposure to cockroach, dust mite, or mouse allergen may not be greater in the winter, and precise quantitation of the time the mother spent in the home, and near other potential sources of allergen exposure, was not obtained. However, the literature suggests that individuals, especially those living in urban environments, spend more time indoors during the winter.36,37 Furthermore, we did not control for all the potential covariates that theoretically could contribute to an effect of birth season, such as prenatal air pollution exposure, viral infections, and potential genetic susceptibility to atopy. In addition, the specificity of CBMC proliferation for indicating antigen-specific memory T-cell immune responses has been questioned because CBMCs can proliferate after in vitro stimulation with nonrecall antigens.38,39 Also, levels of CBMC proliferation may reflect cross-reactivity between dust mite allergens, specifically, purified dust mite proteins Der p 1 and Der f 1, which are contained in our protein extracts.40 Many analyses may have led to spurious findings or a type I error. Finally, owing to sample size, sufficient power was not available to rigorously test the interaction between winter birth and indoor allergen exposure.
In conclusion, an association between winter birth and greater cockroach IL-5 production was observed. In addition, prenatal mouse exposure may predispose to decreased mouse-specific CBMC proliferation. However, in general, greater indoor allergen exposure (evaluated by winter season and allergen levels in home dust) during pregnancy does not seem to significantly affect the development of cockroach and mouse allergic immune responses in utero. Further prospective follow-up of the Columbia Center for Children’s Environmental Health birth cohort will help determine whether cockroach-, dust mite–, or mouse-induced proliferation or cytokine production at birth may be associated with heightened risk of atopy or asthma in the children as they age.
Acknowledgments
Funding Sources: This study was supported by grants P01 ES09600, 5 RO1 ES08977, and P30 ES009089 from the National Institute of Environmental Health Sciences; grants R827027 and RD-832141 from the US Environmental Protection Agency; Bauman Family Foundation; Gladys & Roland Harriman Foundation; New York Community Trust; Educational Foundation of America; The New York Times Company Foundation; The Johnson Family Foundation; Horace W. Goldsmith Foundation; The John Merck Fund; V. Kann Rasmussen Foundation; Marisla Foundation; and Trustees of the Blanchette Hooker Rockefeller Fund.
We thank Adnan Divjan for laboratory analysis, Lori Hoepner for database analysis, Robin Garfinkel for statistical work, and Darrell Holmes for collecting study samples.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. J Allergy Clin Immunol. 2000;105:S493–S498. doi: 10.1016/s0091-6749(00)90049-6. [DOI] [PubMed] [Google Scholar]
- 2.Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med. 2001;164:995–1001. doi: 10.1164/ajrccm.164.6.2011107. [DOI] [PubMed] [Google Scholar]
- 3.Miller RL, Garfinkel R, Horton M, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126:1071–1088. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazala E, Ron-Feldman V, Alterman M, Kama S, Novack L. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41:1125–1128. doi: 10.1002/ppul.20442. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson L, Björkstén B, Hattevig G, Kjellman B, Sigurs N, Kjellman NIM. Season of birth as predictor of atopic manifestations. Arch Dis Child. 1997;76:341–344. doi: 10.1136/adc.76.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björkstén F, Suoniemi I. Time and intensity of first pollen contacts and risk of subsequent pollen allergies. Acta Med Scand. 1981;209:299–303. doi: 10.1111/j.0954-6820.1981.tb11595.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarpong SB, Karrison T. Season of birth and cockroach allergen sensitization in children with asthma. J Allergy Clin Immunol. 1998;101:566–568. doi: 10.1016/S0091-6749(98)70369-0. [DOI] [PubMed] [Google Scholar]
- 8.Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol. 1996;7:109–116. doi: 10.1111/j.1399-3038.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Duren-Schmidt KV, Pichler J, Ebner C, et al. Prenatal contact with inhalant allergens. Pediatr Res. 1997;41:128–131. doi: 10.1203/00006450-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Smillie FI, Elderfield AJ, Patel F, et al. Lymphoproliferative responses in cord blood and at one year: no evidence for the effect of in utero exposure to dust mite allergens. Clin Exp Allergy. 2001;31:1194–1204. doi: 10.1046/j.1365-2222.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 12.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen, I: the prevalence of mouse allergen in inner-city homes: the National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–1074. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 13.Perera FP, Illman SM, Kinney PL, et al. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110:197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew GL, Perzanowski MS, Miller RL, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111:1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strien RTV, Verhoeff AP, Wijnen JHV, Doekes G, Meer GEAD, Brunekreef B. Der p 1 concentrations in mattress surface and floor dust collected from infants’ bedrooms. Clin Exp Allergy. 1995;25:1184–1189. doi: 10.1111/j.1365-2222.1995.tb03042.x. [DOI] [PubMed] [Google Scholar]
- 16.Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol. 1991;87:505–510. doi: 10.1016/0091-6749(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 17.Chew GL, Higgins KM, Gold DR, Muilenberg ML, Burge HA. Monthly measurements of indoor allergens and the influence of housing type in a northeastern US city. Allergy. 1999;10:1058–1066. doi: 10.1034/j.1398-9995.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi D, Wang C, Mao X, Lendor C, Rothman PB, Miller RL. Antigen-specific immune responses to influenza vaccine in utero. J Clin Invest. 2007;117:1637–1646. doi: 10.1172/JCI29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson DJ, Freed DLJ, Taylor G. Respiratory allergy and month of birth. Clin Allergy. 1977;7:29–33. doi: 10.1111/j.1365-2222.1977.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 20.Morrison-Smith JSV. Atopic disease and month of birth. Clin Allergy. 1979;9:153–157. doi: 10.1111/j.1365-2222.1979.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 21.Aalberse RC, Nieuwenhuys EJ, Hey M, Stapel SO. Horoscope effect” not only for seasonal but also for non-seasonal allergens. Clin Exp Allergy. 1992;22:1003–1006. doi: 10.1111/j.1365-2222.1992.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 22.Ownby D, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 and 7 years of age. JAMA. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 23.Perzanowski MS, Ronmark E, Platts-Mills TA, Lundback B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med. 2002;166:696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 24.Scirica CV, Gold DR, Ryan L, et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007;119:81–88. doi: 10.1016/j.jaci.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Businco L, Marchetti F, Pellegrini G, Perlini R. Predictive value of cord blood IgE levels in “at-risk” newborn babies and influence of type of feeding. Clin Allergy. 1983;13:503–508. doi: 10.1111/j.1365-2222.1983.tb02631.x. [DOI] [PubMed] [Google Scholar]
- 26.Michel FB, Bousquet J, Greillier P, Robinet-Levy M, Coulomb Y. Comparison of cord blood immunoglobulin E concentrations and maternal allergy for the prediction of atopic diseases in infancy. J Allergy Clin Immunol. 1980;65:422–430. doi: 10.1016/0091-6749(80)90234-1. [DOI] [PubMed] [Google Scholar]
- 27.Lilja G, Oman H. Prediction of atopic disease in infancy by determination of immunological parameters: IgE, IgE- and IgG-antibodies to food allergens, skin prick tests and T-lymphocyte subsets. Pediatr Allergy Immunol. 1991;2:6–13. [Google Scholar]
- 28.Bergmann RL, Edenharter G, Bergmann KE, et al. Predictability of early atopy by cord blood-IgE and parental history. Clin Exp Allergy. 1997;27:752–760. [PubMed] [Google Scholar]
- 29.Ruiz RG, Richards D, Kemeny DM, Price JF. Neonatal IgE: a poor screen for atopic disease. Clin Exp Allergy. 1991;21:467–472. doi: 10.1111/j.1365-2222.1991.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 30.Kondo N, Kobayashi Y, Shinoda S, et al. Cord blood lymphocyte responses to food antigens for the prediction of allergic disorders. Arch Dis Child. 1992;67:1003–1007. doi: 10.1136/adc.67.8.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Kondo N, Shinoda S, Agata H, Fukutomi O, Orii T. Predictive values of cord blood IgE and cord blood lymphocyte responses to food antigens in allergic disorders during infancy. J Allergy Clin Immunol. 1994;94:907–916. doi: 10.1016/0091-6749(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 32.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- 33.Ly NP, Rifas-Shiman SL, Litonjua AA, et al. Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics. 2007;119:e171–e178. doi: 10.1542/peds.2006-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe J, Kusel M, Holt BJ, et al. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J Allergy Clin Immunol. 2007;119:1164–1173. doi: 10.1016/j.jaci.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Björkstén F, Suoniemi I, Koski V. Neonatal birch-pollen contact and subsequent allergy to birch pollen. Clin Allergy. 1980;10:585–591. doi: 10.1111/j.1365-2222.1980.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 36.Robinson JP, Converse PE, Szalai A. Everyday life in twelve countries. In: Szalai A, editor. The Use of Time: Daily Activities of Urban and Suburban Populations in Twelve Countries. The Hague, the Netherlands: Mouton; 1972. p. xii + 868. [Google Scholar]
- 37.Klepeis NE, Nelson WC, William C, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 38.Ramage JM, Young JL, Goodall JC, Gaston JSH. T cell responses to heat-shock protein 60: differential responses by CD4+ T cell subsets according to their expression of CD45 isotypes. J Immunol. 1999;162:704–710. [PubMed] [Google Scholar]
- 39.Young JL, Daser A, Beverley PC. In vitro proliferative responses of human peripheral blood mononuclear cells to non-recall antigens. J Immunol Methods. 1995;182:177–184. doi: 10.1016/0022-1759(95)00046-d. [DOI] [PubMed] [Google Scholar]
- 40.Heymann PW, Chapman MD, Platts-Mills TA. Antigen Der f 1 from the dust mite Dermatophagoides farinae: structural comparison with Der p 1 from Dermatophagoides pteronyssinus and epitope specificity of murine IgG and human IgE antibodies. J Immunol. 1986;137:2841–2847. [PubMed] [Google Scholar]