Abstract
Objective
Brain arteriovenous malformations (AVM) have high MMP-9, IL-6 and MPO expression, and polymorphic variations in inflammatory genes are associated with increased risk of hemorrhage. In this study, we characterized the presence of inflammatory cells in AVM lesional tissues.
Methods
Immunohistochemistry was used to identify and localize neutrophils (MPO as marker), macrophages/microglia (CD68 as marker), T lymphocytes (CD3 as marker), and B lymphocytes (CD20 as marker). Endothelial cell (EC) marker CD31 was used as an index to assess vascular mass (EC mass). Surgical specimens from 20 unruptured, non-embolized AVMs were examined; seven cortical samples from temporal lobectomy were used as controls. Positive signals for inflammatory cell markers were counted and analyzed by normalizing to the area of the tissue section and the amount of endothelial cells (cells/mm2/EC mass pixels). Levels of MPO and MMP-9 were determined by ELISA.
Results
Neutrophils and macrophages are all frequently identified in the vascular wall of AVM tissues. In contrast, T and B lymphocytes are rarely observed in AVM tissues. AVM tissues displayed more neutrophil and macrophage/microglia markers than epilepsy control tissues (MPO: 434 ± 333 vs 5 ± 4, P=0.0001; CD68: 454 ± 404 vs 4 ± 2, P=0.0001; cells/mm2/EC mass pixels). In ex vivo studies, neutrophil quantity, MPO, and MMP-9 levels were all co-linear(R2=0.98–0.99).
Conclusion
Our study demonstrates that inflammatory cells are present in AVM tissues. Taken together with prior genetic and cytokine studies, these data are consistent with a novel view that inflammation is associated with AVM disease progression and rupture.
Keywords: CD68, inflammation, myeloperoxidase, vascular malformations
Background
Brain arteriovenous malformations (AVM) are complexes of tortuous, tangled vessels representing fistulous connections between arteries and veins lacking a capillary bed (8, 16). These vascular lesions are characterized by a phenotype of excessive angiogenesis and vascular remodeling. The primary clinical implication of AVM is recurrent spontaneous intracranial hemorrhage. The pathophysiology underlying brain AVM is not clearly understood and there is a presumption, with only circumstantial evidence, that they are primarily congenital, static lesions (47).
Our general hypothesis is that inflammation is involved in the clinical course of the disease state, if not the pathogenesis of the lesion. This view is based on several lines of evidence. We previously found an association between promoter polymorphisms in inflammatory cytokine genes and AVM intracranial hemorrhage (ICH) risk; TNF-α was associated with increased risk of new ICH in the natural course of AVMs (1), and IL-6 was associated with presenting ICH (37). We have also demonstrated that brain AVM tissues displayed increased inflammatory markers compared to control tissues by biochemical methods (6, 7). There is a precedent in that intracranial aneurysms, even if unruptured, have an inflammatory component to the phenotype (10, 17).
In order to provide further evidence that inflammation and leukocyte participation is associated with the phenotype of the disease, we present several lines of additional evidence. We examined unruptured tissue specimens in patients that had not undergone pre-surgical embolization, two potential confounders that might increase the level of inflammatory infiltrates in the lesional tissue. We used immunocytochemistry to characterize leukocyte infiltrates. To further explain our previous observations (6, 7), we demonstrate a linear relationship between leukocyte quantity and MPO expression. Because intra- or extravascular blood may affect matrix metallonproteinase 9 (MMP-9) expression (44), we also show that such contamination cannot explain the large increases in MMP-9 levels we measured in lesional tissue.
Materials and Methods
All studies involving patients were IRB-approved and patients gave informed consent. Procedures for the use of laboratory animals were approved by the UCSF Institutional Animal Care and Use Committee.
Subjects
Beginning in June 2000, patients with AVM evaluated at the University of California, San Francisco (UCSF) were entered into an ongoing prospective registry (18). We analyzed a subset of this group who underwent microsurgery and had tissue available for analysis. We selected from our tissue bank cases that were unruptured and did not undergo pre-surgical embolization. Control cerebral cortex was obtained from patients undergoing surgical treatment of epilepsy as previously described (20, 23). Samples were taken from structurally normal temporal lobe remote from the epileptogenic focus. Prior to surgery, Decadron was given to both lobectomy patients and brain AVM patients in the same dose range.
Immunohistochemistry
We used immunocytochemistry to characterize leukocyte infiltrates. CD31 antibody was obtained from DakoCytomation (Denmark) and was used according to the company’s instruction. This antibody primarily labels endothelial cells, and its specificity has been reported.(36) MPO, CD68, CD3, and CD20 were purchased from Serotec (Raleigh, NC) and were used according to the manufacture’s protocol. The specificities of these antibodies have been tested by the manufacture, and have also been reported. (3, 4, 25, 26, 29). Brain sections at 10 μM were fixed with cold acetone for 20 min at 4°C. Endogenous peroxidase activity was quenched by incubating slides in 0.3% H2O2 in methanol for 30 minutes. Slides were rinsed in 2 changes of PBS, pH 7.4, and then incubated in PBS with 0.1% Triton X-100 for 30 minutes at room temperature. After blocking, sections were incubated with primary antibodies diluted in PBS with 0.1% Triton X-100 at the following concentrations: mouse monoclonal anti-human-MPO, 1:2000, mouse monoclonal anti-human-CD68, 1:1000, mouse anti-human CD3, 1:200, mouse anti-human CD20, 1:100, and mouse anti-human CD31, 1:100. After incubating at 4°C overnight and washing in PBS, the sections were incubated with biotinylated goat anti-mouse (Vector Laboratories, Burlingame, CA) diluted 1: 5000 for 1 hour at room temperature, and were treated with the ABC streptavidin detection system (Vector Laboratories) for 1 hour after washing with PBS. The resulting horseradish peroxidase signal was detected using 3,3′-diaminobenzidene. For dual fluorescent staining, after incubating at 4°C overnight with primary antibodies, sections were incubated with Alexa Fluor 594-conjugated goat anti-mouse or Alexa Fluor 488-conjugated goat anti-rabbit IgG (Molecular probes, Eugene, OR) at 1:500 dilution for 1 hour at room temperature. They were then mounted and photographed with fluorescent microscope. Negative controls were performed by omitting the primary antibodies during the immunostaining.
Assessing the amount of neutrophil and macrophage in AVM tissue
The area of each tissue section was measured with NIH image J program, and expressed as mm2. To quantitate the amount of leukocytes present in AVM tissue, neurophil positive signal (brown color associated with blue nuclei) present in the vascular lumen (defined as unassociated with endothelial cells), in the vascular wall (defined as associated with endothelial cells, or smooth muscle cells), and in the parachyma of AVM tissue was counted separately. Neutrophil positive signal was also counted in the control epilepsy tissue in the same manner. The number of positive signals was taken as neutrophil count. The count was divided by the measured area to obtain neutrophil count per unit area. The neutrophil counts from the vascular wall and parenchyma were combined and analyzed because the neutrophils from these two locations are presumably available to exert a substantial biological effect on tissue.
Because the control tissue had a different amount of vascular tissue than the AVM nidus, we normalized the area to an index of blood vessel mass, which is represented by the amount of endothelial cell (EC) marker CD31. Brain AVM tissue and epilepsy tissue were immunostained with CD31. The amount of CD31 was assessed by measuring CD31 digitized positive blood vessels with NIH image J program by computer-aided planimetry. Staining images were digitized under a 20× objective with a spot camera (Leica, Germany) equipped with NIH image J software. To control for the variations during photographing, all images used for cell counting were acquired under the same magnification, light intensities, and exposure time on slides. The thresholds were selected with a “set color threshold” feature in the Image J software, which permits the user to select pixel regions that were considered positive. After establishing the threshold, this same parameter was applied to all images during counting. The tissue slides for each cell marker were processed identically during staining to avoid impact of background color on counting. The positive signal displayed dark brown color which is in sharp contrast to the background light brown color, and thus, the counting is not affected by the small variations in background color. The amount of CD31 was assessed by measuring the pixels of CD31 positive particles, and was expressed as pixels. Neutrophil count per unit area per unit EC mass was calculated. The amounts of macrophage were assessed in the same fashion as for neutrophils.
Isolation of neutrophil from peripheral blood of AVM patients
To further explain our previous observations that suggested the correlation between leukocyte infiltration and inflammatory marker expression (6, 7), we correlated leukocyte quantity and MPO expression ex vivo. Neutrophils were isolated from brain AVM patients’ blood by using Histopaque 1077 (Sigma-Aldrich, St Louis, MO). The isolated cells were resuspended at different density in PBS, and were used for assessing MMP-9 and MPO content by ELISA, as described below.
Enzyme-linked immunoadsorbent assays (ELISA)
MPO levels were measured by ELISA according to the protocols provided by the manufacturer (Calbiochem, San Diego, CA). Protein levels of MMP-9 in tissue specimens were measured using specific ELISA kits (R&D systems Inc., Minneapolis, MN) according to the manufacturer’s protocol.
Animals and blood infusion
Because intra- or extravascular blood in AVM tissue may affect MMP-9 expression (44), we further investigated this effect by injecting whole blood in the mouse brain. Adult male, CD-1 mice, weight 30 to 35 g, were purchased from Charles River Laboratory (Wilmington, MA). Mice (n= 3 per group) were injected with 20μl of whole blood at various dilations: 100% (no dilution), 50%, 25% (diluted with normal saline). A control group was injected with normal saline. The method for whole blood injection was as described by Tejima et al (44). Briefly, after induction of anesthesia with ketamine and xylazine (intraperitoneal), mice were placed in a stereotactic frame (David Kopf Instruments, Tujunga, CA). Hamilton syringe was inserted through a burr hole 1 mm lateral to the sagittal suture, 1 mm posterior to bregma, and 3 mm under the cortex. 20 μl of injectate (whole blood, diluated whole blood, or normal saline as described above) was injected stereotactically into the right caudate putamen. The mice were sacrificed at 3hr after treatment. Tissue sampling is depicted in Figure 8A. Brain tissues were immediately frozen in liquid nitrogen and stored at −80°C. For MMP-9 activity assessment, tissue homogenates were centrifuged at 10,000g for 20 min at 4°C, and the supernatants of the homogenates were used for zymography.
Gelatin zymography
Zymography was performed as previously described (24). Briefly, protein sample of 40 μg was separated under non-reducing conditions in a 10% zymogram gel (Invitrogen, Carlsbad, CA) containing 0.1% gelatin as a substrate. Following electrophoresis, gels were washed, and incubated in developing buffer overnight at 37°C. Then the gels were stained with 0.5% Coomassie Blue R-250 and de-stained. MMP activity can be detected as white bands of lysis against the Coomassie blue stained gel. Protein bands in zymography were quantified by scanning densitometry using KODAK image analysis software (Eastman Kodak Company). In preliminary experiments, controls with increasing amounts of protein verified that quantitative band intensities fell within a linear range.
Statistical analyses
Data are presented as mean ± SD. For neutrophil and macrophage counts and indices, we determined that the distribution of values was skewed and therefore used the non-parametric Mann-Whitney test to compare AVM to control tissues. Linear regression was used to compare the various assayed protein relationships. Results from animal and cell culture studies were analyzed using a one-way ANOVA followed by the Scheffé test. A probability value of less than 5% was considered to represent statistical significance.
Results
Table 1 shows the demographic and brain AVM characteristics of the study cohort. The mean age (years) for AVM patients was 46 ± 15 and 36 ± 13 for control patients (P=0.11). Mean AVM size was 2.2 ± 1.3 cm.
Presence of inflammatory cells in AVM tissues
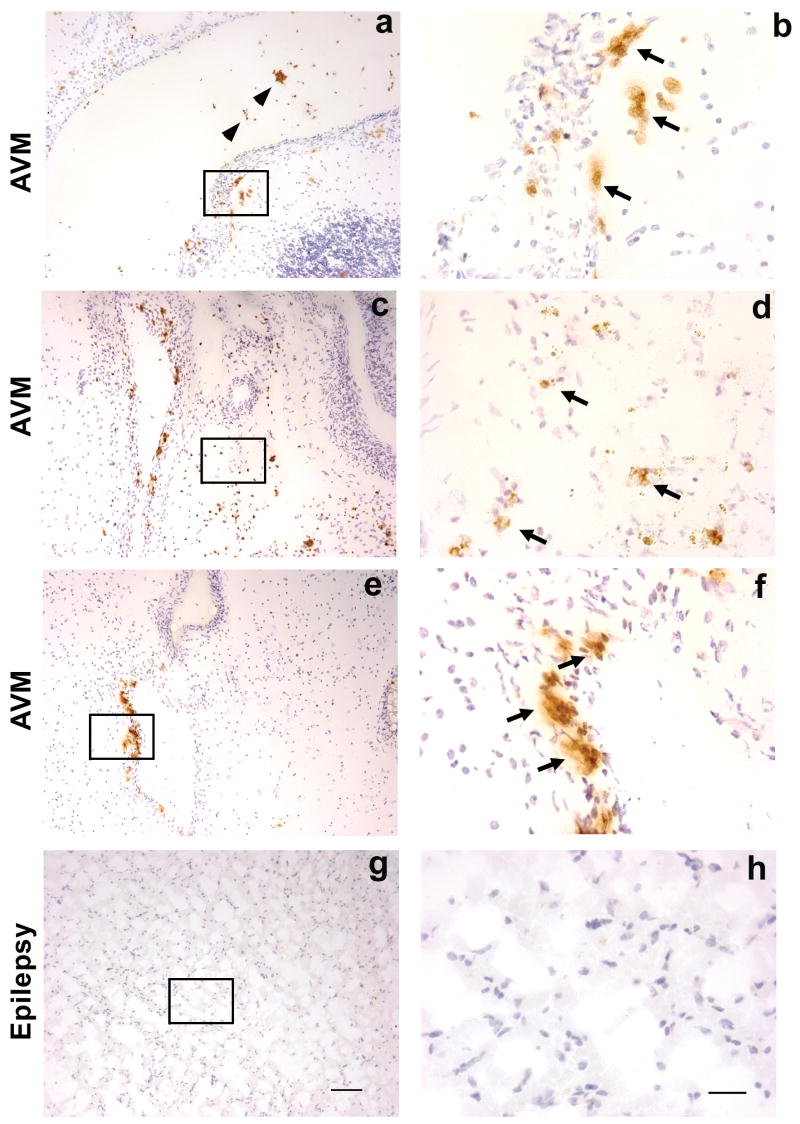
To investigate whether inflammatory leukocytes are present in brain AVM tissue, we performed immunohistochemistry on sections from frozen AVM tissues using neutrophil marker MPO, macrophage/microglia marker CD68, T lymphocyte marker CD3, and B lymphocyte marker CD20. We examined unruptured tissue specimens in AVM patients that had not undergone pre-surgical embolization, two potential confounders that might increase the level of inflammatory infiltrates in the lesional tissue. Neutrophils were present in the vascular wall as well as in parenchymal tissues of AVM, demonstrating regional infiltration of neutrophils (Figure 1a, b, c, d,e,f). Although a majority of MPO signals were detected in the vascular wall and the parenchymal tissues, some of MPO signals were observed in the lumen of the vessel of AVM (brown color indicated by arrowheads) (Figure 1a). These are presumed to be neutrophils that remained in the vascular lumen during resection. There were no MPO positive cells in epilepsy brain samples (Figure 1 g, h).
Figure 1.
Immunohistochemical staining of neutrophil marker MPO on sections from un-ruptured and non-embolized brain AVM tissue (figure a–f) and epilepsy (figure g–h) control tissue. Brown color, indicated by arrows, is positive MPO signal, and blue color is the counter staining with hematoxylin of nuclei. Figure a showed that neutrophils were present in the vascular wall and lumen of AVMs. Figure b was photographed under higher power from selected area of the figure a, indicted by rectangle. Figure c showed neutrophils were present in the vascular wall as well as parenchymal tissue of AVMs, and figure d was photographed under higher power from selected area of c, indicted by rectangle. Figure e showed that neutrophils were predominantly present in the vascular wall, and image f showed selected area from e under higher magnification. Neutrophils were absent in cortical tissue of epilepsy patient (figure g and h). Representative images from 3 AVM patients and an epilepsy patient; size bar for a, c, e, and g: 100μm; size bar for b, d, f, and h: 25μm
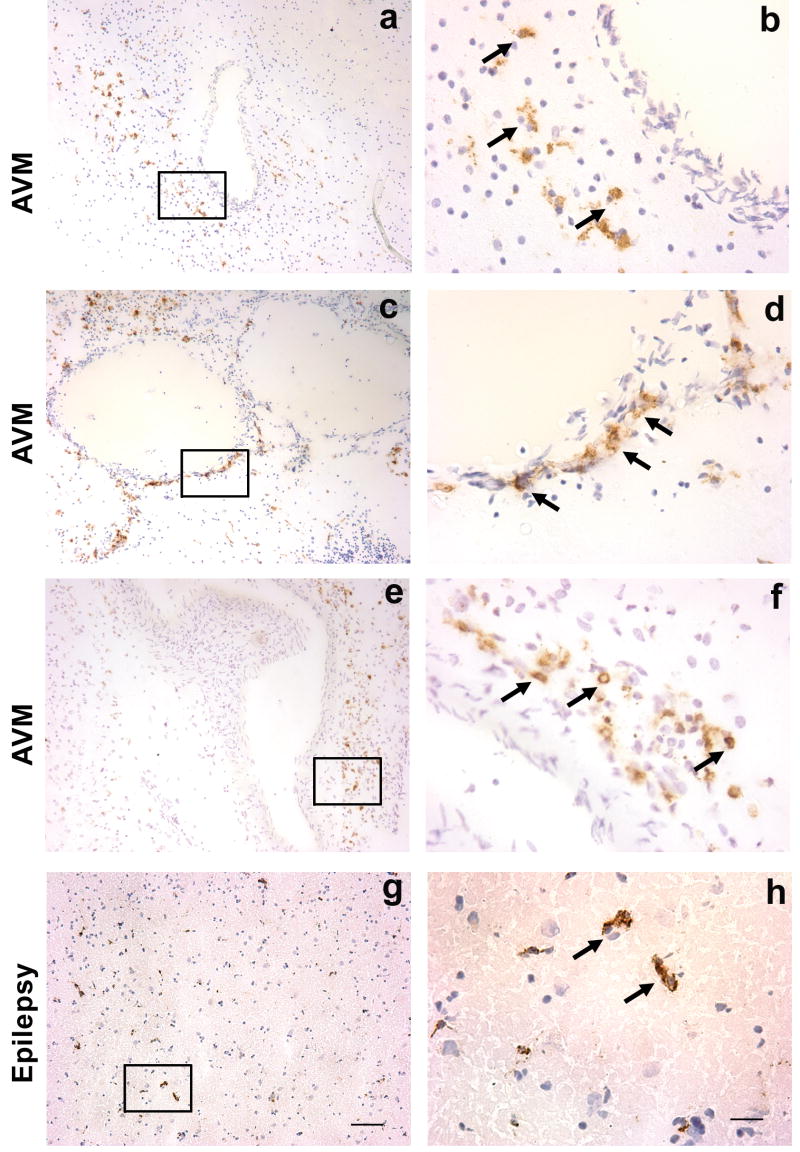
Macrophages, monocytes, and microglia, identified by CD68 staining, were scattered throughout the AVM tissue specimens such as vascular wall and parenchyma (brown color indicated by arrows, Figure 2a, b, c, d, e and f). Figure 2a, c and e are representatives from three brain AVM patients, and g is from an epilepsy patient. Figure 2b, d, f and h are photographed under higher power from areas from Figure 2a, c, e, g respectively (indicated by rectangle).
Figure 2.
Immunohistochemical staining of macrophage/monocyte marker CD68 on sections from un-ruptured and non-embolized brain AVM tissue and epilepsy control tissue. Brown color, shown by arrows, indicates positive CD68 immunoreactivity. Cell nuclei appear blue with hematoxylin counter staining. Figure a showed that macrophages were mainly present in the parenchymal tissue adjacent to the vascular wall, and figure b showed higher magnification of rectangle area from a. Figure c showed that macrophages predominantly reside in the vascular wall and figure d showed higher magnification of rectangle area of c. Similar to figure a, figure e showed that macrophages were mainly present in the parenchymal tissue adjacent to the vascular wall. Figure f was photographed under higher power from selected area, indicted by rectangle, of e. Weak CD68 positive signals were also observed in the cortical tissue from epilepsy patients (figure g and h). Representative images from 3 AVM patients an epilepsy patient; size bar for a, c, e, and g: 100μm; size bar for b, d, f, and h: 25μm
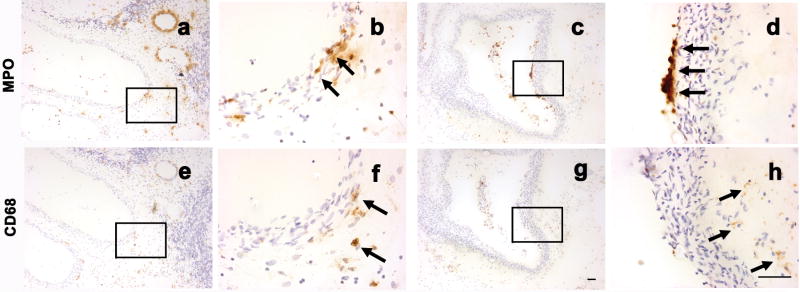
To determine whether neutrophil and macrophage were present in the same vascular loci, serial AVM sections (a and e; c and g) were stained with MPO and CD68 markers respectively. As shown in Figure 3a and 3e, a majority of neutrophils and macrophages were present in proximity in the same vessels. However, in some cases, neutrophils and macrophages were present at different locations: neutrophils mainly at the inner wall, and macrophages mainly at the outer vascular wall (Figure 3c and 3g).
Figure 3.
Co-localization of MPO and CD68 signal. Adjacent AVM tissue sections were stained with MPO, or CD68 marker. Brown color, shown by arrows, indicates positive MPO, or CD68 immunoreactivity. Cell nuclei appear blue with hematoxylin counter staining. Figure a and e showed that neutrophils and macrophages were present in proximity at the same vessels. Figure b and f were photographed under higher power from selected area, indicted by rectangle, of a, e respectively. Figure c and g showed that neutrophils and macrophages were present at different locations: neutrophils were mainly present at the inner wall; macrophages were mainly located at the outer vascular wall. Figure d and h were photographed under higher power from selected area, indicted by rectangle, of c, g respectively. Size bar for a, c, e, and g: 50μm; size bar for b, d, f, and h: 50μm
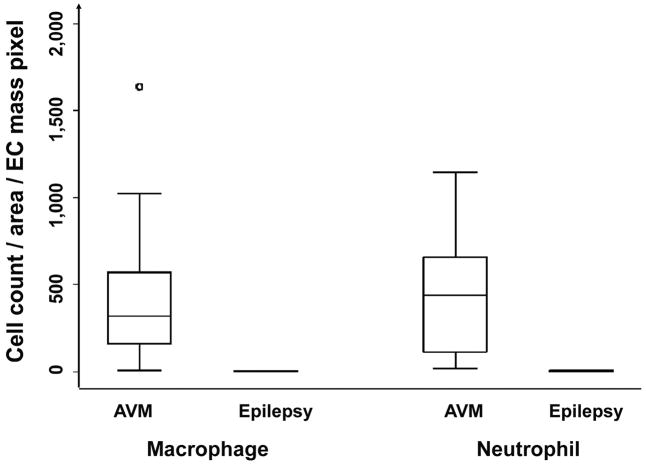
To quantitatively assess leukocyte infilatration in brain AVM tissue, we performed cell counting on slides immunostained with MPO, CD68, or CD31. Table 2 shows results of MPO and CD68 signal representing neutrophils and macrophage indices, respectively, in the vascular wall, lumen, and adjacent parenchyma tissue. As shown in Figure 4, brain AVM tissues had more MPO and CD68 than the cortical tissues from epilepsy patients (MPO: 434 ± 333 vs 5 ± 4, P=0.0001; CD68: 454 ± 404 vs 4 ± 2, P=0.0001; cells/mm2/EC mass pixels). In addition, the amount of neutrophils and macrophages in brain AVMs was not closely correlated (R2 = 0.18, y = 0.515× + 230, n = 20, P = 0.06). We found no association between the amount of neutrophil or macrophage and avm location, size, or drainage (data not shown).
Figure 4.
Comparison of the amount of neutrophil and macrophage between brain AVM patients and epilepsy patients. Cell counting was performed on the slides immunostained with MPO, or CD68. Cell counts were from vascular wall and parenchyma, and normalized by tissue section area (mm2) and the amount of endothelial cells (CD31, pixel). Brain AVM tissues had more MPO and CD68 than the cortical tissues from epilepsy patients (MPO: 434 ± 333 vs 5 ± 4, P=0.0001; CD68: 454 ± 404 vs 4 ± 2, P=0.0001; cells/mm2/EC mass pixel).
In contrast to the abundant MPO and CD68 signals present in brain AVM tissues, the signals for T cells (CD3) and B cells (CD20) were rare in brain AVM tissues (data not shown).
Correlation of neutrophils with MPO and MMP-9 levels
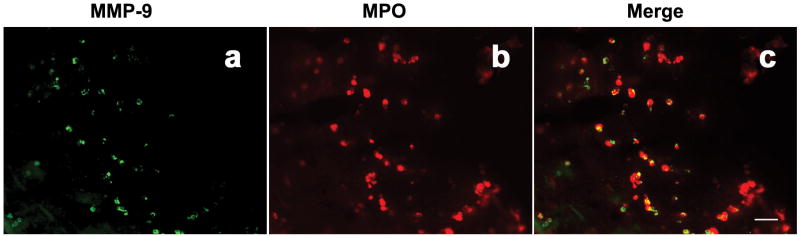
To determine whether neutrophils contribute to MMP-9 production in brain AVM tissue, we performed double immunofluorescent staining of MPO and MMP-9 on AVM tissue sections. As represented in Figure 5, majority of MMP-9 signal (a, green) and MPO signal (b, red) in brain AVM tissue co-localized (c, yellow), indicating that a major source of MMP-9 in AVM tissues was from neutrophils.
Figure 5.
Double staining of MMP-9 (a, green color) and MPO (b, red color) in brain AVM tissue. Yellow color in c indicates co-localization of MMP-9 with MPO. size bar: 50μm
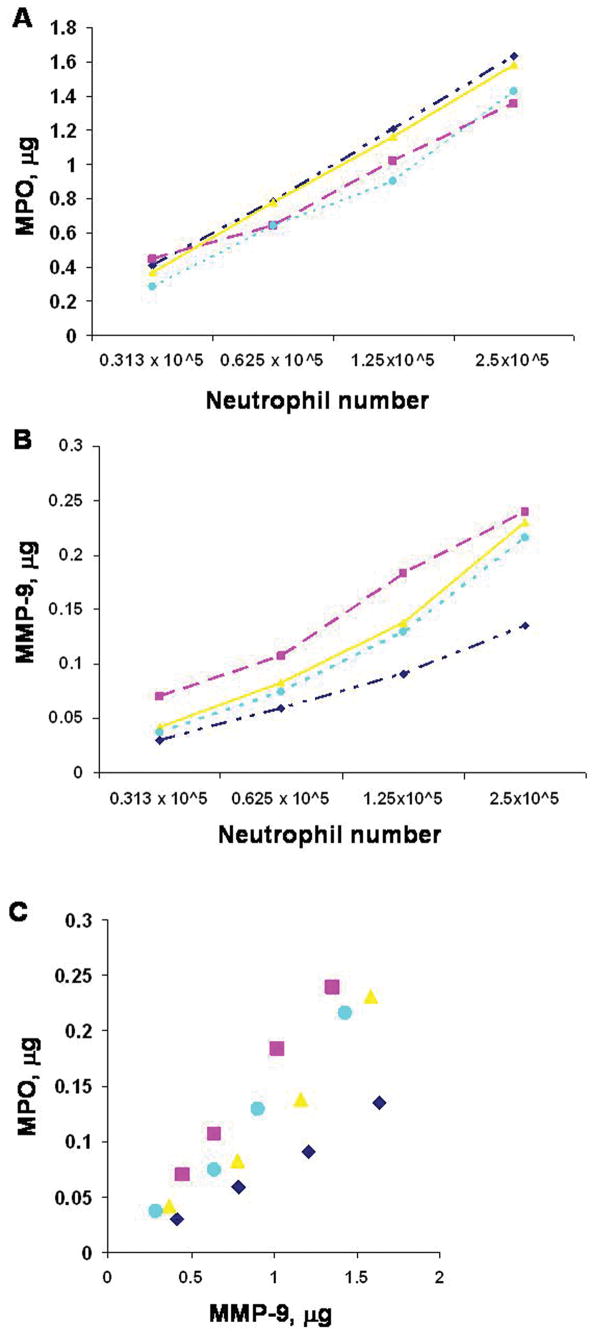
To validate the use of MPO as a marker for quantitative assessment of neutrophils, we measured MPO and MMP levels in neutrophils isolated from patients with brain AVMs. There was a linear relationship among neutrophil counts and MPO and MMP-9 levels (Figure 6A and 6B). Neutrophil count was linearly correlated with MPO and MMP-9 (R2=0.99, R2=0.98). MPO level, in turn, correlated with MMP-9 levels (Figure 6C, R2=0.99). The results indicate that MPO level could be used as an index for assessing the amount of neutrophils, and imply that more neutrophils present in brain avm tissues could be associated with higher MPO and MMP-9 levels.
Figure 6.
A. Correlation of neutrophil number with MPO level. R2 = 0.99, y = 1.85 – 0.37x, n = 4, P<0.01. B. Correlation of neutrophil number with MMP-9 level. R2 = 0.98, y = 0.25 – 0.05x, n = 4, P<0.01. C. Correlation of MMP-9 with MPO. R2 = 0.99, y = 0.14x – 0.02, n = 4, P < 0.01. Neutrophils were isolated from the peripheral blood of four AVM patients at the density of 2.5 ×105. Three serial dilution of each sample was: 1.25×105, 0.625 ×105, 0.312 ×105.
Contribution of the blood pool to tissue measurement of MMP-9
We have previously reported that brain AVM tissues displayed higher levels of inflammatory markers compared to control epilepsy tissues by ELISA (6). Brain AVM tissues dissected in surgery are heterogeneous, and contain varying amount of blood, both intravascular and extravascular. To assess the amount of blood present in AVM tissues, and the contribution of blood source MMP-9 on measurements of total MMP-9 levels, we measured the amount of hemoglobin in brain AVM tissues and used it as an index for the amount of blood and compared it with MMP-9 tissue levels. MMP-9 level was correlated with Hb (Figure 7). However, the blood pool, as reflected by Hb content, contributed to only 6% of the variation in the relationship (R2 = 0.06, y = 0.048× + 9.79, n = 77, P = 0.04), suggesting that blood-borne MMP-9 only partially accounted for the amount of total measured MMP-9 activity in surgical brain AVM specimens.
Figure 7.
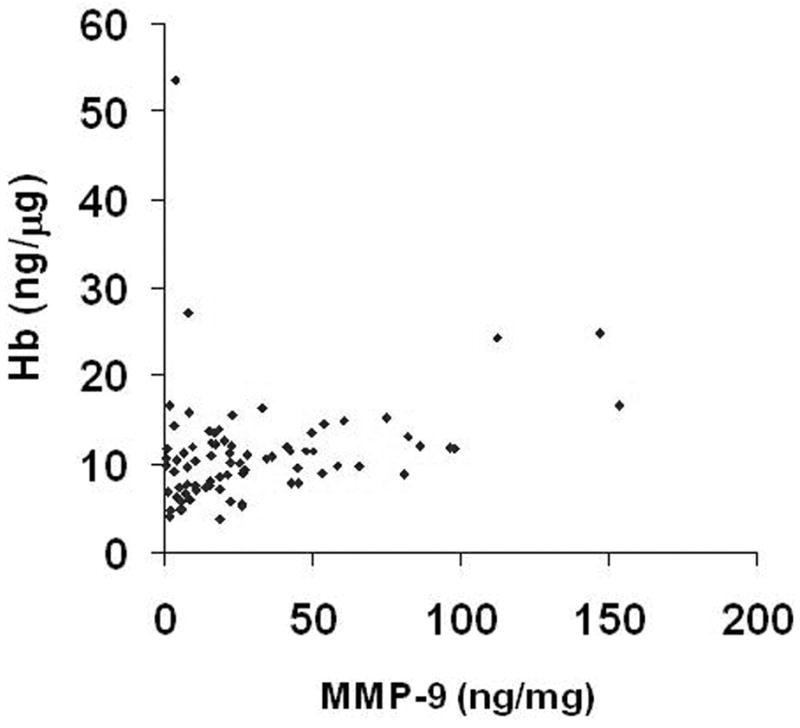
Correlation of MMP-9 with hemoglobin in brain AVM tissue. R2 = 0.057, y = 0.048x + 9.79, n = 77, P = 0.04
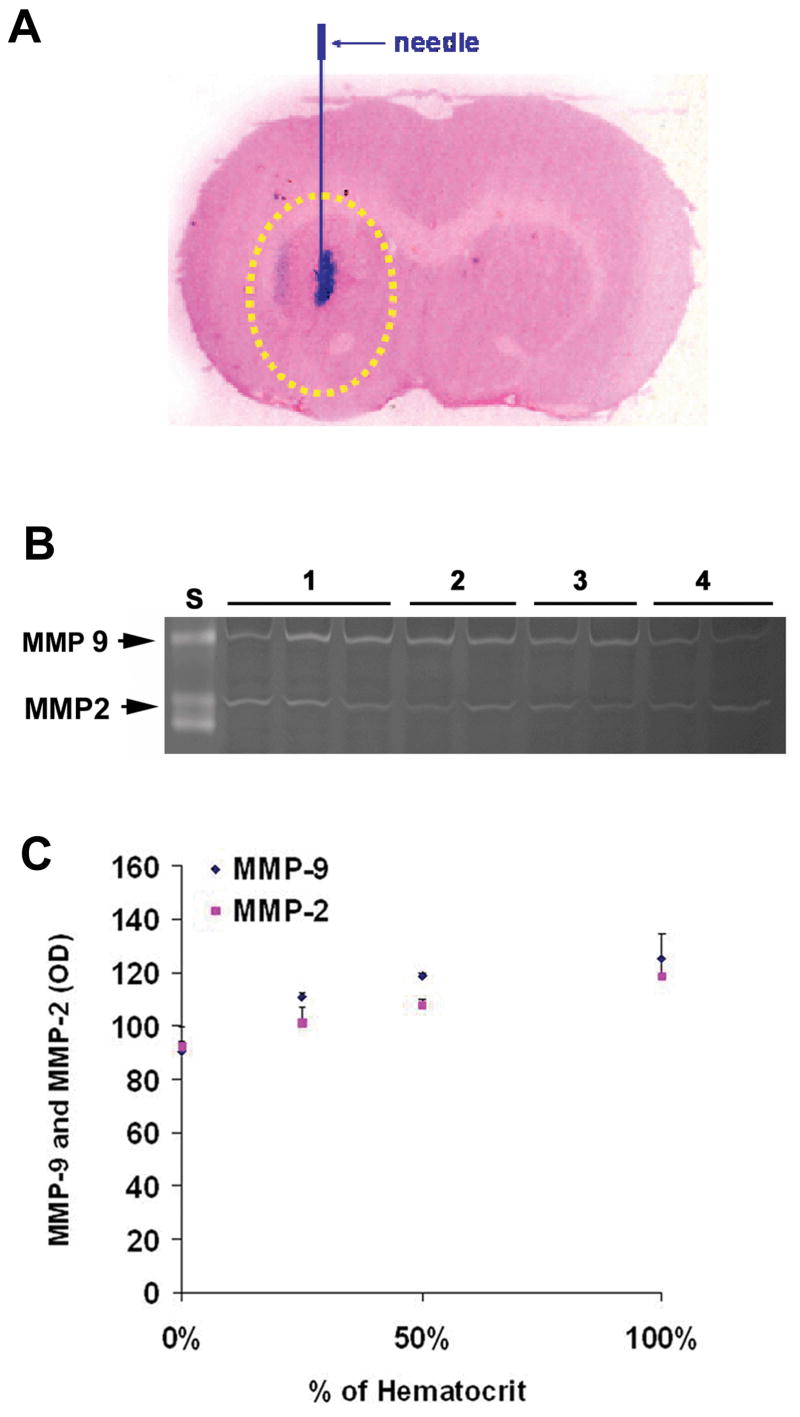
To further examine the potential confounding effects of retained blood cells in AVM tissues on MMP-9 tissue levels, we injected blood at varying hematocrits into the mouse brain and measured MMP-9 tissue levels. Figure 8A shows the site of blood infusion and tissue sampling. Blood injection increased MMP-9 level by 22% to 37% (100% blood 137 ± 11 vs 50% blood 131 ± 1.3 vs 25% blood 122 ± 1.8 vs normal saline 100 ± 10). MMP-2 level increased in a parallel fashion as MMP-9 (Figure 8B and 8C). The results indicate that about 78% to 63% MMP-9 level came from parenchyma tissue, and suggest that even large amounts of extravascular blood could not account for the large increases in MMP-9 level that we have previously described (6).
Figure 8.
A. coronal section of mouse brain showing the site of blood infusion and tissue sampling. Arrow indicates the position of blood (in red) infusion into striatum of the mouse brain. Yellow circled area represents the area of tissue sampling for assessing the level of MMPs. B. MMP-9 and MMP-2 level assessed by zymography in mice brain 3hr following whole blood injection. N= 3 for each treatment group. S: MMP standards, 1: 100% of original hematocrit, 2: 50% of original hematocrit, 3: 25% of original hematocrit, 4: normal saline. C. Quantitation (Optical density) of MMP-9 and MMP-2 levels.
Discussion
This study reports the first quantitative description of neutrophils and macrophages in the nidus of brain AVM tissue removed during microsurgical resection. We found that neutrophils and macrophages were present in the vascular wall of brain AVM tissues, as well as adjacent parenchyma to a greater degree than control tissue. In contrast, we did not observe a significant amount of T and B lymphocytes present in brain AVM tissues. Because prior hemorrhage or embolic material might confound interpretation of inflammatory infiltrates in the lesional tissue, we examined leukocyte infiltrates in brain AVM tissue from unruptured and un-embolized patients. Our current findings are consistent with our prior reports which suggest that inflammatory response is present in brain AVMs and plays a role in its pathophysiology. (6, 7, 20) Our gene microarray studies found that several genes involved in inflammation such as chemokine receptor 1, TGF-β, angiopoitein 2, and integrin α are upregulated in brain AVMs. (20) In addition, our ELISA analysis showed that elevated concentrations of inflammatory biomarkers in brain AVMs.(6, 7) Consistent with our data, Shenkar et al reported that several inflammatory genes such as NF-kB, IL-8 receptor, and TNF-related receptor were increased in brain AVMs.(40) The novel finding of this study is the observation of inflammatory leukocytes in un-ruptured, and non-embolized brain AVM patients.
Inflammatory leukocytes are present in brain AVM tissues
Inflammation has been considered as shared pathological mechanisms in many vascular disorders including aneurysm (10, 21). Inflammatory leukocytes have been implicated as pathological mediators in cardiovascular disease and brain injury (14, 30, 42). Under physiological conditions, leukocytes circulate freely and do not interact with the endothelial cells. During inflammatory cascades, leukocytes transmigrate to the cerebral microvasculature, and accumulate at the inflammatory site in response to cytokines, chemokines, as well as products of tissue breakdown. They secrete enzymes such as MPO, MMPs, and release cytokines including IL-6 that contribute to brain injury (5, 9, 41).
Our immunohistochemical staining demonstrates that neutrophils and macrophages are present in the vascular wall as well as adjacent tissue of brain AVMs. Our data are consistent with the view that inflammation may play a role in brain AVM pathophysiology. The rarity of T and B cells in brain AVMs was somewhat surprising. However, their absence does not preclude an effect of chronic inflammation: macrophages, neutrophils, as well as vascular endothelia are sufficient to produce and release inflammatory mediators.(38) A significant cellular component of chronic inflammation associated with pathological angiogenesis is the macrophage.(2, 11, 32, 33) We do not have definitive explanation or a precedent why pathways for T & B lymphocyte recruitment in brain AVMs are not as pronounced as those for macrophages and neutrophils. Further studies are needed to elucidate the precise stimulatory mechanisms for macrophages and neutrophils recruitment.
Although dexamethasone was given to both lobectomy patients and brain AVM patients in the same dose range, we cannot exclude the possibility that the drug may differentially impact AVM and temporal lobe differently, as the degree of inflammation in the control and brain AVM patients was clearly different and but there is a possibility that there is some difference in the dose response to diminishing the number cells. Because brain AVM tissues appear to have a much higher inflammatory load, we would surmise that dexamethasone probably leads to an underestimation of inflammation in AVM, and thus not affect our conclusions. Inflammatory cells could release various pro-inflammatory cytokines, MMPs, and other proteolytic enzymes capable of weakening or destroying the vascular wall leading to vascular rupture and hemorrhage. MMP-9 is an effector as well as a regulator of leukocyte function. It is stored in the tertiary granules of neutrophils, and is considered a specific marker of neutrophil maturation (35). MMP-9 synthesis in leukocytes is stimulated by lipopolysaccaride and cytokines (12, 15, 39, 45). Enhanced MMP-9 activity could lead to brain AVM hemorrhage.
The amount of neutrophils correlates with MPO and MMP-9 levels
Myeloperoxidase (MPO) is a heme protein expressed at high levels in neutrophils (13, 27, 43), and is a potent proinflammatory mediator (28, 34). To validate the use of MPO as an index for quantitative assessment of neutrophils, we determined MPO and MMP levels in neutrophils isolated from patients with brain AVMs. Our results demonstrate a linear relationship between neutrophil quantity and MPO expression. In addition, the amount of neutrophils has been found to be tightly associated with the level of MMP-9. MMP-9 is a major component of neutrophilic tertiary granules, and is released from neutrophils in inflammation in response to pro-inflammatory cytokines.(35, 39, 45) In our recent animal model studies, we have demonstrated that neutrophil depletion lead to decreased MMP-9 activities.(19) In addition, it has been demonstrated that neutrophils release neutrophil gelatinase-associated lipocalin (NGAL), which protects MMP-9 from degradation, thereby resulting in enhanced MMP-9 enzymatic activity.(46) The data indicate that MPO level serves as a practical means of measuring neutrophil load. The data also imply that more neutrophils present in brain AVM tissues could lead to higher MPO and MMP-9 levels.
Contribution of the blood pool to tissue measurement of MMP-9
Because intra- or extravascular blood may affect MMP-9 expression (44), we assessed the potential impact of blood pool-derived MMP-9 by correlating hemoglobin level with MMP-9 level. The purpose of injecting blood into mice brain and measuring MMP-9 levels (data presented in Figure 8) was to determine possible contribution of MMP-9 in blood, from either intra- and extravascular, on measurements of total MMP-9 levels. We measured MMP-9 levels in mice brain tissues which contained injected blood. This was to mimic MMP-9 determination in brain AVMs which contained a combination of AVM lesional tissue, intravascular blood, and extravascular blood. Since none of our cases in the present study presented with hemorrhage, the estimate of blood pool MMP-9 is a “worst-case” estimate.
In our previous study, we have reported that brain AVM samples had higher levels of MMP-9, TIMP-1, and TIMP-3. MMP-2 and TIMP-2 levels were below detection in brain AVMs.(22) MMP-9 has been implicated to play a critical role in vascular remodeling and rupture, thus we focused on MMP-9 in this study. Our data indicate that MMP-9 derived from the blood pool contributes only to a small degree to the otherwise large amount of total MMP-9 expressed in brain AVM tissue amount, as determined by the ELISA method. This notion is supported by our animal study which showed that increasing dosage of blood only partially elevated MMP-9 level. MMP-9 was determined at 3 hr after blood infusion. We chose this time point to mimic the period for AVM tissue harvest at clinical settings. This is also the time period for neutrophil in the blood to infiltrate and transmigrate to vascular wall (31). Infusion of whole blood into the mouse brain increased MMP-9 level to 2.4 ng/mg compared to1.6 ng/mg with normal saline injection. The mean MMP-9 level measured in brain AVM tissue was 28 ng/mg (6). These data suggest that no more than 1/10 of total MMP-9 derives from the blood pool. We cannot directly translate from mouse to human tissue, but the result serves as an estimate of the order of magnitude. Taken together, our results suggest that the influence of blood contamination, either by induction of astrocytic MMP or direct transfer, might be estimated to be in the range of 6% (by MMP-9 correlation with Hb in AVM tissue analysis) to 37% (by animal model using blood injection), a range that is an order of magnitude lower than previous estimates of MMP and MPO expression in AVM compared to normal brain tissue (6).
Conclusions and Limitations
The data presented here, when taken together with previously reported data linking cytokine genotype with clinical course, are consistent with a view that inflammation is a contributory cause for disease pathogenesis or progression. Inflammation may act alone or perhaps in concert with congenital defects, environmental exposures, or chronic hemodynamic derangements to result in the clinical phenotype. Our results do not prove that inflammatory mechanisms are causative of disease or directly influence the disease progression, but there is a growing body of evidence that is consistent with such hypotheses (10, 17). In our study, choice of control tissue for brain AVM nidus has inherent limitations. We have described previously our use of brain taken from surgical epilepsy patients (22, 23), but others have proposed using superficial temporal arteries (40). Neither of these tissues is ideal, as neither contains intracranial blood vessels of the caliber found in brain AVM nidus. It is unlikely that precise choice of normal comparator tissue would change the conclusions; as inflammation should be absent in both normal parenchyma and conductance vessels. Further, the primary purpose to include tissues from surgical epilepsy patients is to use as a quality control assay to rule out the possible effects of surgical trauma. Future work needs to address the mechanisms by which pro-inflammatory pathways might influence brain AVM growth, progression or rupture. Improved understanding of the disease pathogenesis has the potential to assist in the development of novel therapeutics.
Acknowledgments
This study was supported in part by PHS grants: R01 NS27713 (WLY), R01 NS34949 (WLY), P01 NS44155 (WLY, GYY, TH).
The following members of the UCSF BAVM Study Project contributed to portions of this work including data collection and analysis: Nancy J. Quinnine, RN; Yongfeng Fan, MD, PhD, Yiqian Zhu, MD, Trudy Poon, MS; Van V. Halbach, MD; Randall T. Higashida, MD; Brad Dispensa, BS; Helen Kim, PhD, and Phillipe Jolivalt. The authors thank the other members of the UCSF BAVM Study Project (http://avm.ucsf.edu), and Voltaire Gungab for assistance with manuscript preparation.
Abbreviations
- AVM
arteriovenous malformations
- EC
endothelial cell
- Hb
hemoglobin
- ICH
intracranial hemorrhage
- MMP-9
matrix metalloproteinase 9
- MPO
myeloperoxidase
References
- 1.Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, Johnston SC, Lee C, Lawton MT, Sidney S, Marchuk DA, Kwok PY, Young WL. TNFa-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations (Abstract) Stroke. 2006;37:638–639. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 2.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2007 doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Audrain MA, Baranger TA, Moguilevski N, Martin SJ, Devys A, Lockwood CM, Muller JY, Esnault VL. Anti-native and recombinant myeloperoxidase monoclonals and human autoantibodies. Clin Exp Immunol. 1997;107:127–134. doi: 10.1046/j.1365-2249.1997.d01-895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campana D, Thompson JS, Amlot P, Brown S, Janossy G. The cytoplasmic expression of CD3 antigens in normal and malignant cells of the T lymphoid lineage. J Immunol. 1987;138:648–655. [PubMed] [Google Scholar]
- 5.Cavanagh SP, Gough MJ, Homer-Vanniasinkam S. The role of the neutrophil in ischaemia-reperfusion injury: potential therapeutic interventions. Cardiovasc Surg. 1998;6:112–118. doi: 10.1016/s0967-2109(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang GY, Young WL. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 8.Choi JH, Mohr JP. Brain arteriovenous malformations in adults. Lancet Neurol. 2005;4:299–308. doi: 10.1016/S1474-4422(05)70073-9. [DOI] [PubMed] [Google Scholar]
- 9.Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45:1137–1146. doi: 10.1097/00006123-199911000-00024. discussion 1146-1137. [DOI] [PubMed] [Google Scholar]
- 11.Dahlqvist K, Umemoto EY, Brokaw JJ, Dupuis M, McDonald DM. Tissue macrophages associated with angiogenesis in chronic airway inflammation in rats. Am J Respir Cell Mol Biol. 1999;20:237–247. doi: 10.1165/ajrcmb.20.2.3081. [DOI] [PubMed] [Google Scholar]
- 12.Dasu MR, Barrow RE, Spies M, Herndon DN. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns. 2003;29:527–531. doi: 10.1016/s0305-4179(03)00154-2. [DOI] [PubMed] [Google Scholar]
- 13.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang C-M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 15.Ferroni P, Basili S, Martini F, Cardarello CM, Ceci F, Di Franco M, Bertazzoni G, Gazzaniga PP, Alessandri C. Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. J Investig Med. 2003;51:295–300. doi: 10.1136/jim-51-05-17. [DOI] [PubMed] [Google Scholar]
- 16.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 17.Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 18.Halim AX, Singh V, Johnston SC, Higashida RT, Dowd CF, Halbach VV, Lawton MT, Gress DR, McCulloch CE, Young WL. Characteristics of brain arteriovenous malformations with coexisting aneurysms: a comparison of two referral centers. Stroke. 2002;33:675–679. doi: 10.1161/hs0302.104104. [DOI] [PubMed] [Google Scholar]
- 19.Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, Young WL, Yang GY. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly T, Jr, Stewart CL, Dressman HK, Barbaro NM, Marchuk DA, Young WL. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54:410–423. doi: 10.1227/01.neu.0000103421.35266.71. discussion 423-415. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto T, Meng H, Young WL. Intracranial aneurysms: links between inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006;28:372–380. doi: 10.1179/016164106X14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, Barbaro NM, Higashida RT, Dowd CF, Halbach VV, Young WL. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56:1058–1065. 1058–1065. [PubMed] [Google Scholar]
- 24.Hashimoto T, Yang GY, Young WL. Abnormal expression of MMP9 and TIMPs in brain arteriovenous malformations (Abstract) Anesthesiology. 2002;96:A721. [Google Scholar]
- 25.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 26.Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, Swedenborg J. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. doi: 10.1016/s0741-5214(03)00791-2. [DOI] [PubMed] [Google Scholar]
- 27.Klebanoff SJ. Myeloperoxidase. Proc Assoc Am Physicians. 1999;111:383–389. doi: 10.1111/paa.1999.111.5.383. [DOI] [PubMed] [Google Scholar]
- 28.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 29.Lifson JD, Rossio JL, Piatak M, Jr, Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, Hirsch VM, Reimann KA, Schmitz JE, Ghrayeb J, Bischofberger N, Nowak MA, Desrosiers RC, Wodarz D. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;75:10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucchesi BR, Mullane KM. Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- 31.Maier CM, Hsieh L, Yu F, Bracci P, Chan PH. Matrix metalloproteinase-9 and myeloperoxidase expression: quantitative analysis by antigen immunohistochemistry in a model of transient focal cerebral ischemia. Stroke. 2004;35:1169–1174. doi: 10.1161/01.STR.0000125861.55804.f2. [DOI] [PubMed] [Google Scholar]
- 32.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, Shibuya M. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. 2006;108:1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- 34.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 35.Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- 36.Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990;43:752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok PY, Young WL. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 38.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 39.Pugin J, Widmer MC, Kossodo S, Liang CM, Preas HLn, Suffredini AF. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999;20:458–464. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- 40.Shenkar R, Elliott JP, Diener K, Gault J, Hu LJ, Cohrs RJ, Phang T, Hunter L, Breeze RE, Awad IA. Differential gene expression in human cerebrovascular malformations. Neurosurgery. 2003;52:465–478. doi: 10.1227/01.NEU.0000044131.03495.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimakura A, Kamanaka Y, Ikeda Y, Kondo K, Suzuki Y, Umemura K. Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res. 2000;858:55–60. doi: 10.1016/s0006-8993(99)02431-2. [DOI] [PubMed] [Google Scholar]
- 42.Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tejima E, Zhao BQ, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, Montaner J, Wang X, Lo EH. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J Cereb Blood Flow Metab. 2007;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- 45.Wize J, Sopata I, Smerdel A, Maslinski S. Ligation of selectin L and integrin CD11b/CD18 (Mac-1) induces release of gelatinase B (MMP-9) from human neutrophils. Inflamm Res. 1998;47:325–327. doi: 10.1007/s000110050336. [DOI] [PubMed] [Google Scholar]
- 46.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 47.Young WL, Yang GY. Are there genetic influences on sporadic brain arteriovenous malformations? Stroke. 2004;35:2740–2745. doi: 10.1161/01.STR.0000145054.35083.32. [DOI] [PubMed] [Google Scholar]