Summary
Decreased tissue levels of docosahexaenoic acid (DHA; 22:6n-3) are implicated in the etiologies of non-puerperal and postpartum depression. With the aim of determining neurobiological sequelae of decreased brain DHA content, this study examined the effects of a loss of brain DHA content and concurrent reproductive status in adult female Long-Evans rats. An α-linolenic acid-deficient diet and breeding protocols were used to produce virgin and parous female rats with cortical phospholipid DHA levels 23–26% lower than virgin and parous rats fed a control diet containing adequate α-linolenic acid. Parous dams were tested/euthanized at weaning (postnatal day 20) of the second litter; virgin females, during diestrus. Decreased brain DHA was associated with decreased hippocampal BDNF gene expression and increased relative corticosterone response to an intense stressor, regardless of reproductive status. In virgin females with decreased brain DHA, serotonin content and turnover in frontal cortex were decreased compared to virgin females with normal brain DHA. In parous dams with decreased brain DHA, the density of 5-HT1A receptors in the hippocampus was increased, corticosterone response to an intense stressor was increased, and the latency to immobility in the forced swim test was decreased compared to parous dams with normal DHA. These findings demonstrate neurobiological alterations attributable to decreased brain DHA or an interaction of parous status and brain DHA level. Furthermore, the data are consistent with findings in depressed humans, and thus support a role for DHA as a factor in the etiologies of depressive illnesses, particularly postpartum depression.
Keywords: omega-3 polyunsaturated fatty acid, brain-derived neurotrophic factor, serotonin 1A receptor, forced swim, postpartum, corticosterone
Introduction
Depression, which occurs more commonly in women than men (Merikangas and Herrell, 2004), has a complex etiology that likely involves the interaction of genetic and environmental factors (Charney and Manji, 2004). Stressful life events have emerged as an important environmental factor associated with the disorder (Mazure and Maciejewski, 2003a, b, Heim et al., 2004). In addition, a highly consistent literature suggests that altered polyunsaturated fatty acid (PUFA) status, particularly low docosahexaenoic acid (DHA, 22:6n-3), may also represent a non-genetic factor in both non-puerperal and postpartum depression. Of note, postmortem DHA content of the orbitofrontal cortex was 22% lower in depressed patients than in controls, and was the only fatty acid found to be altered (McNamara et al., 2007a). Likewise, erythrocyte and adipose DHA levels were 33–36% lower in depressives than controls (Edwards et al., 1998, Peet et al., 1998, Frasure-Smith et al., 2004, Mamalakis et al., 2006) and were correlated with the severity of symptoms or attempted suicide (Adams et al., 1996, Edwards et al., 1998, Maes et al., 1999, Mamalakis et al., 2002, Huan et al., 2004, Sublette et al., 2006). Prevalence of depression was inversely related to fish consumption, a major dietary source of n-3 long chain PUFAs (Hibbeln, 1998, Tanskanen et al., 2001a, b, Timonen et al., 2004). Furthermore, treatment with n-3 PUFAs improved depressive symptoms in a majority of controlled clinical trials (Freeman et al., 2006b). Similar to non-puerperal depression, postpartum depression has been associated with decreased DHA levels, or decreased ratio of DHA to the n-6 long chain PUFA docosapentaenoic acid (DPA, 22:5n-6), in plasma or breast milk (Hibbeln, 2002, De Vriese et al., 2003, Otto et al., 2003, Miyake et al., 2006). Treatment with n-3 long chain PUFA supplements also reduced depressive symptoms in women with postpartum depression in a pilot study (Freeman et al., 2006a).
DHA, a long chain PUFA derived from the essential fatty acid α-linolenic acid (18:3n-3), is a component of membrane phospholipids. It is the most abundant PUFA in the brain, representing roughly 15% of total brain fatty acids (Sinclair, 1975). It accumulates in the brain primarily during pre-and neonatal development, and is supplied by the mother to the developing fetus in utero, and to the neonate via breast milk. (Clandinin et al., 1980a, b). DHA content influences the physicochemical properties of neuronal membranes, and thus modulates the function of membrane bound proteins, such as receptors and ion channels. DHA, and other long chain PUFAs such as arachidonic acid (20:4n-6), also serve as precursors for a variety of signaling molecules (e.g., prostaglandins) and activate transcription factors (Salem et al., 2001, Horrocks and Farooqui, 2004). Low availability of DHA results in compensatory substitution of DPA, a product derived from the n-6 essential fatty acid linoleic acid (18:2n-6), into the membrane phospholipids (Galli et al., 1971). We have shown that the content of brain phospholipids in virgin female rats can be reduced if the animals are fed an n-3 PUFA-deficient diet for an adequate period of time (Levant et al., 2006a). The loss of DHA from the brain is accelerated in reproducing females, presumably due to the physiological demands of pregnancy and lactation such that maternal brain DHA content can be decreased about 20% after gestation and nursing of one litter (Levant et al., 2006a), similar to the decrease observed in humans with major depression (McNamara et al., 2007a). Maximal changes in overall brain PUFA composition occur after two litters (Levant et al., 2006c).
Although much is known about the role of DHA in brain development (McNamara and Carlson, 2006), it is not known how neurobiology is altered when the normal adult brain experiences a loss of DHA or how the neurobiological sequelae of a reduction of brain DHA interact with the hormonal and other changes occurring during the postpartum period. To further assess the potential role of decreased brain DHA levels in non-puerperal and/or postpartum depression, this study used a rat model to examine the effects of a clinically-relevant decrease in brain phospholipid DHA content and its interactions with reproductive status (virgin or parous) on several neurobiological end points most consistently found to be altered in depression: hippocampal brain-derived neurotrophic factor (BDNF), the monoamine neurotransmitter systems, and the hypothalamic-pituitary-adrenal (HPA) axis (Garlow et al., 2004). The effects of brain DHA levels on behavior in the forced swim test, a rodent model for assessment of antidepressant efficacy, were also determined.
Methods
Animals
Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Adult, male and female Long-Evans rats (Harlan, Indianapolis, IN) were housed in a temperature-and humidity-controlled animal facility with a 12-h dark-light cycle (on at 06:00h), and given food and water ad libitum. Rats were obtained at least five days prior to any treatments and were handled regularly.
Experimental Design
A between-groups design was used to assess the effects of reproductive status (virgin or parous) and brain DHA content (normal or decreased). Brain DHA content was manipulated by feeding diets varying in n-3 PUFA content (see below).
Parous dams underwent two sequential reproductive cycles (gestation and nursing), with the initial mating occurring on postnatal day (P) 75–80. Dams were placed on the experimental diets at the time of the first mating. Litters were weighed and culled to eight pups on P1 (with preference for males, which were used in other experiments), and weaned on P20. The second mating occurred eight-10 days after weaning of the first litter. Parous dams were tested and/or euthanized on P20 after the second litter. This treatment produces maximal alterations in brain PUFA composition in dams fed the deficient diet (Levant et al., 2006a). Dams producing litters of fewer than eight pups in either litter were not used for the determination of neurobiological end points.
Virgin females with normal DHA were fed the control diet for 13 weeks, corresponding to the time required for two reproductive cycles, beginning at P75–80, based on our prior finding that brain PUFA composition does not vary in virgin females fed the control diet for periods of six weeks to six months (Levant et al., 2006a). Virgin females with decreased DHA were produced by feeding the deficient diet for six months, the time required to produce a decrease in brain DHA comparable to that observed in parous dams fed the deficient diet (Levant et al., 2006a), starting at P56–60, thus bracketing the treatment period for the other groups.
At the completion of the diet and breeding treatments (i.e., on P20 after the second litter) and between 1000 and 1100h, rats were weighed, and either euthanized by decapitation or subjected to the forced swim test and then euthanized. Accordingly, the maternal brain and behavior were examined at the end of the period of greatest offspring demand for DHA (i.e., weaning in the rat), which is similar in this respect to the postpartum period (one - three months after birth) in humans (Makrides et al., 1994, Green and Yavin, 1996), and thus parallels the clinical onset of postpartum depression (Miller, 2002). Virgin females were tested/euthanized during diestrus, determined by vaginal lavage performed at the same time each day (0900 – 1000h). Virgins were considered to be in diestrus on the day following estrus, and were not subjected to vaginal lavage on the day of testing/euthanasia. Trunk blood was collected, and brains were rapidly removed and frozen on dry ice. Brain regions of interest were isolated by freehand dissection on ice. All samples were stored at −70° C. Four separate cohorts of rats were used.
Experimental diets
The control diet was prepared from a purified basal mix (TD00235; Teklad, Indianapolis, IN) with pure unhydrogenated soybean oil (70 g/kg), and was identical in composition to Teklad AIN-93G, which meets all current nutrient standards for rat pregnancy and growth (Reeves et al., 1993). Accordingly, the control diet contained 4.20 g/kg α-linolenic acid (18:3n-3) and 33.81 g/kg linoleic acid (18:2n-6). The deficient diet was the same as the control diet, except it was prepared with safflower oil (66.5 g/kg) and soybean oil (3.5 g/kg), and thus contained 0.38 g/kg α-linolenic acid (18:3n-3) and 45.96 g/kg linoleic acid (18:2n-6).
Brain total phospholipid fatty acid composition
As previously described (Levant et al., 2006b), phospholipids were extracted from occipital cortex, a brain region not required for the determination of neurochemical end points, and isolated by thin layer chromatography. The band containing total phospholipids was removed and transmethylated with boron trifluoride methanol (Sigma, St. Louis, MO) to yield fatty acid methyl esters. Individual fatty acid methyl esters were separated on a Varian 3400 gas chromatograph with an SP-2330 capillary column (30 m, Supelco, Inc., Belfonte, PA), with helium used as the carrier gas. The resulting peaks were identified by comparison to authentic standards (PUFA 1 and 2; Supelco, Inc. and 22:5n-6, Nu-Chek Prep, Elysian, MN) and expressed as percent of total fatty acids on the basis of peak area.
Solution hybridization - nuclease protection assays for BDNF mRNAs
Total cellular RNAs were extracted from hippocampal tissue samples using a rapid guanidinium isothiocyanate phenol/chloroform method. The resultant total RNAs were analyzed for BDNF and β-actin mRNA levels by solution hybridization – nuclease protection assays using 32P-labeled antisense cRNA probes as previously described (McCarson and Krause, 1994, Duric and McCarson, 2005). Specific mRNA amounts were determined by comparison to cRNA quantitation standards. Data are reported as pg BDNF mRNA/ng β-actin mRNA.
BDNF peptide ELISA
BDNF peptide levels were determined using a Sandwich ELISA kit (Chemicon International/Millipore, Billerica, MA). Data are reported as ng BDNF/g tissue (original wet weight).
Receptor binding assays
The affinity and density of 5-HT1A receptors were assessed by Scatchard analysis using 4 concentrations of [propyl-2,3-ring-1,2,3-3H]8-OH-DPAT (Perkin Elmer Life Sciences; Boston, MA; 125 Ci/mmol; 0.2 – 5 nM) in an equilibrium, filtration assay according to a modification of the methods of Alper and Nelson (Alper and Nelson, 2000). The assay buffer was 50 mM Tris, pH 7.4 at 23° C. Nonspecific binding was defined in the presence of 10 µM 5-HT. Membrane protein concentrations were determined by the BCA method (Pierce, Rockford, IL) and were ~50 µg/tube. Specific binding is expressed as fmol/mg protein and analyzed for KD and Bmax using SigmaPlot (v. 8.0.2).
The affinity and density of 5-HT2A receptors were assessed using [ethylene-3H]ketanserin (Perkin Elmer Life Sciences; Boston, MA; 67 Ci/mmol; 0.06- 2.4 nM) according to a modification of the methods of Leysen et al. (Leysen et al., 1982). Assays were performed as described above, except the assay buffer was 50 mM Tris, 120 mM NaCl, 5 mM KCl, pH 7.4 at 23° C; and nonspecific binding was defined in the presence of 1 µM spiperone.
Measurement of monoamine neurotransmitters and metabolites
Levels of serotonin, 5-HIAA, norepinephrine, and dopamine were quantified using an isocratic HPLC-EC system (ESA Coulochem III, Chelmsford, MA) coupled to a Coulochem III dual-channel electrochemical array detector (ESA Inc., Chelmsford, MA; Model 5100A, E1 - 150 mV and E2 +275 mV using a 5011 dual analytical cell) according to a modification of published methods (Enna et al., 2006). Tissues were extracted in perchloric acid, diluted with mobile phase, and analytes separated using a C18 reverse phase column (ESA Inc., Chelmsford, MA, HR-80l, 4.6 mm × 80 mm, 3 µm particles) with a pH 4.1 citrate-acetate mobile phase containing 6.0% methanol and 0.35 mM 1-octane-sulfonic acid. The flow rate was 1.8 ml/min. The internal standard was 3,4-dihydroxybenzylamine. Protein concentration of the extracted tissue was determined by the BCA method (Pierce, Rockford, IL). Monoamine concentrations are expressed as ng/mg protein. Neurotransmitter turnover was calculated as the ratio of the metabolite to the neurotransmitter.
Forced swim test
The modified forced swim test was performed as previously described (Cryan et al., 2005). Rats were placed in a cylindrical water tank (45 cm tall × 19 cm) filled with 25°C tap water to a depth of 32 cm. Between 1000–1100h rats were placed in the tank for 15 min. Twenty-four h later, rats were subjected to a five-min test session. Cylinder water was changed after each rat. Video tapes of the test session were scored by an independent blind observer for time spent swimming (moving through the water), climbing (struggling at the sides of the tank), floating immobile (including small movements required to maintain floating), and latency to immobility. Immediately following the test session, rats were euthanized by decapitation and trunk blood collected for determination of corticosterone levels.
Corticosterone Assays
Serum corticosterone levels were determined in trunk blood collected from rats subjected to minimal stress, defined as normal husbandry and handling, or after the forced swim test using a rat corticosterone radioimmunoassay kit (Siemens/Diagnostic Products Corp., Los Angeles, CA).
Data analysis
Reproductive status and brain phospholipid DHA content are used as the descriptors for the independent variables to emphasize the focus of this study on the physiological condition, rather than the treatments used to produce those conditions. Data are expressed as the mean ± SEM. Data were analyzed for statistically significant effects by two- or three-way ANOVA with factors of brain DHA content, reproductive status, and where appropriate, stressor intensity (Systat, v.10.2). Outliers identified by Systat were excluded. Significant effects were further analyzed post-hoc using one-way ANOVA with all groups, followed by the Tukey-Kramer multiple comparisons test. Differences between groups were considered significant at P<0.05.
Results
Brain phospholipid fatty acid composition
In agreement with previous studies of whole brain (Levant et al., 2006a), treatment of virgin or parous rats with the deficient diet for 6 months or two sequential reproductive cycles, respectively, produced decreases in cortical DHA content of similar magnitude (23–26%), depending on treatment cohort, compared to virgin females fed the control diet in each of the four cohorts of rats used in these studies (P<0.01). The percentage of DHA in the cortex of parous dams fed the control diet was not different than virgin females fed the control diet. Representative data from one of the four cohorts of rats used in these experiments are shown in Table 1. The compensatory incorporation of DPA was 33% greater in parous dams with decreased DHA than in virgin females with decreased DHA. The percentage of arachidonic acid in cortical phospholipids was not altered by these treatments.
Table 1.
Effects of diet and breeding treatments on the percentage of docosahexaenoic acid (DHA, 22:6n-3), docosapentaenoic acid (DPA, 22:5n-6), and arachidonic acid (AA; 20:4n-6) in cortical phospholipids of female rats.
| Group | DHA | DPA | AA |
|---|---|---|---|
| (Percent of Total Fatty Acids) | |||
| Virgin – Normal DHA | 14.3 ± 0.67 | 0.34 ± 0.023 | 11.3 ± 0.27 |
| Virgin – Decreased DHA | 10.8 ± 0.54* | 1.8 ± 0.11* | 10.8 ± 0.34 |
| Parous – Normal DHA | 14.6 ± 0.65† | 0.45 ± 0.044† | 11.7 ± 0.52 |
| Parous – Decreased DHA | 10.7 ± 0.64*† | 2.4 ± 0.18*†‡ | 11.6 ± 0.25 |
Data are presented as the mean ± SEM (n = 8–9 per group) and are representative of those obtained from each of the four cohorts of rats used in these studies.
Different from Virgin – Normal DHA (P<0.01).
Different from Virgin-Decreased DHA (P<0.01).
Different from Parous-Normal DHA (P<0.01).
Parous dams underwent two sequential reproductive cycles (gestation and nursing), beginning at P75–80. Virgin females with normal DHA were fed the control diet for 13 weeks, corresponding to two reproductive cycles, beginning at P75–80. Virgin females with decreased DHA were produced by feeding the deficient diet for 6 months, starting at P56–60, thus bracketing the treatment period for the other groups.
Body weight and reproductive success
Two-way ANOVA indicated a significant main effect of reproductive status on body weight at the end of the diet and breeding treatments (F(1,55) = 6.48, P<0.05), with parous dams exhibiting 6% higher body weight than virgin females, but no main effect diet (Table 2). The number of pups per litter produced by the reproducing dams was not different between diet groups. All pups were healthy. There were no effects of diet on pup weight on P1 or at weaning on P20 (Figure 1).
Table 2.
Effects of diet and breeding treatments on body weight in female rats.
| Group | Weight (g) |
|---|---|
| Virgin – Normal DHA | 300 ± 7 |
| Virgin – Decreased DHA | 318 ± 6 |
| Parous – Normal DHA | 323 ± 6 |
| Parous – Decreased DHA | 334 ± 11* |
Data are presented as the mean ± SEM (n = 15 per group). Body weight was determined at the completion of the diet and breeding treatments immediately prior to euthanasia, and is representative of those obtained from each of the four cohorts of rats used in these studies.
Different from Virgin – Normal DHA (P<0.05).
Figure 1. Effects of diet in reproducing females on number of pups per litter (A), average pup weight on postnatal day 1 (B), and male pup weight at weaning on postnatal day 20 (C).
Data are presented as the mean ± SEM (n = 15 per group). Male weanling pups were each from a different litter. No significant effects of diet or litter number were detected by ANOVA.
Hippocampal BDNF expression
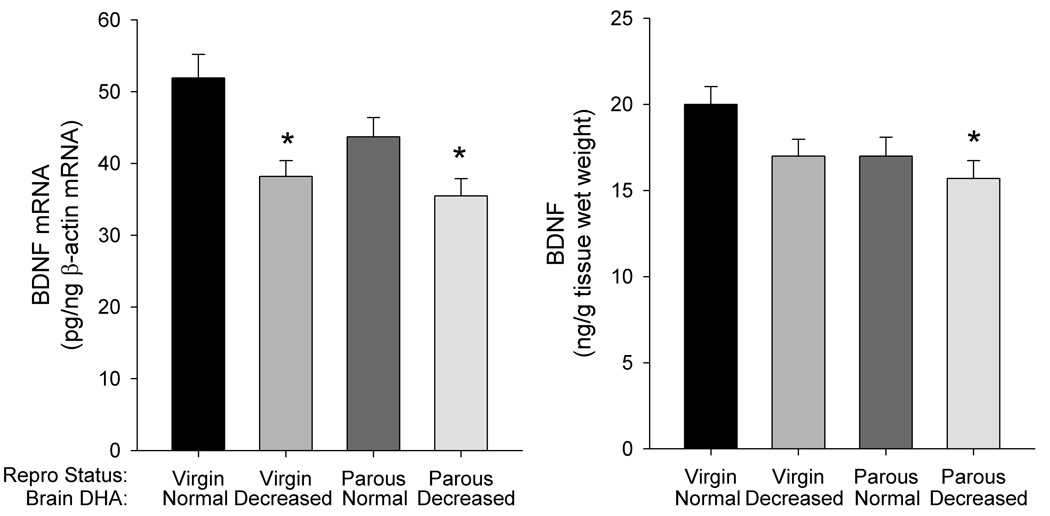
Two-way ANOVA indicated a significant main effect of brain DHA content (F(1,37) = 16.36, P<0.0001) on levels of hippocampal BDNF mRNA. A trend towards an effect of reproductive status (F(1,37) = 4.10, P = 0.050) was also detected. Post hoc analysis indicated that hippocampal BDNF mRNA levels were decreased 27% in virgin females with decreased DHA, and 32% in parous rats with decreased DHA compared to virgin rats with normal DHA (P<0.01) (Figure 2). A trend towards a decrease in BDNF mRNA level was also observed in the parous dams with normal DHA (P = 0.163).
Figure 2. Effects of brain DHA status and reproductive status on BDNF mRNA and peptide levels in hippocampus.
Data are presented as the mean ± SEM (n = 10–11 per group for mRNA, 8–10 for peptide). *Different from Virgin – Normal DHA (P<0.05).
Two-way ANOVA indicated significant main effects of brain DHA content (F(1,32) = 4.219, P < 0.05) and reproductive status (F(1,32) = 4.251, P<0.05) on BDNF peptide content. Post hoc analysis indicated that hippocampal BDNF was decreased 21% in parous dams with decreased DHA compared to virgin females with normal DHA (P<0.05). A trend towards a decrease in BDNF was also observed in virgin females with decreased DHA (P = 0.183) and in parous dams with normal DHA (P = 0.226).
Regional serotonin receptor binding
For hippocampal 5-HT1A receptors (Table 3), two-way ANOVA for binding density (Bmax) indicated a significant main effect of brain DHA content (F(1,33) = 4.91, P<0.05), and an interaction of brain DHA content and reproductive status (F(1,33) = 12.77, P<0.001). A significant main effect of brain DHA content was also detected for receptor affinity (KD) (F(1,33) = 4.85, P<0.05). Post hoc analysis indicated that the density of 5-HT1A receptors in hippocampus was 45% and 35% higher in parous dams with decreased DHA than in parous dams with normal DHA or virgin females with decreased DHA, respectively (P<0.05). The density of 5-HT1A receptors was also 22% higher in parous dams with decreased DHA than in virgin females with normal DHA, but this difference was not quite significant (P = 0.087). The affinity of 5-HT1A sites was not different between groups.
Table 3.
Effects of brain DHA content and reproductive status on the affinity and density of serotonin receptors in frontal cortex and hippocampus.
| Reproductive status/brain DHA content | Bmax (fmol/mg protein) | KD (nM) |
|---|---|---|
| Frontal Cortex | ||
| 5-HT1A receptor | ||
| Virgin – Normal DHA | 93 ± 6.2 | 1.4 ± 0.23 |
| Virgin – Decreased DHA | 98 ± 6.3 | 1.1 ± 0.16 |
| Parous – Normal DHA | 97 ± 8.5 | 1.2 ± 0.13 |
| Parous – Decreased DHA | 95 ± 6.7 | 1.2 ± 0.17 |
| 5-HT2A receptor | ||
| Virgin – Normal DHA | 385 ± 48 | 2.3 ± 0.22 |
| Virgin – Decreased DHA | 403 ± 31 | 1.9 ± 0.23 |
| Parous – Normal DHA | 413 ± 23 | 2.3 ± 0.24 |
| Parous – Decreased DHA | 393 ± 21 | 2.0 ± 0.24 |
| Hippocampus | ||
| 5-HT1A receptor | ||
| Virgin – Normal DHA | 153 ± 8.6 | 1.2 ± 0.10 |
| Virgin – Decreased DHA | 139 ± 15 | 1.3 ± 0.21 |
| Parous – Normal DHA | 129 ± 6.9 | 0.9 ± 0.11 |
| Parous – Decreased DHA | 187 ± 5.4†‡ | 1.5 ± 0.18 |
| 5-HT2A receptor | ||
| Virgin – Normal DHA | 50 ± 3.8 | 1.0 ± 0.14 |
| Virgin – Decreased DHA | 61 ± 3.3 | 1.4 ± 0.11 |
| Parous – Normal DHA | 57 ± 3.7 | 1.3 ± 0.09 |
| Parous – Decreased DHA | 51 ± 4.2 | 1.2 ± 0.05 |
Data are presented as the mean ± SEM (n = 8–10 per group).
Different from Virgin - Decreased DHA (P<0.01).
Different from Parous - Normal DHA (P<0.01).
For hippocampal 5-HT2A receptor binding, two-way ANOVA for binding density (Bmax) indicated an interaction of brain DHA content and reproductive status (F(1,29) = 4.57, P<0.05); however, post hoc analysis indicated no significant differences between groups.
In frontal cortex, no significant effects of brain DHA content or reproductive status on the density or affinity of 5-HT1A or 5-HT2A receptors were observed.
Regional serotonin and norepinephrine content
In frontal cortex, two-way ANOVA indicated a significant interaction of brain DHA content and reproductive status for serotonin content (F(1,38) = 31.36, P<0.001), serotonin turnover (F(1,38) = 11.81, P<0.001), and norepinephrine content (F(1,37) = 9.72, P<0.01); and a significant main effect of reproductive status for serotonin turnover (F(1,38) = 4.72, P<0.05) (Table 4). Post hoc analysis indicated that the serotonin content of frontal cortex was decreased 12% in virgin females with decreased DHA compared to virgin females with normal DHA (P<0.05). In contrast, the serotonin content of frontal cortex was 30% higher in parous dams with decreased DHA compared to parous dams with normal DHA (P<0.05), and serotonin turnover was decreased 25% (P<0.05). Norepinephrine content of frontal cortex was 17% lower, and serotonin turnover 45% higher, in parous dams with normal DHA compared to virgin females with normal DHA (P<0.05).
Table 4.
Effects of brain DHA content and reproductive status on levels of norepinephrine, dopamine, serotonin, and 5-hydroxyindole acetic acid (5-HIAA) in specific brain regions.
| Norepinephrine | Dopamine | Serotonin | 5-HIAA | 5-HIAA/5-HT | |
|---|---|---|---|---|---|
| Reproductive status/Brain DHA content | (ng/mg protein) | ||||
| Frontal Cortex | |||||
| Virgin – Normal DHA | 3.5 ± 0.11 | 1.1 ± 0.34 | 3.3 ± 0.13 | 3.7 ± 0.11 | 1.1 ± 0.06 |
| Virgin – Decreased DHA | 3.2 ± 0.13 | 0.91 ± 0.22 | 2.9 ± 0.08* | 3.9 ± 0.11 | 1.3 ± 0.05 |
| Parous – Normal DHA | 2.9 ± 0.11* | 0.77 ± 0.19 | 2.7 ± 0.09* | 4.2 ± 0.27 | 1.6 ± 0.13* |
| Parous – Decreased DHA | 3.3 ± 0.12 | 0.71 ± 0.03 | 3.5 ± 0.10†‡ | 4.3 ± 0.23 | 1.2 ± 0.07‡ |
| Hypothalamus | |||||
| Virgin – Normal DHA | 16.1 ± 0.48 | 4.0 ± 0.16 | 5.6 ± 0.31 | 8.2 ± 0.51 | 1.5 ± 0.11 |
| Virgin – Decreased DHA | 14.3 ± 0.57 | 3.9 ± 0.26 | 4.9 ± 0.37 | 9.0 ± 0.55 | 2.0 ± 0.31 |
| Parous – Normal DHA | 18.8 ± 0.58*† | 3.9 ± 0.31 | 5.0 ± 0.36 | 9.9 ± 0.40 | 2.2 ± 0.25 |
| Parous – Decreased DHA | 18.5 ± 0.48*† | 4.3 ± 0.28 | 5.8 ± 0.41 | 9.7 ± 0.31 | 1.7 ± 0.12 |
| Hippocampus | |||||
| Virgin – Normal DHA | 1.4 ± 0.13 | 0.23 ± 0.02 | 1.0 ± 0.08 | 2.1 ± 0.17 | 2.24 ± 0.18 |
| Virgin – Decreased DHA | 1.3 ± 0.10 | 0.21 ± 0.01 | 0.9 ± 0.10 | 2.5 ± 0.17 | 3.02 ± 0.32 |
| Parous – Normal DHA | 1.6 ± 0.14 | 0.23 ± 0.02 | 1.1 ± 0.13 | 2.8 ± 0.25 | 2.85 ± 0.34 |
| Parous – Decreased DHA | 1.8 ± 0.15 | 0.31 ± 0.04 | 1.2 ± 0.13 | 3.0 ± 0.26 | 2.54 ± 0.24 |
| Temporal Lobe | |||||
| Virgin – Normal DHA | 4.4 ± 0.26 | 0.93 ± 0.09 | 3.3 ± 0.30 | 5.8 ± 0.21 | 1.9 ± 0.18 |
| Virgin – Decreased DHA | 4.0 ± 0.27 | 0.87 ± 0.11 | 2.8 ± 0.25 | 6.1 ± 0.31 | 2.5 ± 0.38 |
| Parous – Normal DHA | 4.4 ± 0.29 | 0.96 ± 0.10 | 3.2 ± 0.27 | 6.1 ± 0.34 | 1.8 ± 0.20 |
| Parous – Decreased DHA | 4.8 ± 0.30 | 1.02 ± 0.13 | 3.8 ± 0.39 | 6.2 ± 0.26 | 1.9 ± 0.31 |
| Brain Stem | |||||
| Virgin – Normal DHA | 6.4 ± 0.39 | 0.34 ± 0.04 | 4.4 ± 0.29 | 6.3 ± 0.42 | 1.43 ± 0.06 |
| Virgin – Decreased DHA | 7.3 ± 0.52 | 0.39 ± 0.03 | 5.1 ± 0.25 | 7.4 ± 0.40 | 1.54 ± 0.08 |
| Parous – Normal DHA | 6.4 ± 0.25 | 0.36 ± 0.03 | 4.8 ± 0.07 | 6.3 ± 0.26 | 1.35 ± 0.09 |
| Parous – Decreased DHA | 7.3 ± 0.48 | 0.39 ± 0.04 | 5.5 ± 0.40 | 7.4 ± 0.65 | 1.40 ± 0.09 |
Data are presented as the mean ± SEM (n = 9–11 per group).
Different from Virgin – Normal DHA (P<0.05).
Different from Virgin - Decreased DHA (P<0.01).
Different from Parous - Normal DHA (P<0.01).
In hypothalamus, two-way ANOVA indicated a significant main effect of reproductive status on norepinephrine content (F(1,36) = 30.80, P < 0.0001), with parous dams with normal and decreased DHA having hypothalamic norepinephrine levels 17% and 29% than the respective virgin groups (P < 0.05).
No effects of brain DHA content or reproductive status were detected for monoamine neurotransmitters in the hippocampus, temporal lobe (amygdala and piriform cortex), or brain stem.
Forced swim test
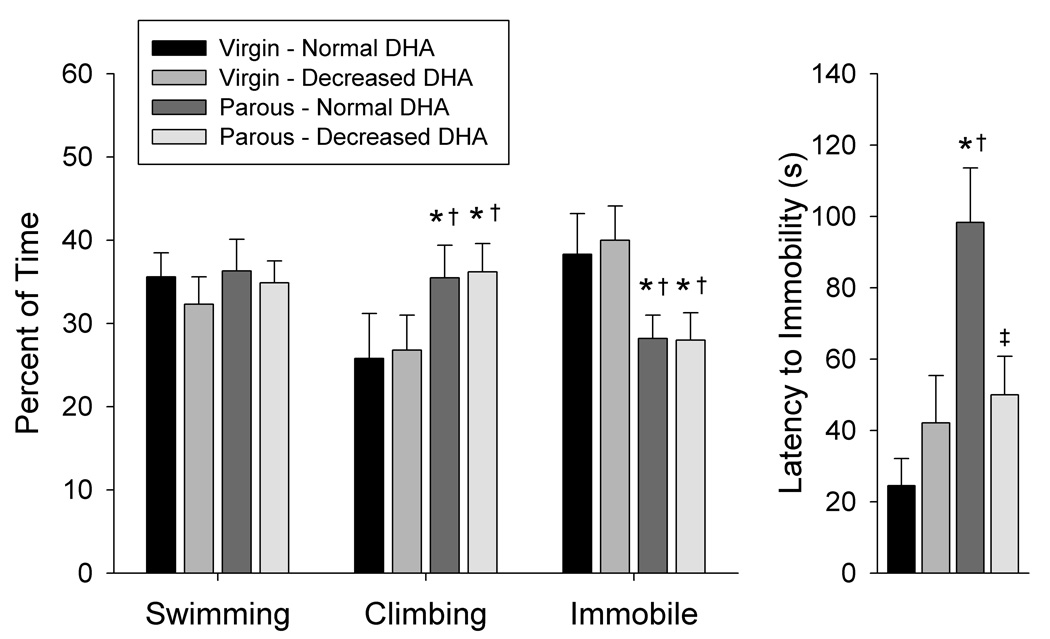
Two-way ANOVA indicated a significant main effect of reproductive status for time spent climbing (F(1,43) = 6.40, P<0.05) and time spent immobile (F(1,43) = 10.67, P<0.01), with parous dams exhibiting more climbing and less immobility than virgin females (Figure 3). A significant main effect of reproductive status (F(1,43) = 10.75, P<0.01), and an interaction of brain DHA content and reproductive status (F(1,43) = 6.99, P<0.05) were also detected for latency to immobility. Post hoc analysis indicated that latency to immobility was 51% shorter in parous dams with decreased DHA than in parous dams with normal DHA (P<0.05).
Figure 3. Effects of brain DHA status and reproductive status on behavioral response in the forced swim test.
Data are presented as the mean ± SEM (n = 12–14 per group). *Different from Virgin – Normal DHA (P < 0.05). †Different from Virgin – Decreased DHA (P < 0.05). ‡Different from Parous – Normal DHA (P < 0.05).
Corticosterone response to stress
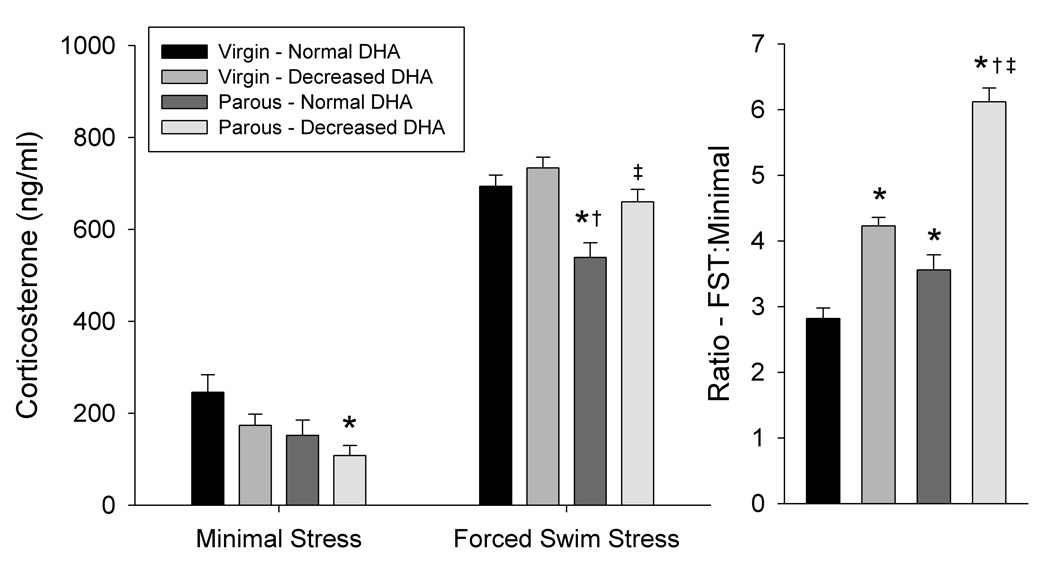
Three-way ANOVA indicated significant main effects of stressor intensity (F(1,84) = 576, P<0.0001), reproductive status (F(1,84) = 23.1, P<0.0001), and an interaction of brain DHA content and stressor intensity (F(1,84) = 11.63, P<0.001) (Figure 4). Post hoc analysis indicated that serum corticosterone levels after minimal stress were 56% lower in parous dams with decreased DHA compared to virgin females with normal DHA (P<0.05). Corticosterone levels were also different between groups after the intense stress associated with the forced swim test, with parous dams with normal DHA having lower corticosterone levels than virgin females with normal or decreased DHA (P<0.01). In addition, corticosterone levels of parous dams with decreased DHA were 22% higher in dams with decreased DHA than in parous dams with normal DHA after the forced swim test. The relative increase in corticosterone response between the minimal stress and forced swim stress conditions was greater in both virgin females and parous dams with decreased DHA compared to virgins and parous dams with normal DHA, respectively (P<0.01). In agreement with the significant main effect of reproductive status, the relative increase in corticosterone response was also greater in parous dams with normal DHA than in virgin females with normal DHA. (P<0.05).
Figure 4. Effects of brain DHA status and reproductive status on corticosterone secretion after minimal or intense stressors.
Minimal stress was defined normal handling. The five-min test session of the forced swim test was used at the intense stressor. Data are presented as the mean ± SEM (n = 10–13 per group). *Different from Virgin – Normal DHA (P < 0.05). †Different from Virgin – Decreased DHA (P < 0.05). ‡Different from Parous – Normal DHA (P < 0.05).
Discussion
The diet and breeding treatments used in this study produced virgin and parous female rats with decreases in brain DHA content of a magnitude similar to that observed in depressed humans (McNamara et al., 2007a). The present findings demonstrate several neurobiological alterations associated with reduced brain phospholipid DHA content, or the interaction of brain DHA content and reproductive status, in these animals. A number of the observed effects, such as decreased hippocampal BDNF gene expression, altered serotonin content and receptors in specific brain regions, and augmented HPA axis response to stress, are generally consistent with findings in depression (e.g., (Garlow et al., 2004, Duman and Monteggia, 2006). Other neurobiological findings in depression, such as increased cortical 5-HT1A and 5-HT2A receptors and decreased serotonin content in the brain stem (Stockmeier, 2003), were not found in either virgin or parous dams with decreased brain DHA, suggesting that other factors must underlie those neurobiological alterations. In fact, a variety of neurochemical parameters were not altered in rats with decreased brain DHA content, regardless of reproductive status, demonstrating that a loss of DHA from the brain of an adult animal does not result in gross brain dysfunction. Furthermore, there was no main effect of diet on body weight, indicating that the diet treatment had no gross effects on health or feeding of the animals. Likewise, there were similar number of pups per litter, and pup weight at birth and at weaning, indicating no detectable effect of the diets on maternal offspring burden.
Effects on hippocampal BDNF levels
Depression-associated decreased expression of BDNF in the hippocampus, a component of the limbic system that is integral in memory, affect, and regulation of the HPA axis (Sapolsky, 2000), is reversed by antidepressant treatment (Chen et al., 2001), and is believed to contribute to the hippocampal atrophy observed in the disease (Sheline et al., 1996, 1999, Karege et al., 2005). Similar results have been observed in animal studies with various models of depression, stress paradigms, and antidepressant drug treatments (Nibuya et al., 1995, 1995, Smith et al., 1995). In addition, exercise, which is clinically efficacious in reversing depressive symptoms (Brosse et al., 2002), also increased hippocampal BDNF levels (Johnson et al., 2003).
A reduction in brain DHA content, regardless of reproductive status, decreased levels of BDNF mRNA in hippocampus consistent with neurobiological findings in depression (Duman and Monteggia, 2006). The magnitude of this decrease in mRNA level was similar to that observed in male rats after acute restraint stress (Duric and McCarson, 2006). Consistent with this observation, BDNF peptide levels were also decreased in parous dams with decreased DHA and a trend towards a decrease was noted in virgin females with decreased DHA. This difference in the effects of manipulation of brain DHA content and reproductive status on BDNF mRNA and peptide suggests that transcription of this neurotrophic factor gene is more sensitive to manipulation via DHA-dependent mechanisms than are steady-state BDNF protein levels. Total BDNF peptide levels are likely also regulated by mechanisms other than de-novo synthesis in this system.
The observation of a decrease in hippocampal expression of BDNF is consistent with a role for decreased brain DHA content in the multifactorial pathogenesis of depression, and suggests that decreased expression of BDNF in hippocampus may be a contributing factor in both the non-puerperal and postpartum disorders. The trend toward a decrease in hippocampal BDNF mRNA and peptide levels in parous dams with normal brain DHA levels suggests that reproductive activity may also affect BDNF gene expression, though to a lesser extent than a loss of brain DHA. Concordant with this possibility, serum BDNF levels were decreased in women before and after childbirth (Lommatzsch et al., 2006). Assuming the central role for hippocampal BDNF in the pathogenesis of depression, these observations suggest that reduced expression of BDNF in this brain region may contribute to the increased vulnerability of postpartum women to depression (Cooper et al., 1988), particularly if brain DHA content is reduced. Also consistent with the present findings, decreased levels of BDNF in the frontal cortex have been reported in rats with decreases in brain DHA content induced prior to maturity (Rao et al., 2007).
Effects on corticosterone response to stress
Dysregulation of the HPA axis in depression is suggested by increased basal circulating levels of cortisol, urinary free cortisol concentrations, and the concentration of corticotrophin releasing factor (CRF) in cerebral spinal fluid (CSF) of depressed patients (Carpenter and Bunney, 1971, Carroll et al., 1976, Nemeroff et al., 1984). Activity of the HPA axis is also affected by reproductive status, with increased levels of cortisol-binding globulin, CRF, and adrenocorticotrophic hormone during pregnancy and the first few weeks after childbirth (Smith et al., 1987, Smith and Thomson, 1991, Bloch et al., 2003). In rats, elevated corticosterone levels are reported throughout lactation (Meaney et al., 1989), although stress-induced corticosterone secretion may be attenuated (Walker et al., 1995). Consistent with previous studies (Walker et al., 1995), the corticosterone response after the forced swim test was lower in parous dams with normal brain DHA than in virgin females, although corticosterone secretion after minimal stress was similar or lower in parous dams compared to virgin females in this study.
In addition to the effects of reproductive status on the HPA axis, the present data indicate a greater relative response to an intense stressor, compared to a minimal stressor, in rats with decreased brain DHA regardless of reproductive status. Furthermore, the corticosterone response to the intense stressor was greater in parous dams with decreased DHA compared to parous dams with normal DHA indicating an interaction of brain DHA level and reproductive status, in addition to the main effect of brain DHA level on relative stress response. These observations indicate that a loss of brain DHA contributes to an exaggerated HPA axis response to stress, and is concordant with a role for reduced brain DHA in the etiologies of depressive illnesses, particularly postpartum depression. Consistent with this observation, low plasma DHA levels were correlated with increased CRF concentrations in CSF in humans (Hibbeln et al., 2004). This observation also concurs with reports that rats fed an n-3 PUFA-enriched diet exhibited lower levels of the anxiety-and stress-like behavioral effects of interleukin-1, which increases corticosterone levels, in the elevated plus maze and the open field test (Song et al., 2004).
Effects on monoaminergic function
Alterations in the monoaminergic systems in postmortem brains of depressives or suicide victims are reported, including decreased neurotransmitter content and increased density of several receptors, most notably 5-HT1A, 5-HT2A, and β, in prefrontal cortex. These receptors down-regulate after treatment with antidepressants, which increase the synaptic availability of serotonin, and in some instances norepinephrine (Baldessarini, 2001, Stockmeier, 2003, Duman, 2004, Garlow et al., 2004).
Several monoaminergic parameters were altered by a decrease in brain DHA content; however, effects were different in virgin and parous females. The density of hippocampal serotonin 5-HT1A receptors in parous dams with decreased brain DHA was increased compared to both virgin females with decreased brain DHA and parous dams with normal brain DHA levels. Although increased density of 5-HT1A in prefrontal cortex is a consistent finding in depression (Mann and Arango, 1999), most postmortem studies report no alteration in hippocampal 5-HT1A binding (Dillon et al., 1991, Lowther et al., 1997, Stockmeier et al., 1997) or a decrease in 5-HT1A gene expression (Lopez-Figueroa et al., 2004). Thus, the increase in hippocampal 5-HT1A receptor density observed in parous dams with decreased brain DHA may contribute uniquely to the yet-to-be determined etiology of postpartum depression.
Serotonin concentrations in frontal cortex were also decreased in virgin females with decreased brain DHA content compared to virgins females with normal brain DHA, in agreement with findings in depressed humans (Garlow et al., 2004). Consistent with this observation, variation in DHA levels have been associated with altered serotonin function in humans. Of note, decreased plasma DHA levels resulting from alcoholism were correlated with increased cerebrospinal fluid concentrations of the serotonin metabolite 5-HIAA (Hibbeln et al., 1998). Likewise, plasma DHA levels were correlated with the density of platelet serotonin transporter binding (De Vriese et al., 2004).
A difference in serotonin content in frontal cortex was also observed between parous dams with normal and decreased DHA; however, brain DHA and reproductive status interacted such that serotonin levels were higher in those with decreased DHA compared to those with normal DHA, where serotonin levels were also decreased compared to virgin females with normal DHA. While an increase in serotonin appears counter to current theories of depression, this DHA-related effect may reflect differences in the etiologies of the non-puerperal and postpartum disorders—an issue that must be resolved in future studies.
Interestingly, alterations in serotonin levels and turnover, but no alterations in 5-HT1A or 5-HT2A receptors, were observed in frontal cortex; whereas altered 5-HT1A receptor binding, but no alteration in serotonin levels or turnover, were observed in hippocampus. While numerous receptor alterations are known to result from changes in receptor stimulation as a result of agonist or antagonist treatment or dennervation, the resulting effect on any given receptor by a particular manipulation cannot be presumed [e.g., (Leysen et al., 1987, 1988)]. Likewise, it cannot be presumed that the receptor and neurotransmitter alterations observed in this study are directly causally related to each other. The loss of brain DHA produced by the treatments used in this study occur throughout the brain and affect a variety of systems by multiple mechanisms (see Introduction). The net result of these effects are the neurochemical changes reported here.
Altered brain DHA status in various animal models has also been associated with changes in serotonergic function. Of note, diets that increased cortical DHA content increased cortical serotonin content in piglets (de la Presa Owens and Innis, 1999). Rats raised from conception with diet and breeding protocols that resulted in a 75% decrease in brain DHA exhibited altered serotonergic neurotransmission and increased density of frontal cortical 5-HT2A receptors (Delion et al., 1994, 1997); however it must be noted that the effects in these animals reflect a much larger decrease in brain DHA content than that occurring in this study, as well as its interactions with brain development.
Effects in the forced swim test
The forced swim test is an extensively validated predictor of antidepressant efficacy, in which drugs with antidepressant efficacy decrease the time spent immobile, and has also been used as a putative model of depressive behavior in rodents (Cryan et al., 2005). In this study using female Long-Evans rats, reproductive status produced a pronounced effect on behavior in the test, with parous dams exhibiting more time climbing and less time floating immobile (or making other small movements necessary to maintain floating), than virgin females. Previous studies have reported differences between the behavior of virgin females and nursing dams of various rat strains at various time points during lactation in the forced swim test (Walker et al., 1995, Galea et al., 2001); however, virgins and parous dams at weaning have not been directly compared. Behavior in the forced swim test varies depending on a number of factors including the rat strain, sex, dimensions of the apparatus, room lighting, hormonal status, and possibly estrous cycle (Alonso et al., 1991, Pare and Redei, 1993, Walker et al., 1995, Porsolt et al., 2000, Galea et al., 2001). These factors likely underlie the differences in the specific behaviors observed in this and previous studies.
In addition to the main effect of reproductive status, an interaction of reproductive status and brain DHA content was observed. Whereas a decrease in brain DHA produced no alterations in behavior in the forced swim test in virgin females, parous dams with decreased brain DHA had significantly shorter latencies to immobility compared to those with normal brain DHA levels. This behavioral difference could reflect the increase in hippocampal 5-HT1A receptors or the increased corticosterone response detected in that group. Similar to this observation, previous studies with male rats with manipulations in DHA status induced at various points in development reported increased immobility in the forced swim test in those with lower brain DHA contents (DeMar et al., 2006, Huang et al., 2006).
Implications of the present findings
The neurobiological effects observed in both virgin and parous rats with decreased brain DHA (i.e., decreased hippocampal BDNF gene expression and increased relative corticosterone response to an intense stressor) are attributable to changes in brain phospholipid fatty acid composition involving a loss of DHA. In the instances where decreased brain DHA content and reproductive status interacted to result in additional effects of decreased brain DHA in the parous dams (e.g., increased hippocampal 5-HT1A binding, decreased latency to immobility in the forced swim test, and increased corticosterone response to an intense stressor), the hormonal and physiological changes related to the postpartum state likely play a role. However, differences in the changes in overall brain phospholipid fatty acid composition between virgin and parous rats with decreased brain DHA, specifically the greater compensatory incorporation of DPA in parous dams compared to virgin females, could result in greater alteration in the physicochemical properties of the neuronal membranes, and thus contribute to the effects observed only in the parous dams with decreased DHA in addition to, or in concert with postpartum status.
The similarity between the effects of reduced brain DHA observed in this study and the neurobiological findings observed in humans with depression is concordant with the hypothesis that a loss of brain phospholipid DHA content represents a contributing factor in the etiology of depressive disorders in females. Neurobiological changes similar to those reported here may also occur in males that experience a loss of brain DHA; however, this remains to be determined. Previous studies with similar diet treatments in male rats failed to produce a decrease in brain DHA content (Bourre et al., 1992). This suggests that females may be more vulnerable to a loss of DHA than males, which could be one factor contributing to the higher incidence of depression in women. An α-linolenic-deficient diet has been shown to decrease brain DHA in adult male mice (McNamara et al., 2007b), suggesting that strain or species differences may also exist. Although diet was used to manipulate brain DHA status in this study, reduced levels of DHA in postpartum women may result from conditions other than a dietary deficiency (e.g., a preexisting metabolic disorder, hypothetical pregnancy-associated changes in fatty acid metabolism or transport, etc.). Even so, the present findings are likely relevant to conditions involving decreased brain DHA regardless of its underlying cause. The present data also suggest that the apparent therapeutic effects of treatment with long chain PUFA supplements in depressive disorders (Freeman et al., 2006b) result from reversal of the neurobiological effects of reduced brain DHA observed in this study. However, the ability to restore the brain DHA content of an adult animal has yet to be demonstrated experimentally. In addition, while it is interesting to speculate that a decrease in brain DHA occurring at any point in the life-span may contribute to the development of depressive illness, it must be noted that the DHA levels of specific brain regions are differentially affected when the adult brain experiences a loss of DHA compared to a developing brain that had inadequate initial accumulation of DHA (Levant et al., 2006b, 2007). Thus, it is likely that the neurobiological consequences of low brain DHA content may differ in these models. It is also likely that the interaction of low brain DHA with developmental processes in the immature animal contributes to distinct neurobiological changes than those occurring in an adult animal that experiences a loss of brain DHA. Finally, the present data are consistent with a role for a decrease in brain DHA as a factor in the etiology of depressive disorders; however, they do not establish the requirement for altered DHA status in these diseases.
Conclusion
The present findings demonstrate specific alterations in neurobiology attributable to a decrease in phospholipid brain DHA content of a similar magnitude to that reported in depressed humans (McNamara et al., 2007a), parous status, or their interaction. These results are generally consistent with neurobiological findings in depression and suggest that a loss of brain DHA may represent one of the factors contributing to the etiologies of depressive illnesses. In view of the low levels of n-3 PUFAs in Western diets, particularly relative to n-6 PUFAs, which compete for metabolism into long chain PUFAs (Simopoulos, 2002), a loss of brain DHA represents a viable causative factor in these disorders. Furthermore, a decrease in brain DHA content interacts with other physiological changes occurring during the postpartum period. The greater number of neurobiological and behavioral alterations observed in parous dams with decreased brain DHA, combined with our previous observation of the increased vulnerability for reproducing females to experience a loss of brain DHA (Levant et al., 2006a), suggests that decreased brain DHA may be particularly relevant to the pathogenesis of postpartum depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31 Suppl:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Castellano MA, Afonso D, Rodrigues M. Sex differences in behavioral despair: relationships between despair and open field activity. Physiol Behav. 1991;49:69–72. doi: 10.1016/0031-9384(91)90232-d. [DOI] [PubMed] [Google Scholar]
- Alper RH, Nelson DL. Inactivation of 5-HT(1A) receptors in hippocampal and cortical homogenates. Eur J Pharmacol. 2000;390:67–73. doi: 10.1016/s0014-2999(00)00032-7. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders: depression and anxiety disorders. In: Hardman JG, Limbird LE, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 447–484. [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Dumont OS, Piciotti MJ, Pascal GA, Durand GA. Dietary alpha-linolenic acid deficiency in adult rats for 7 months does not alter brain docosahexaenoic acid content, in contrast to liver, heart and testes. Biochim Biophys Acta. 1992;1124:119–122. doi: 10.1016/0005-2760(92)90087-c. [DOI] [PubMed] [Google Scholar]
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32:741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Bunney WE., Jr Adrenal cortical activity in depressive illness. Am J Psychiatr. 1971;128:31–40. doi: 10.1176/ajp.128.1.31. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. J Psychol Med. 1976;6:43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004:re5. doi: 10.1126/stke.2252004re5. 2004. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev. 1980a;4:131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980b;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Campbell EA, Day A, Kennerly H, Bond A. Non-psychotic psychiatric disorder after childbirth: a prospective study of prevalence, incidence, course, and nature. Br J Psychiatry. 1988;152:799–806. doi: 10.1192/bjp.152.6.799. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- de la Presa Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J Nutr. 1999;129:2088–2093. doi: 10.1093/jn/129.11.2088. [DOI] [PubMed] [Google Scholar]
- De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci. 2003;73:3181–3187. doi: 10.1016/j.lfs.2003.02.001. [DOI] [PubMed] [Google Scholar]
- De Vriese SR, Christophe AB, Maes M. In humans, the seasonal variation in poly-unsaturated fatty acids is related to the seasonal variation in violent suicide and serotonergic markers of violent suicide. Prostaglandins Leukot Essent Fatty Acids. 2004;71:13–18. doi: 10.1016/j.plefa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Delion S, Chalon S, Herault J, Guilloteau D, Bresnard JC, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotonergic neurotransmission in rats. J Nutr. 1994;124:2466–2475. doi: 10.1093/jn/124.12.466. [DOI] [PubMed] [Google Scholar]
- Delion S, Chalon S, Guilloteau D, Lejeune B, Besnard JC, Durand G. Age-related changes in phospholipid fatty acid composition and monoaminergic neurotransmission in the hippocampus of rats fed a balanced or an n-3 polyunsaturated fatty acid-deficient diet. J Lipid Res. 1997;38:680–689. [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Duman RS. The neurochemistry of mood disorders: preclinical studies. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2004. pp. 421–339. [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005;133:999–1006. doi: 10.1016/j.neuroscience.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7:544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- Enna SJ, Reisman SA, Stanford JA. CGP 56999A, a GABA(B) receptor antagonist, enhances expression of brain-derived neurotrophic factor and attenuates dopamine depletion in the rat corpus striatum following a 6-hydroxydopamine lesion of the nigrostriatal pathway. Neurosci Lett. 2006;406:102–106. doi: 10.1016/j.neulet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand. 2006a;113:31–35. doi: 10.1111/j.1600-0447.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006b;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Galli C, Trzeciak HI, Paoletti R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: reciprocal replacement of n-6 and n-3 polyunsaturated fatty acids. Biochim Biophys Acta. 1971;248:449–454. [Google Scholar]
- Garlow SJ, Musselman DL, Nemeroff CB. The neurochemistry of mood disorders: clinical studies. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2004. pp. 440–460. [Google Scholar]
- Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids. 1996;31:859–865. doi: 10.1007/BF02522981. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Hibbeln J. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Umhau JC, Linnoila M, George DT, Ragan PW, Shoaf SE, Vaughan MR, Rawlings R, Salem N., Jr A replication study of violent and nonviolent subjects: cerebrospinal fluid metabolites of serotonin and dopamine are predicted by plasma essential fatty acids. Biol Psychiatry. 1998;44:243–249. doi: 10.1016/s0006-3223(98)00143-7. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Bissette G, Umhau JC, George DT. Omega-3 status and cerebrospinal fluid corticotrophin releasing hormone in perpetrators of domestic violence. Biol Psychiatry. 2004;56:895–897. doi: 10.1016/j.biopsych.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP. Omega-3 fatty acids on the forced-swimming test. J Psychiatr Res. 2006 doi: 10.1016/j.jpsychires.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. J Nutr. 2006a;136:2236–2242. doi: 10.1093/jn/136.8.2236. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Jones KA, Carlson SE. Differential effects of modulation of docosahexaenoic acid content during development in specific regions of rat brain. Lipids. 2006b;41:407–414. doi: 10.1007/s11745-006-5114-6. [DOI] [PubMed] [Google Scholar]
- Levant B, Radel JD, Carlson SE. Reduced brain DHA content after a single reproductive cycle in female rats fed a diet deficient in N-3 polyunsaturated fatty acids. Biol Psychiatry. 2006c;60:987–990. doi: 10.1016/j.biopsych.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J Nutr. 2007;137:130–134. doi: 10.1093/jn/137.1.130. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM. [3H]Ketanserin (R 41468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- Leysen JE, van Gompel P, Gommeren W, Laduron PM. Differential regulation of dopamine-D2 and serotonin-S2 receptors by chronic treatment with the serotonin-S2 antagonists, ritanserin, and setoperone. Psychopharmacol Ser. 1987;3:214–224. doi: 10.1007/978-3-642-71288-3_25. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Janssen PF, Van Gompel P, Janssen PA. Receptor interactions of dopamine and serotonin antagonists: binding in vitro and in vivo and receptor regulation. Psychopharmacol Ser. 1988;5:12–26. doi: 10.1007/978-3-642-73280-5_2. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Hornych K, Zingler C, Schuff-Werner P, Hoppner J, Virchow JC. Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology. 2006;31:388–394. doi: 10.1016/j.psyneuen.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, Lopez JF, Watson SJ. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lowther S, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls. J Affect Disord. 1997;42:199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- Mamalakis G, Kalogeropoulos N, Andrikopoulos N, Hatzis C, Kromhout D, Moschandreas J, Kafatos A. Depression and long chain n-3 fatty acids in adipose tissue in adults from Crete. Eur J Clin Nutr. 2006;60:882–888. doi: 10.1038/sj.ejcn.1602394. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Arango V. Abnormalities of brain structure and function in mood disorders. In: Charney DS, Neslter EJ, Bunney BS, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 1999. pp. 385–393. [Google Scholar]
- Mazure CM, Maciejewski PK. The interplay of stress, gender and cognitive style in depressive onset. Arch Womens Ment Health. 2003a;6:5–8. doi: 10.1007/s00737-002-0161-3. [DOI] [PubMed] [Google Scholar]
- Mazure CM, Maciejewski PK. A model of risk for major depression: effects of life stress and cognitive style vary by age. Depress Anxiety. 2003b;17:26–33. doi: 10.1002/da.10081. [DOI] [PubMed] [Google Scholar]
- McCarson KE, Krause JE. NK-1 and NK-3 type tachykinin receptor mRNA expression in the rat spinal cord dorsal horn is increased during adjuvant or formalin-induced nociception. Journal of Neuroscience. 1994;14:712–720. doi: 10.1523/JNEUROSCI.14-02-00712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 Fatty Acid docosahexaenoic Acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007a;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult mice: Prevention by chronic lithium treatment. J Psychiatr Res. 2007b doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Aitken DH, Bhatnagar S. Glucocorticoid receptors in brain and pituitary of the lactating rat. Physiol Behav. 1989;45:209–212. doi: 10.1016/0031-9384(89)90187-x. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Herrell R. Contributions of epidemiology to the neurobiology of mental illness. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. Oxford: Oxford University Press; 2004. pp. 103–111. [Google Scholar]
- Miller LJ. Postpartum depression. JAMA. 2002;287:762–765. doi: 10.1001/jama.287.6.762. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Sasaki S, Yokoyama T, Tanaka K, Ohya Y, Fukushima W, Saito K, Ohfuji S, Kiyohara C, Hirota Y. Risk of postpartum depression in relation to dietary fish and fat intake in Japan: the Osaka Maternal and Child Health Study. Psychol Med. 2006;36:1727–1735. doi: 10.1017/S0033291706008701. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element-binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SJ, de Groot RH, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot Essent Fatty Acids. 2003;69:237–243. doi: 10.1016/s0952-3278(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Pare WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Models of affective illness: forced swimming and tail suspension in rodents. In: Enna SJ, Williams M, Ferkany J, Kenakin T, Porsolt RD, Sullivan JP, editors. Current Protocols in Pharmacology. New York: John Wiley & Sons; 2000. pp. 5.8.1–5.8.9. [Google Scholar]
- Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr, Litman B, Kim H-Y, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–960. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medical healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ. Long chain polyunsaturated fatty acids in the mammalian brain. Proc Nutr Soc. 1975;34:287–291. doi: 10.1079/pns19750051. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress alters the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Owens PC, Brinsmead MW, Singh B, Hall C. The nonsuppressibility of plasma cortisol persists after pregnancy. Horm Metab Res. 1987;19:41–42. doi: 10.1055/s-2007-1011733. [DOI] [PubMed] [Google Scholar]
- Smith R, Thomson M. Neuroendocrinology of the hypothalamo-pituitary-adrenal axis in pregnancy and the puerperium. Baillieres Clin Endocrinol Metab. 1991;5:167–186. doi: 10.1016/s0950-351x(05)80102-8. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress. 2004;7:43–54. doi: 10.1080/10253890410001667188. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamaki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001a;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamaki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001b;52:529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Rasanen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Walker CD, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. J Neuroendocrinol. 1995;7:615–622. doi: 10.1111/j.1365-2826.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]