Summary
β1 integrin is established as a regulator of angiogenesis based upon the phenotypes of complete knockout of β1 heterodimer partners or ligands and antibody inhibition studies in mice. Its direct function in endothelial cells (ECs) in vivo has not been determined because β1−/− embryos die before vascular development. Excision of β1 from ECs and a subset of hematopoietic cells, using Tie2-Cre, resulted in abnormal vascular development by embryonic day 8.5 (e8.5) and lethality by e10.5. Tie1-Cre mediated a more restricted excision of β1 from ECs and hematopoietic cells and resulted in embryonic lethal vascular defects by e11.5. Capillaries of the yolk sacs were disorganized, and the endothelium of major blood vessels and of the heart was frequently discontinuous in mutant embryos. We also found similar vascular morphogenesis defects characterized by EC disorganization in embryonic explants and isolated ECs. β1-null ECs were deficient in adhesion and migration in a ligand-specific fashion, with impaired responses to laminin and collagens, but not fibronectin. Deletion of β1 reduced EC survival, but did not affect proliferation. Our findings demonstrate that β1 is essential for EC adhesion, migration, and survival during angiogenesis, and further validate that therapies targeting β1 integrins may effectively impair neovascularization.
Keywords: angiogenesis, integrin, endothelial cell, vascular development, extracellular matrix
Introduction
Integrins are cell surface receptors that bind to the extracellular matrix (ECM) and propagate many crucial intracellular signals. Several integrins and their ECM protein ligands are expressed in endothelial cells (ECs) during the formation of new blood vessels, or angiogenesis (Hynes, 2002). This observation has led to intense investigation on integrin-ECM interactions as critical regulators of angiogenesis. Although it is established that integrin-ECM interactions are involved angiogenesis, it is unclear which integrin-ligand pairs are required for the angiogenic process. For example, the integrins αvβ3 and αvβ5 are upregulated in ECs during wound healing and in certain tumor vasculatures, and antibody or small molecule inhibition of these integrins blocks angiogenesis in vivo (Eliceiri and Cheresh, 2001). However, the unexpected finding that β3−/−/β5−/− mice are viable and fertile has challenged the notion that αv integrins are required for angiogenesis and calls for further investigation into the precise role of integrins in angiogenesis.
At least six β1 integrins (α1β1, α2β1, α3β1, α5β1, α6β1, and αvβ1) are expressed in ECs, and several αβ1 heterodimers are thought to be pro-angiogenic. The collagen receptors, α1β1 and α2β1, are upregulated by vascular endothelial growth factor, and antibody-based inhibition of these integrins blocks tumor angiogenesis (Senger et al., 1997). In addition, tumor angiogenesis in α1−/− mice is reduced compared to controls (Pozzi et al., 2000). Fibronectin and its primary integrin receptors, α4β1 and α5β1, are upregulated in blood vessels of several human tumors, and inhibition of α4β1, α5β1, or fibronectin with antibody- or peptide- based approaches blocks angiogenesis in vivo (Garmy-Susini et al., 2005; Kim et al., 2000a). In addition, β1 integrins, and α5β1 in particular, are critical for vascular network formation in embryoid bodies (Bloch et al., 1997; Francis et al., 2002). Finally, α5−/− or fibronectin−/− mice are embryonic lethal due to cardiovascular and neuronal defects, although the precise role of these genes in vascular development remains unclear because of their widespread embryonic expression (Francis et al., 2002; George et al., 1997; George et al., 1993; Yang et al., 1999; Yang et al., 1993).
Direct in vivo genetic evidence for an angiogenic role for endothelial β1 integrins is precluded because β1−/− die at embryonic day (e) 5.5 due to implantation defects, prior to vascular development (Fassler and Meyer, 1995; Stephens et al., 1995). In this study, we deleted β1 in a cell lineage-specific manner, using both Tie2-Cre and Tie1-Cre, to bypass the early implantation defects of β1 complete null embryos, and gained significant insights into its role in EC adhesion, migration, proliferation, and survival during vascular development.
Results
EC expression of β1 integrin is required for early embryonic vascular development
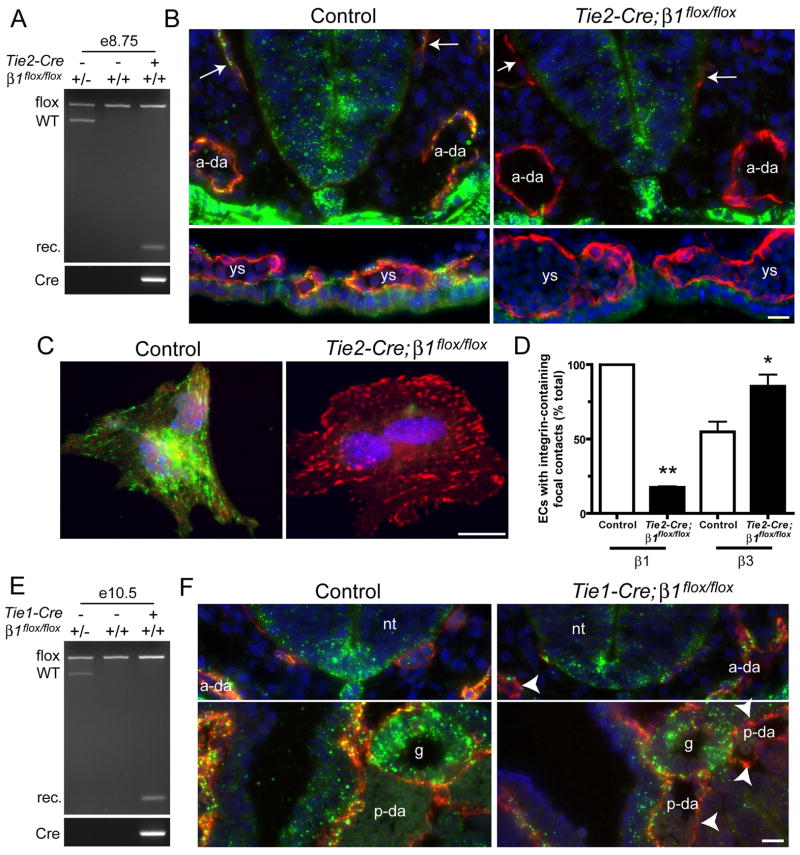
In order to delete β1 integrins in ECs, we bred Tie2-Cre;β1flox/+ male mice with β1flox/flox female mice (Graus-Porta et al., 2001). Cre was also active in a subset of hematopoietic cells in this line (Braren et al., 2006). No live Tie2-Cre;β1flox/flox (from now on referred to as “Tie2-Cre mutant”) mice were born indicating that the mutation was embryonic lethal. Since Cre activity is observed as early as e7.5 in our Tie2-Cre line (Braren et al., 2006), we analyzed e8.5 embryos for β1 deletion by several methods. First, we performed genomic PCR analysis of whole embryos using primers that flank the loxP sites and are capable of detecting wild type β1, floxed β1, and the recombined β1 alleles. We found that only embryos carrying two floxed alleles and Tie2-Cre demonstrated recombination (Fig. 1A). Next, we performed immunofluorescence with antibodies against CD31, a pan-EC marker, and β1 integrins to determine the cell type specificity of gene deletion. β1 integrins were expressed in ECs of the primary head veins, dorsal aorta, and yolk sac blood islands as well as in other tissues in littermate controls, which consisted of embryos lacking Tie2-Cre or having only one floxed β1 allele (Fig. 1B). Conversely, β1 integrins were absent from ECs of Tie2-Cre mutants despite their persistent expression in non-EC tissues. Finally, we digested e8.5 embryos, plated the cells onto fibronectin-coated dishes, and stained the mixed population with anti-CD31, anti-β1 integrin, and anti-β3 integrin antibodies. While control ECs displayed prominent β1 integrin focal contact staining, only about 10% of Tie2-Cre mutant ECs had detectable β1 integrins in focal contacts (Fig. 1C,D). We also observed more prominent focal contact staining of β3 integrins in Tie2-Cre mutant ECs compared with control ECs, suggesting compensation for the absence of β1. These findings indicate that Tie2-Cre mediates efficient deletion of β1 integrins from ECs in embryos by e8.5.
Figure 1. Tie2- and Tie1-Cre mediate efficient deletion of β1 integrins in embryos.
Gene deletion analyses of Tie2- (A–D) and Tie1- (E,F) mutants. (A) Genomic PCR analysis of e8.5 embryos demonstrates recombination (rec.) of β1 in embryos carrying Tie2-Cre and two floxed alleles of β1. (B) e8.5 cryosections were stained with anti-CD31 (red), anti-β1 integrin (Ha2/5, green), and DAPI (blue). (C) Collagenase-dissociated e8.5 embryonic cells were plated onto FN and stained with anti-β1 (HMβ1-1, green), anti-β3 (red), and DAPI (blue). EC identity in C was determined by co-staining with anti-CD31 (not shown). (D) Focal contacts in isolated ECs. Bars are means + SEM of 2 (β1) or 3 (β3) experiments. **p < 0.01 by one sample T-Test and *p < 0.05 by Student’s T-test. (E) Genomic PCR analysis of e10.5 embryos from Tie1-Cre matings. (F) e9.5 cryosections were stained with anti-CD31 (red), anti-β1 integrin (green), and DAPI (blue). Arrows, primary head veins; arrowheads, β1-negative endothelium; ys, yolk sac blood islands; a-da, anterior dorsal aortae; p-da, posterior dorsal aortae; nt, neural tube; g, gut; ve, visceral endoderm. Also see Fig. 3F for diagram of embryonic structures. Bars, 20 μm.
Tie2-Cre mutant embryos were indistinguishable from controls upon dissection at e8.5 (data not shown). At e9.0, Tie2-Cre mutants had normal sizes but appeared to have slightly enlarged pericardial sacs (see Fig. S1 in supplementary material). By e9.5, almost all Tie2-Cre mutants displayed enlarged pericardial sacs indicative of edema, which is a common phenotype of mutations that affect the vasculature at this stage (Conway et al., 2003). Approximately half of e9.5 Tie2-Cre mutants were also growth-retarded, a phenotype that became more obvious as the embryos neared death around e10.5. No live Tie2-Cre mutants were observed at e11.5 (see Table S1 in supplementary material).
To verify that EC expression of β1 integrins is required for vascular development, we also used the Tie1-Cre line, which mediates recombination of floxed genes in the majority of ECs and a small fraction of hematopoietic cells in embryos (Gustafsson et al., 2001). We bred Tie1-Cre;β1flox/+ males with β1flox/flox females and found that among 8 litters of newborn pups, only one live Tie1-Cre;β1flox/flox (from now on referred to as “Tie1-Cre mutant”) mouse was born, indicating that this genotype was also embryonic lethal. Timed embryo dissections yielded zero Tie1-Cre mutants at e14.5 or e12.5. At e11.5, 4 of 4 Tie1-Cre mutants were apparently dead in that they lacked a heartbeat and were growth retarded. e10.5 Tie1-Cre mutants were also slightly growth retarded, and the majority of embryos displayed hemorrhaging in the head veins (see Fig. S1 in supplementary material). Genomic PCR analysis of e10.5 embryos demonstrated recombination of β1 in embryos carrying two floxed β1 alleles and Tie1-Cre (Fig. 1E). Unlike Tie2-Cre mutants, the size and appearance of e9.5-e10 Tie1-Cre mutants were similar to those of controls, perhaps because β1 deletion was incomplete in Tie1-Cre mutant ECs at e9.5 (Fig. 1F). Thus, most Tie1-Cre mutants die around e11.5, approximately one day later than Tie2-Cre mutants (see Table S1 in supplementary material).
EC disorganization occurs throughout the entire cardiovascular system in the absence of EC β1 integrins
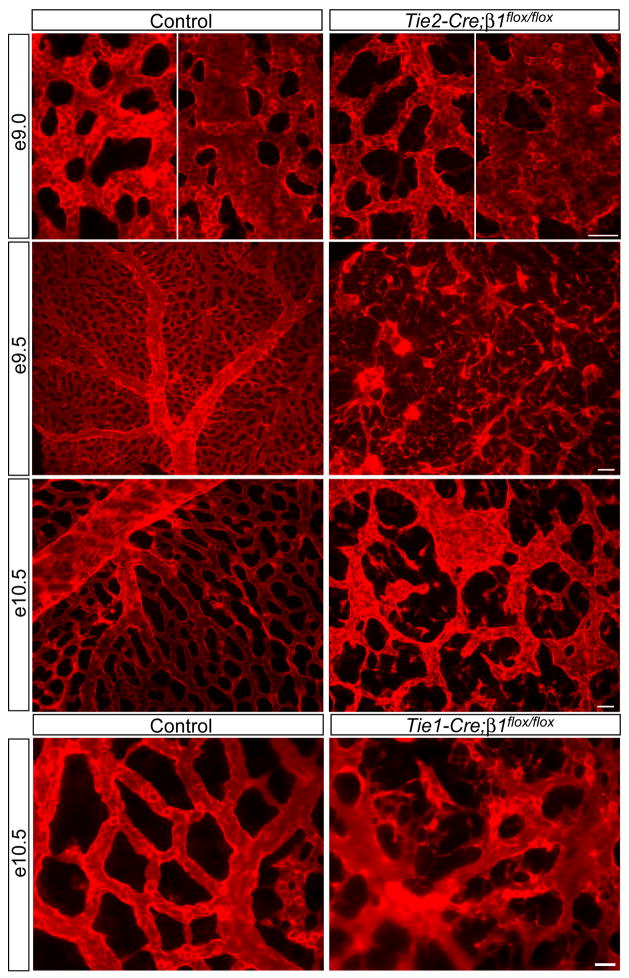
The embryonic yolk sac undergoes extensive angiogenic remodeling from e8.5–e10.5, and therefore we examined this tissue by anti-CD31 immunofluorescence. Although we observed rather homogenous capillary plexuses at e8.5 in both control and Tie2-Cre mutant yolk sacs, mutant capillaries were slightly thinner than controls in some regions (data not shown). More strikingly, subsequent arteriovenous remodeling of yolk sac blood vessels was severely affected in the Tie2-Cre mutants. Whereas large tubular vessels were evident in control yolk sacs at e9.0, mutant blood vessels failed to organize into tubular structures and instead assembled into sac-like structures (Fig. 2). At e9.5 and e10.5, a hierarchical network of arteries, veins, and capillaries was developed in control yolk sacs, whereas Tie2-Cre mutant yolk sacs contained a disorganized network of thin capillaries and sac-like structures containing CD31-positive ECs. Connections appeared to be missing among mutant blood vessels, and we frequently observed individual or small clusters of ECs between vessels at e9.5 and e10.5. We also examined the yolk sac vasculature of Tie1-Cre mutants and found that blood vessels were disorganized, irregularly shaped, and frequently contained prominent EC protrusions in between vessels (Fig. 2). Taken together, these findings suggest that β1 integrins are required within ECs for proper vascular morphogenesis and that in their absence, ECs are disorganized and detached from one another.
Figure 2. Endothelial deletion of β1 integrins causes EC disorganization leading to cardiovascular defects and lethality at midgestation.
Whole mount anti-CD31 immunostained yolk sacs at the indicated embryonic stages. The left panels of e9.0 are capillary regions and the right panels are regions apparently undergoing arteriovenous remodeling. Note the disconnected capillaries and overall vascular disorganization in all mutants. Images are representative of at least 5 control/mutant embryo pairs at each stage. Bars, 100 μm (e9.5), 50 μm (e9.0 and e10.5, Tie2-Cre), and 25 μm (Tie1-Cre).
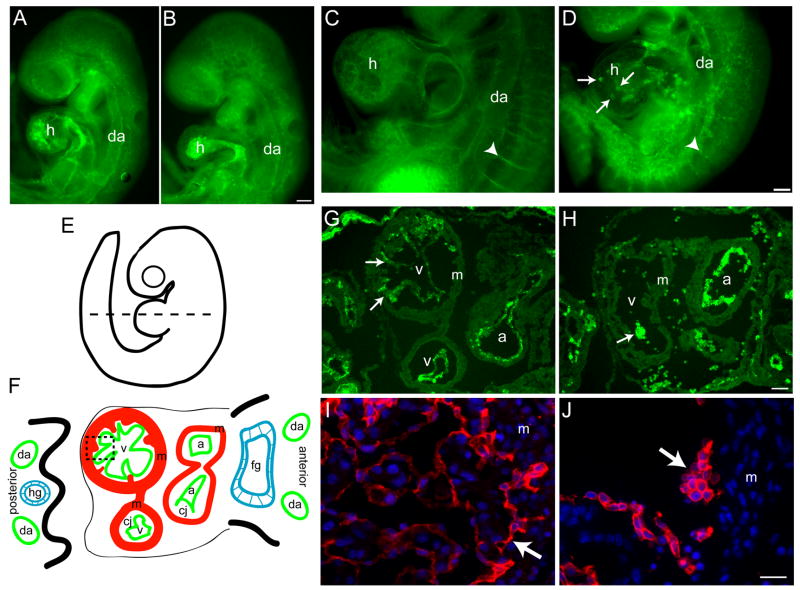
Tie2- and Tie1-Cre were active in the endocardium, and therefore we also analyzed cardiac development. In controls, the primitive heart tube looped and developed into a four-chamber heart by e9.0–e9.5 (see Fig. S1 in supplementary material and Fig. 3A,C). During this period, the ventricular myocardium underwent trabeculation and the endocardium became more tightly associated with the myocardium (Fig. 3C,G). In Tie2-Cre mutants, the primitive heart remained tubular at e9.0, and the endocardium appeared less intricate than the control endocardium (Fig. 3B). Heart looping and four-chamber development eventually occurred in the mutants, but it was delayed by about a half day relative to controls (Fig. 3D,H and data not shown). The most striking phenotype in Tie2-Cre mutant hearts was the disorganization of endocardial cells, which appeared as speckles in whole mount anti-CD31 stains at e9.5 and later (Fig. 3D). Cross-sectional analyses revealed that mutant endocardial cells formed clusters that failed to associate tightly with the ventricular or atrial myocardium (Fig. 3H). This contrasted the control endocardium, which formed a monolayer that closely apposed the entire trabecular surface of the myocardium (Fig. 3G,I). Like the Tie2-Cre mutants, Tie1-Cre mutants had cardiac defects that were characterized by poorly trabeculated ventricles containing rounded endocardial cells that had detached from the myocardium (Fig. 3J).
Figure 3. Endocardial cell patterning defects and abnormal cardiac morphogenesis in the absence of β1 integrins.
Endothelial staining of Tie2-Cre (A–F) and Tie1-Cre (G,H) mutants. Whole mount anti-CD31 immunostained control (A,C) and Tie2-Cre mutant (B,D) embryos at e9.0 (A,B) and e9.5 (C,D). (E,F) Diagram of an e9.5 embryo. Dashed line indicates the approximate plane of the cross-section diagrammed in F, and of the e9.5 control (G) and Tie2-Cre mutant (H) cryosections stained with anti-CD31 antibodies. The dashed box in F indicates the approximate location of the e10.5 control (I) and Tie1-Cre mutant (J) paraffin sections stained with anti-VE-cadherin antibodies and DAPI. Arrows, endocardium; arrowheads, intersomitic vessels; da, dorsal aorta; h, heart; a, atrium; v, ventricle; m, myocardium; fg, foregut; hg, hindgut; In F, the endocardium (green) is separated from the myocardium (red) by the cardiac jelly (cj). Bars, 100 μm (A–D, G,H), 20 μm (I,J). All images are representative of at least 4 control/mutant embryo pairs.
Next, we examined the patterning and structure of embryonic blood vessels. Tie2-Cre mutant dorsal aortae were formed and patent at e9.0 and the capillary beds appeared similar to controls (Fig. 3A,B). However, at e9.5 (Fig. 3D) and e10.5 (data not shown), Tie2-Cre mutant dorsal aortae had narrowed, capillaries were disorganized, and intersomitic vessels failed to form (Fig. 3D).
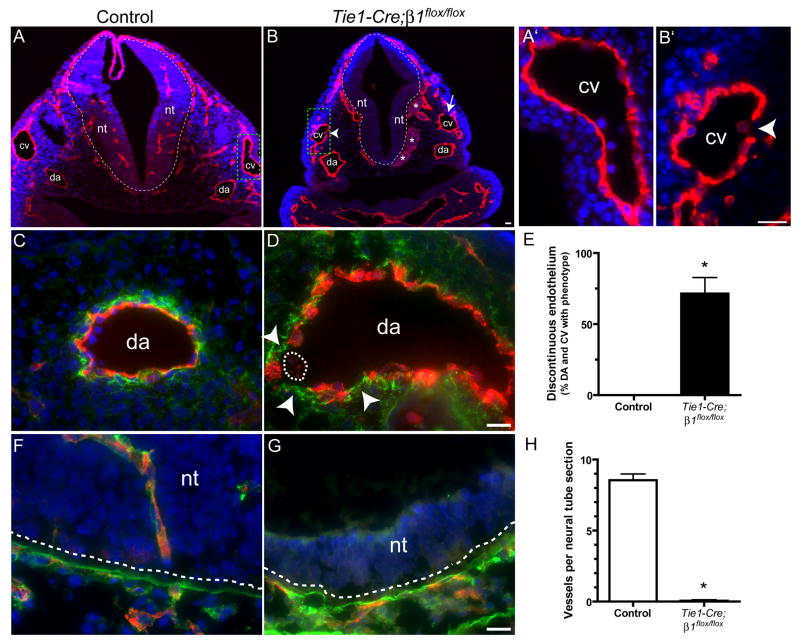
Several prominent vascular defects were observed in Tie1-Cre mutants at e10.5. First, the endothelium of Tie1-Cre mutant (Fig. 4B,B′) blood vessels was frequently discontinuous, unlike the continuous monolayer observed in controls (Fig. 4A,A′). This phenotype was most clearly observed when the basement membrane to which ECs adhere was labeled with anti-fibronectin antibodies (Fig. 4C,D), and was detected in approximately 75% of all dorsal aortae and cardinal vein sections analyzed (Fig. 4E). Second, the fibronectin staining was more diffuse and less prominent in mutant basement membranes as compared with controls, where it stained brightly beneath the continuous EC monolayer (Fig. 4C). A similar reduction in the intensity of fibronectin staining was observed in the vascular walls of Tie2-Cre mutants as well (data not shown). Third, cranial blood vessels in mutants were frequently dilated (Fig. 4B), unlike controls. Finally, we detected blood vessel patterning defects in mutant neural tubes. Whereas numerous capillaries that co-stained prominently with laminin were present within control neural tubes (Fig. 4A,F,H), mutant neural tubes were completely devoid of capillaries (Fig. 4B,G,H), even though the laminin boundary between the mesenchyme and neural tube was present (Fig. 4G). In summary, deletion of β1 integrins via Tie2- or Tie1-Cre leads to widespread EC disorganization resulting in embryonic lethality at mid-gestation.
Figure 4. EC detachment and absence of neural tube invasion upon Tie1-Cre-mediated deletion of β1 integrins at e10.5.
(A,B) Embryos were whole mount stained with anti-VE-cadherin (red), embedded in paraffin, sectioned, and counterstained with DAPI (blue). The dashed line indicates the boundary between the neural tube (nt) and the mesenchyme. The arrow indicates an area of discontinuous endothelium in the cardinal vein (cv) and the arrowhead indicates an endothelial cell apparently undergoing detachment. Asterisks indicate dilated blood vessels. Dorsal aortae (da) are labeled. The cardinal veins in the dashed boxes are shown at high magnification in A′ and B′. (C,D,F,G) Embryo cryosections stained with anti-CD31 (red), DAPI (blue), and anti-fibronectin (green, C,D) or anti-laminin (green, F,G). Arrowheads in D indicate discontinuous endothelium along the vascular basement membrane, and the dashed line encircles an endothelial cell within the lumen that has apparently detached. In F and G, the laminin-rich basement membrane (green) indicated by the dashed lines separates the nt from the mesenchyme. Note the absence of ECs in the neural tubes in mutants, and that significant laminin surrounds ECs within the control neural tubes. (E,H) Bars are the mean values + SEM of 4 control/mutant pairs at e10.5. *p < 0.001 by Student’s T-test. Bars, 20 μm.
Abnormal vascular morphogenesis is due to EC-intrinsic defects upon β1 integrin deletion
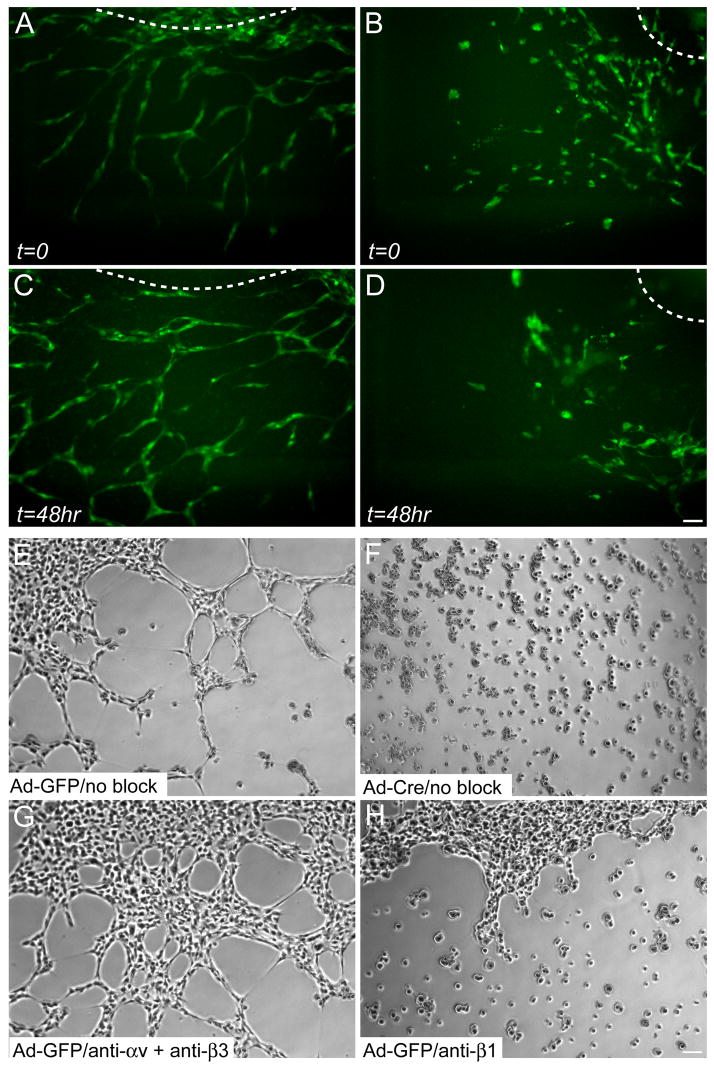
Fluid shear stress resulting from blood flow is known to contribute to vascular remodeling in the chick and mouse yolk sacs (le Noble et al., 2004). In order to eliminate the influence of shear stress and examine the EC-intrinsic effects of β1 integrin deletion on capillary morphogenesis, we performed two in vitro angiogenesis assays. In the first, we monitored vascular development in para-aortic splanchnopleural (P-Sp) explants (Takakura et al., 1998). To do so, we dissected the P-Sp region of e8.5 control and Tie2-Cre mutant embryos and cultured it on top of a feeder layer of OP9 mouse stromal cells. We then monitored vascular development in cultures expressing the green fluorescent protein (GFP) in ECs via a Tie1-GFP transgene (Iljin et al., 2002) in real-time with timelapse fluorescence videomicroscopy. In cultures lacking Tie1-GFP, we used endpoint whole mount anti-CD31 immunostaining. Vascular network formation began in control cultures when spindle-shaped tip ECs sprouted outwards from the P-Sp. The tip ECs retained contact with ECs at their rears, and the sprouts grew more or less as single-file lines of ECs. The complexity of control networks was increased when two or more sprouts came into contact with each other, and multiple ECs within each sprout made stable connections with neighboring ECs (Fig. 5A,C and Movie 1,2). In contrast to the ordered development of EC networks in control P-Sp cultures, Tie2-Cre mutant ECs, while highly motile, were rounded and did not maintain their migration paths (Fig. 5B,D and Movie 1,2). Mutant ECs migrated in clusters and either failed to make EC-EC connections, or made transient ones. We also observed that mutant ECs died more frequently than control ECs (Movie 1,2). The inability of mutant ECs to make and maintain stable EC-EC connections led to the appearance of EC clusters at the endpoint of the cultures (Fig. 5D), which contrasted the vascular networks that formed in control cultures (Fig. 5C).
Figure 5. EC-autonomous role for β1 integrins in angiogenic remodeling.
Still images of control (A,C) and Tie2-Cre mutant (B,D) P-Sp explant cultures at the indicated timepoints. GFP-positive ECs were visualized by a Tie1-GFP transgene. Aberrant EC clusters are evident in Tie2-Cre mutant explants, but not in control explants. The OP9 feeder cells, not visible in the fluorescence images, were a confluent monolayer on top of which the ECs grew out from the P-Sp. The dashed lines indicate the approximate location of the P-Sp explants. See also supplemental online movies. (E–H) Capillary morphogenesis of embryonic β1flox/flox ECs immortalized with PyMT and subsequently infected with adenovirus (Ad), in the absence of any antibodies (E,F) or in the presence of anti-αv plus anti-β3 (G) or anti-β1 (H) function-blocking integrin antibodies. Phase contrast images were captured after 4 hr in culture. Bars, 100 μm.
We next tested whether β1-null embryonic ECs were capable of forming capillary networks on Matrigel, a gelatinous basement membrane mixture consisting of mostly laminin and collagen IV that promotes EC network formation. These experiments required large numbers of purified ECs, so we generated immortalized embryonic ECs from e9.5 β1flox/flox embryos (called ECβ1flox/flox), and infected them with control adenovirus containing GFP (Ad-GFP or control) or Ad-Cre (or mutant). Within 4 hr of seeding single cell suspensions onto Matrigel, control ECsβ1flox/flox formed elaborate networks reminiscent of capillary beds (Fig. 5E). In contrast, mutant ECsβ1flox/flox remained as single cells or isolated groups at 4 hr (Fig. 5F). The mutant cells did not form networks at later time points, indicating that the morphogenic process is blocked in the absence of β1 integrins and not simply delayed (data not shown). To confirm the role of β1 integrins in this process, we seeded control ECsβ1flox/flox in the presence of function-blocking anti-integrin antibodies, and found that anti-β1 antibodies (Fig. 5H), but not the combination of anti-αv plus anti-β3 antibodies, phenocopied the response of mutant ECsβ1flox/flox (Fig. 5G). To verify that the anti-αv and anti-β3 antibodies were function-blocking, we included the combination in a cell adhesion assay and found that they significantly inhibited the adhesion and spreading of control ECsβ1flox/flox on vitronectin (data not shown). The EC disorganization observed in the P-Sp and Matrigel assays support our in vivo findings, and indicate that β1 integrins are required in an EC-intrinsic manner for angiogenesis.
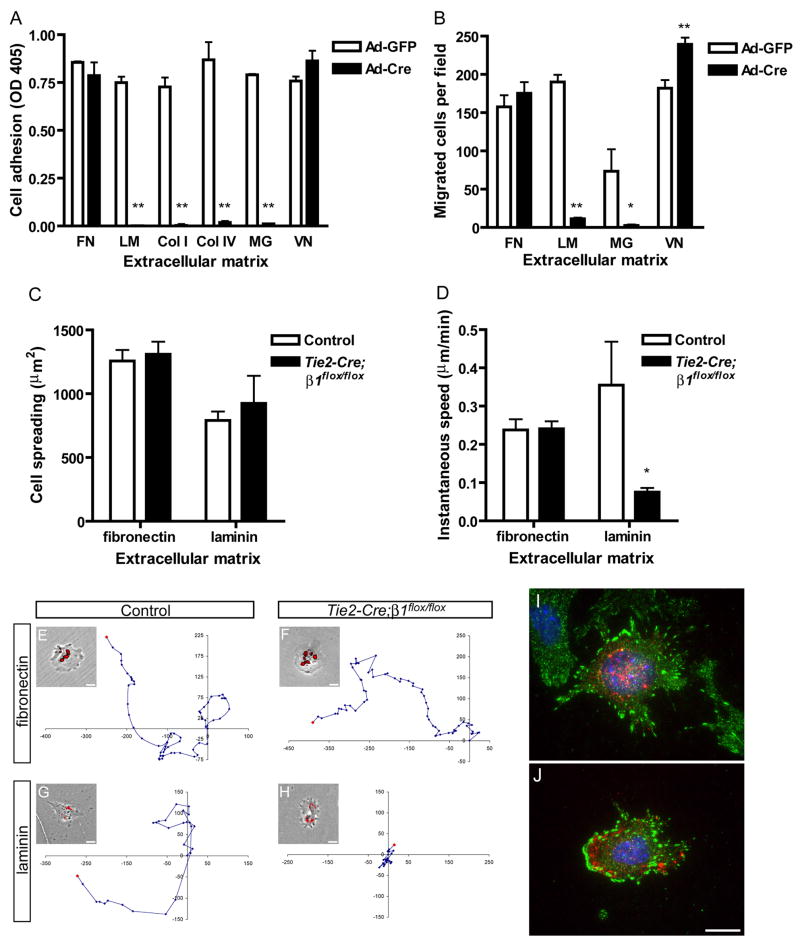
β1-null ECs are defective on collagens and laminin, but not fibronectin in vitro
The embryonic vascular ECM consists of fibronectin, laminin, and collagen, and β1 integrins are thought to be the primary EC receptors for each of these proteins (George et al., 1997; Kalluri, 2003). We therefore examined the requirement for β1 integrins for interactions with these ECM proteins in vitro. To determine whether β1 integrins are required for EC adhesion, we plated control and mutant ECsβ1flox/flox onto ECM-coated plates in serum-free medium. We found that while control ECsβ1flox/flox adhered well to all tested ECM, mutant ECsβ1flox/flox failed to adhere to laminin, collagen I, collagen IV, or Matrigel, even though they adhered as well as controls to fibronectin or vitronectin (Fig. 6A). Next, we tested whether β1 integrins are required for EC haptotaxis, or migration in response to an immobilized substrate, in the absence of serum. Control and mutant ECsβ1flox/flox were plated onto ECM-coated filters and allowed to migrate for 4 hr. Fibronectin, laminin, Matrigel, or vitronectin each supported the migration of control ECsβ1flox/flox (Fig. 6B). Conversely, mutant ECsβ1flox/flox were incapable of migrating on laminin or Matrigel, even though they migrated as well as controls on fibronectin and significantly more so than controls on vitronectin.
Figure 6. β1 integrins are required for EC adhesion and migration in a matrix-specific manner.
Analysis of immortalized embryonic β1flox/flox ECs (A, B) or primary embryonic ECs (C–J). (A) Adhesion of adenovirus (Ad) infected embryonic β1flox/flox ECs in serum-free medium after 30 min. Microplate coating concentrations were: fibronectin (FN), 20 nM; laminin (LM), 25 nM; collagen I (Col I), 55 nM; collagen IV (Col IV), 40 nM; Matrigel (MG), 125 μg/ml; vitronectin (VN), 20 nM. (B) Haptotactic migration of embryonic β1flox/flox ECs in serum-free medium after 4 hr. The ECM that served as the stimulus for migration was coated to the underside of a filter at the following concentrations: FN, 40 nM; LM, 50 nM; MG, 500 μg/ml; VN, 20 nM. (C) Cell spreading after 20 hr culture. (D) Migration speed measured over 24 hr (fibronectin) or 14 hr (laminin) by timelapse videomicroscopy. (E–H) Representative migration tracks along with a phase contrast image overlaid with DiI-Ac-LDL uptake fluorescence. Units on migration tracks are pixels. Coating concentrations were: fibronectin, 40 nM; laminin, 15 nM. Focal adhesion formation in control (I) and mutant (J) embryonic ECs on fibronectin as assessed by anti-FAKpY397 (green), anti-CD31 (red), and DAPI (blue) staining. Values in panels A and B are means + SD, and in panels C and D are means + SEM. **p < 0.01 and *p < 0.05 by Student’s T-test. All experiments were performed at least twice and representative results are shown. Bars, 20 μm.
Next, we examined the phenotype of primary ECs by dissociating e9.0 embryos, plating them onto ECM-coated plates in serum-containing medium, and labeling ECs with DiI-Ac-LDL. We then monitored EC behaviors with timelapse fluorescence videomicroscopy. We found that mutant ECs spread on fibronectin or laminin as well as control ECs (Fig. 6C). The ability of primary mutant ECs, but not mutant ECsβ1flox/flox (Fig. 6A), to adhere to laminin may be due to the presence of serum or to the longer duration of attachment required for the culture of primary cells. We also measured the motility of primary ECs, and found that the average speed of mutant ECs was similar to controls on fibronectin (Fig. 6D–F). In contrast, mutant ECs were significantly less motile than control ECs on laminin (Fig. 6D,G,H). Finally, we examined focal contact formation on fibronectin, but did not detect any obvious differences in the staining patterns of activated focal adhesion kinase (FAK, Fig. 6I,J) or paxillin (data not shown) among control and mutant ECs.
β 1 integrins regulate EC growth by affecting cell survival, but not proliferation
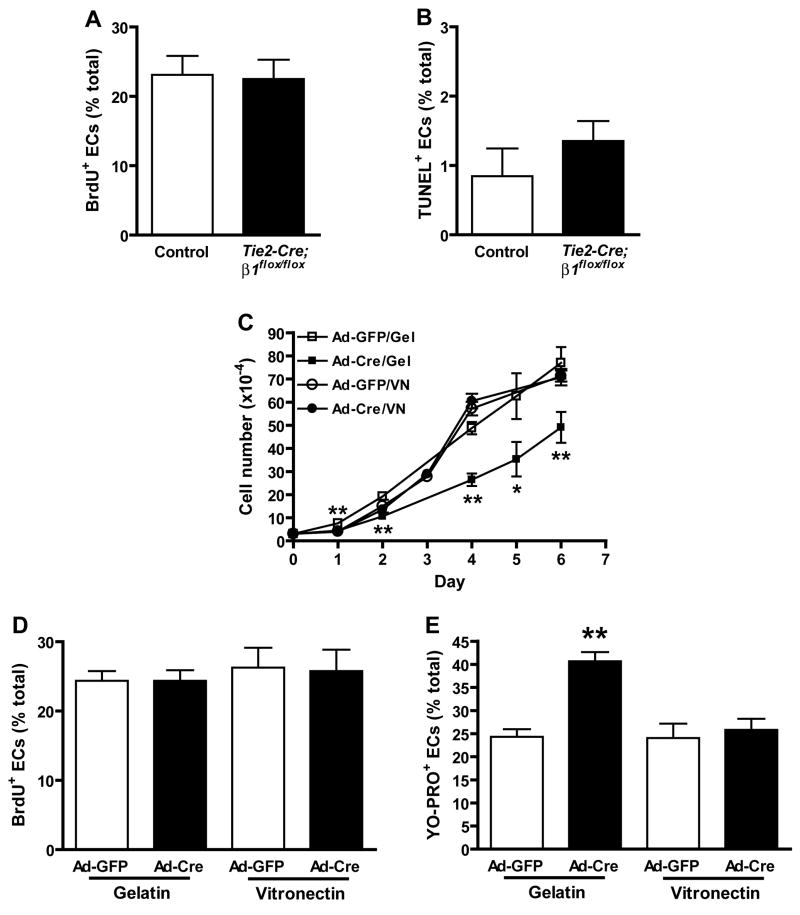
Integrins regulate cell proliferation and survival in adherent cell types (Giancotti and Ruoslahti, 1999). To test whether decreased proliferation or survival contributed to the vascular defects of mutants, we performed BrdU incorporation and TUNEL assays on control and Tie2-Cre mutant embryos at e9.0. Approximately 25.1 +/− 1.8% of control and 23.9 +/− 1.4% of Tie2-Cre mutant EC incorporated BrdU within 2 hr (Fig. 7A). We observed a slight increase in mutant EC apoptosis, as 0.85 +/− 0.4% of control and 1.35 +/− 0.3% of Tie2-Cre mutant ECs were TUNEL positive (Fig. 7B). Mutant ECs were substantially more apoptotic than controls at e9.5, but mutant non-ECs were highly apoptotic, which may indicate that systemic defects also contributed to cell death at this stage (data not shown).
Figure 7. β1 integrins regulate EC growth through effects on survival rather than proliferation.
In vivo (A, B) and in vitro (C–E) effects of β1 deletion on EC growth. The ratios of BrdU/CD31 (A) and TUNEL/CD31 (B) double positive ECs relative to total ECs were calculated from multiple immunostained cryosections prepared from 3 (A) or 4 (B) pairs of control and Tie2-Cre mutant embryos at e9.0 prior to the onset of overt morbidity. Bars in panels A and B are means + SEM, and differences are not statistically significant by Student’s T-test (A, n = 1200 control and 965 Tie2-Cre mutant EC, p = 0.625; B, n = 1299 control and 1116 mutant EC, p = 0.36). (C) Growth rate of embryonic β1flox/flox ECs cultured on gelatin (Gel) or vitronectin (VN). (D) Incorporation of BrdU into DNA after 6 hr culture. (E) Entry of the apoptotic cell permeant dye, YO-PRO-1, into proliferating EC cultures. Panel C-E data are means + SD of replicate measurements, and each experiment was repeated with similar results. *p < 0.01 and *p < 0.05 by Student’s T-test.
We also measured the growth rate of ECsβ1flox/flox cultured in two different conditions: either on gelatin, a direct ligand for β3 integrins (Davis, 1992) and an indirect ligand for β1, β3, and β5 integrins due to its high affinity for fibronectin present in serum (Engvall and Ruoslahti, 1977), or on vitronectin, a ligand for β3 and β5, but not β1, integrins. We found that while mutant ECs grew significantly slower than control ECs on gelatin, their growth was unaffected on vitronectin (Fig. 7C). Mutant ECs incorporated BrdU into DNA as efficiently as controls on both ECM (Fig. 7D). Mutant ECs were significantly more permeable than control ECs to the apoptotic cell dye YO-PRO-1 on gelatin, but not on vitronectin (Fig. 7E). These findings, considered together with the P-Sp results, suggest that β1 integrin deletion impairs EC growth primarily through effects on cell survival pathways.
Discussion
We set out to determine what role β1 integrins play within ECs during embryonic vascular development using a cell lineage-specific gene deletion approach. Both Tie2- and Tie1-Cre-mediated gene excisions show that β1 integrins in ECs are essential for embryonic angiogenesis by regulating cell adhesion, migration, and survival. β1-null ECs fail to interact properly with β1 ligands such as collagens and laminin, but behave normally on a non-β1 ligand, vitronectin. β3 integrins compensate for the absence of β1 during EC interactions with fibronectin in vitro, but may be insufficient to completely mediate the essential roles of fibronectin in vivo.
Integrins are multi-functional proteins, and our results suggest that β1 integrins primarily regulate EC adhesion, migration, and survival during embryonic angiogenesis. For example, we demonstrate a discontinuous endothelium in the mutant blood vessels, isolated ECs in between blood vessels in mutant yolk sacs, and clusters of rounded endocardial cells in mutant hearts. These phenotypes are consistent with the adhesion defects that we observe in β1-null ECs in vitro. Furthermore, ECs do not invade the neuroepithelium in Tie1-Cre mutants. Since capillary growth into the neural tube occurs exclusively via sprouting angiogenesis (Kurz et al., 1996), it is likely that β1-null ECs are defective in cell migration. Cell migration is a dynamic process not readily tractable in mouse embryos, and therefore we examined this behavior in vitro. β1-null ECs do not migrate on laminin or Matrigel and fail to maintain their migration paths in P-Sp explants. A rounded cellular phenotype in vivo and similar in vitro cell adhesion and migration defects are observed when β1 integrins are deleted in vascular smooth muscle cells, indicating that they also control these processes in the other major cell type of the vascular wall in vivo (Abraham et al., 2008). In addition, we observe a slight increase in EC apoptosis in e9.0 Tie2-Cre mutant embryos and more substantial EC apoptosis in mutant P-Sp explants and cultured ECs. While others have reported no effect of β1 deletion on vascular smooth muscle cell survival (Abraham et al., 2008), our results in ECs are consistent with findings in mammary epithelial cells, which are 1.5-fold more apoptotic than controls when β1 is deleted in vivo (Li et al., 2005), and are substantially more apoptotic when β1 is blocked with antibodies in vitro (Boudreau et al., 1995). Finally, we find that proliferation is unaffected by β1 deficiency, which is consistent with results from β1 integrin-deficient enteric neural crest cells (Breau et al., 2006). Conversely, keratinocyte (Raghavan et al., 2000), mammary epithelial cell (Li et al., 2005), and cerebral granule cell (Blaess et al., 2004) proliferation is reduced, and vascular smooth muscle cell proliferation is increased (Abraham et al., 2008) in the absence of β1 integrins. It is possible that β3 integrins, which we find to be upregulated in the absence of β1, can compensate for β1 function in EC proliferation, but not survival. All together, our results offer a mechanistic explanation for the multitude of studies indicating that inhibition of β1 integrins with antibody-or peptide- based approaches blocks angiogenesis in vivo (Garmy-Susini et al., 2005; Kim et al., 2000b; Pozzi et al., 2000; Senger et al., 1997).
The ECM ligands for β1 integrins that are likely to be most relevant to vascular development are collagens, laminin, and fibronectin, since all of these molecules are expressed in the EC basement membranes of e8.5 mouse embryos (Francis et al., 2002). The main EC collagen receptors are α1β1 and α2β1, and therefore it is not surprising that β1-null ECs fail to adhere to collagens. Similarly, the main EC receptors for laminin, α3β1 and α6β1, are deleted in our system. However, αvβ3 and α6β4 are also expressed in EC and have demonstrated functions as laminin receptors. For example, the G domain of the α4 laminin chain, which is pro-angiogenic, binds αvβ3 and α3β1, and EC adhesion to this domain is blocked by anti-αvβ3 or -β1 (Gonzales et al., 2001; Gonzalez et al., 2002). In addition, β4 integrins colocalize with laminin in tumor blood vessels, and blood vessels in mice expressing a truncated β4 integrin lacking the intracellular domain do not develop in Matrigel implants to the extent that they do in control mice (Nikolopoulos et al., 2004). These results suggest that αvβ3 and α6β4 interactions with laminin are pro-angiogenic. However, we find that β1-null EC express β3 integrins and adhere and migrate on vitronectin, the main ligand for αvβ3 and αvβ5, yet fail to mount appropriate angiogenic responses. Therefore, αvβ3 and α6β4 interactions with laminin, if they indeed exist in embryonic ECs, are not sufficient to support embryonic angiogenesis.
Although α5−/− or fibronectin−/− mice are embryonic lethal (Francis et al., 2002; George et al., 1997; George et al., 1993; Yang et al., 1999; Yang et al., 1993), it was unclear from these studies whether the cardiovascular phenotypes were due to gene deletion within ECs or supporting tissues. Since the phenotypes of our mutants are similar to these mutants in many regards, it is likely that EC-intrinsic defects play a major role in the lethality of α5−/− and fibronectin−/−embryos. For example, cranial blood vessels in α5−/− are dilated, branch less frequently, and contain less fibronectin than wild type vessels (Francis et al., 2002), which is similar to what we observe in our mutants. In addition, ECs within α5−/− or fibronectin−/− embryoid bodies are disorganized and fail to form networks (Francis et al., 2002), which reflect our observations of β1-null ECs in P-Sp cultures or grown on Matrigel. Somewhat unexpectedly, we find that EC adhesion, migration, and focal contact formation on fibronectin appear to be independent of β1 integrin expression. These data are consistent with studies of α5−/− embryonic cells (Yang and Hynes, 1996), but differ from data obtained with β1−/− embryonic cells (Fassler et al., 1995; Stephens et al., 1993), which do not adhere or migrate on fibronectin. Despite the apparent lack of a requirement for β1 integrins in EC interactions with fibronectin in vitro, it is a prominent component of embryonic vascular basement membranes. Since we show EC adhesion and migration defects in our mutant embryos, we hypothesize that compensatory pathways that support fibronectin interactions with β1-null ECs in vitro fail to compensate fully for β1 interactions with fibronectin in vivo. This may be due to additional in vivo factors, such as hemodynamics, a three dimensional environment, and the complex ECM composition of vascular basement membranes, which are difficult to model in vitro, but are essential to consider when assessing the function of EC β1. Our results, considered together with the phenotypes of α5−/− and fibronectin−/− embryos and tumor models in which these molecules are inhibited (Garmy-Susini et al., 2005; Kim et al., 2000b), strongly support the notion that EC β1 integrin interactions with fibronectin are required for angiogenesis.
Our conclusion that β1 integrins within ECs are required for angiogenesis is supported by two additional reports that were published after we submitted our manuscript (Lei et al., 2008; Tanjore et al., 2008). Lei et al., using different Tie2-Cre and β1flox/flox lines, report that deletion of β1 integrin is lethal around e9.5, with growth retardation and gross vascular defects that include dilation and reduced branching in the head region as well as abnormal patterning in the yolk sac (Lei et al., 2008). Using the same Tie2-Cre line as Lei et al. and a different β1flox/flox line from both Lei et al. and us, Tanjore et al. report that mutants die by e10.5 and are growth retarded. Large vessels in mutants are developed equally well as controls, but mutants display less sprouting and branching of smaller vessels (Tanjore et al., 2008). While the general conclusion regarding the requirement for β1 integrins within ECs is similar among the published reports and ours, our work provides several unique morphological, cellular, and molecular findings. First, a major phenotype in both of our Tie2- and Tie1-Cre mutants is the histological evidence of EC adhesion defects, seen most prominently in the hearts of Tie2-Cre mutants and in the dorsal aortae and cardinal veins of Tie1-Cre mutants. By contrast, Tanjore et al. report a lack of detectable histological abnormalities in mutant embryos at e9.5, and instead conclude that general hypoxia is the major contributor to lethality (Tanjore et al., 2008). Second, we provide evidence from P-Sp explant and in vitro analyses that β1 deficient ECs are defective in cell adhesion and migration. Third, we identify that cell survival defects contribute to the slowed growth of β1-null EC in vitro and are likely to contribute to the demise of Tie2-Cre mutants in vivo. Finally, we demonstrate reduced fibronectin in mutant blood vessels, and suggest a critical role of EC β1 for interactions with this molecule, despite the apparent compensation by β3 integrins, which also bind to fibronectin. Thus, our results not only demonstrate that β1 integrins within ECs are required for vascular development, but also extend the published reports by providing mechanistic insights into the functions of EC β1 integrins.
Our findings have implications that extend beyond embryonic vascular development and may be of therapeutic importance. For example, anti-angiogenic compounds targeting α5β1 are currently in development for cancer therapy (Ramakrishnan et al., 2006). The data presented herein provide genetic evidence of an EC-intrinsic requirement for β1 integrins during angiogenesis, and contribute a mechanistic explanation for the anti-angiogenic actions of β1-targeted therapies. Moreover, they support the further development of targeted therapies to block EC β1 function during pathological angiogenesis or to promote its activity in ischemia or other diseases in which the growth of new blood vessels is desirable.
Materials and Methods
Mice
Tie2-Cre (Braren et al., 2006), β1flox/flox (Graus-Porta et al., 2001), Tie1-Cre (Gustafsson et al., 2001), and Tie1-GFP (Iljin et al., 2002) have been described. Genotyping was performed with PCR for 40 cycles under the following conditions: denature: 95°C, 1 min; anneal: 56°C, 1 min; extend 72°C, 1 min. The Cre primers were 5′-GCCTGCATTACCGGTCGATGCAACGAGTG and 5′-CTGGCAATTTCGGCTATACGTAACAGGGTG. For routine genotyping, the β1flox/flox primers were 5′-GGAAAGTAGGCTGGACATGG and 5′-TGCCACTCCAAACATAGAGC. For detection of the wild type, floxed, and recombined β1 alleles in the same reaction, 5′-TTCTGCAAGTGTGGTGTGAAG and 5′-TGCCACTCCAAACATAGAGC were used. The GFP primers were 5′-GCCGACCACTACCAGCAGAACACCCCCATC and 5′-ATTTTATGTTTCAGGTTCAGGGGGAGGTGT. All animals were treated in accordance with the guidelines of the University of California, San Francisco Institutional Animal Care and Use Committee.
Immunological staining of embryo whole mounts and sections
Embryo harvest, processing, and immunostaining were performed as described (Braren et al., 2006). The following antibodies were used at a dilution of 1:100: anti-CD31 (MEC13.3, BD Biosciences), anti-VE-cadherin (11D4.1, BD Biosciences, San Jose, CA), anti-β1 integrin (Ha2/5, biotinylated, BD Biosciences, San Jose, CA), and anti-BrdU (Zymed/Invitrogen, Carlsbad, CA). Polyclonal anti-laminin (1:1000) and anti-fibronectin (1:2500) antibodies were obtained from Dr. Alex Morla. Secondary reagents were obtained from Invitrogen (Carlsbad, CA) and 4′,6-diamidino-2-phenylindole (DAPI)-containing Vectashield mounting medium was from Vector Laboratories (Burlingame, CA).
Primary EC isolation and culture
Embryonic cells were prepared as described (Braren et al., 2006), and cultured in F12 + 10% FBS + penicillin/streptomycin + 150 μg/ml endothelial mitogen (Biomedical Technologies, Stoughton, MA) + 25 mM HEPES + 0.1 mM 2-Mercaptoethanol + non-essential amino acids, sodium pyruvate, and glutamine. For immortalization, cells were infected with a retrovirus containing polyoma middle T antigen (pMIG-PyMT-IRES-GFP, obtained from Dr. Alana Welm at UCSF), which selectively immortalizes embryonic EC (Balconi et al., 2000). Further purification was performed with anti-CD31 antibodies and anti-rat IgG conjugated magnetic Dynabeads (Invitrogen, Carlsbad, CA), and purity was confirmed by anti-CD31 and anti-VE-cadherin immunofluorescence and DiI-Ac-LDL (Biomedical Technologies, Stoughton, MA) uptake (data not shown). Immortalized EC were cultured on 0.1% gelatin (Sigma, St. Louis, MO) in DMEM:F12 + 10% FBS + penicillin/streptomycin + 50 μg/ml heparin (Sigma, St. Louis, MO) + 25 μg/ml endothelial mitogen. Adenoviral-mediated deletion of β1 integrins was performed with Ad-GFP or Ad-Cre-GFP (obtained from Dr. Hillary Beggs at UCSF) at a multiplicity of infection of 100. Infections were performed in serum-free medium for 2 hr. Up to 4 rounds of infection were performed to achieve >95% β1 deletion by FACS (data not shown).
P-Sp explants
P-Sp explants obtained from e8.5 embryos were cultured and monitored as described (Braren et al., 2006; Takakura et al., 1998). EC fluorescence was derived from a Tie1-GFP transgene bred into the Tie2-Cre;β1flox/+ line. Anti-CD31 immunostaining was performed on cultures lacking GFP.
In vitro capillary morphogenesis
ECs were seeded onto Matrigel in 24-well plates and cultured in a timelapse chamber at 37°C in normal growth medium in a humidified mixture of 5% C02/95% air. Phase contrast images were captured at 15 min intervals, 1–24 hr after plating. Function-blocking anti-β1 (Ha2/5), -β3 (2C9.G2), and –αv (H9.2B8) integrin antibodies, used at 10 μg/ml, were from BD Biosciences (San Jose, CA).
Cell adhesion, spreading, migration, and focal contact formation
Timelapse analyses of primary EC were performed as described (Braren et al., 2006). Imaging and measurements were performed using Slidebook software (Intelligent Imaging Innovations, Denver, CO). Cell adhesion and modified Boyden migration (ChemoTx 101-8, Neuro Probe, Gaithersburg, MD) studies were performed as described (Carlson et al., 2001), except that DMEM:F12 + 0.5% bovine serum albumin (Sigma, St. Louis, MO) was the medium. Immunofluorescence for focal contacts was performed as described (Carlson et al., 2001) after overnight culture of primary ECs on 10μg/ml fibronectin-coated glass coverslips. The following antibodies, diluted at 1:100 were used: anti-β1 integrin (HMβ1-1-biotinylated, BioLegend, San Diego, CA), anti-β3 (2C9.G2-Alexa Fluor 647, BioLegend, San Diego, CA), anti-FAKpY397 (BD Biosciences, San Jose, CA), or anti-paxillin (Zymed/Invitrogen, Carlsbad, CA). ECs were identified with anti-CD31 (MEC13.3, BD Biosciences, San Jose, CA) at 1:100. The sources of ECM were: Human fibronectin, Roche (Indianapolis, IN); Natural mouse laminin, Invitrogen (Carlsbad, CA); Rat collagen I, Upstate Biotechnology/Millipore (Billerica, MA); Mouse collagen IV, Sigma (St. Louis, MO); Growth factor reduced Matrigel, BD Biosciences (San Jose, CA); Human vitronectin, Chemicon/Millipore (Billerica, MA).
Cell proliferation and survival
In vivo analyses were performed as described (Braren et al., 2006), except that BrdU was injected at 100 μg/g body weight 2 hr prior to dissection at e9.0. Apoptosis was detected with a Fluorescein In Situ Apoptosis Detection Kit (Chemicon/Millipore, Billerica, MA) in combination with anti-CD31 immunofluorescence. In vitro EC growth was measured by counting cells with a hemacytometer every day for one week after seeding 30,000 cells/well in 0.1% gelatin- or 1μg/ml vitronectin- coated 12-well tissue culture plates. 0.5% bovine serum albumin (heat-inactivated for 10 min at 85°C) was added to vitronectin-coated wells to block the additional adsorption of serum components prior to cell seeding. In vitro proliferation was assessed by culturing ECs on ECM coated glass coverslips with 125μM BrdU (Sigma, St. Louis, MO) for 6 hr prior to staining with anti-BrdU antibodies (Zymed/Invitrogen, Carlsbad, CA). In vitro survival was assessed by culturing ECs on ECM coated glass coverslips overnight prior to staining with YO-PRO-1 (Vybrant Apoptosis Assay kit #4, Invitrogen, Carlsbad, CA). In both assays, the coverslips were mounted with Vectashield containing DAPI, and the percentages of BrdU or YO-PRO-1 positive ECs were calculated after manual counting.
Supplementary Material
Acknowledgments
We thank Drs. Ulrich Muller and Louis Reichardt, Reinhard Fässler, and Kari Alitalo for providing β1flox/flox, Tie1-Cre, and Tie1-GFP mice, respectively. We also thank the members of our laboratory for helpful discussions and Christin Munkittrick for assistance in the preparation of this manuscript. This work was supported by the Pacific Vascular Research Institute, HHMI UCSF BRSP, and NIH R01 HL075033 to R.W., and NIH 5T32 HL007731-13 and AHA 0625021Y to T.C.
References
- Abraham S, Kogata N, Fassler R, Adams RH. Integrin {beta}1 Subunit Controls Mural Cell Adhesion, Spreading, and Blood Vessel Wall Stability. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- Balconi G, Spagnuolo R, Dejana E. Development of endothelial cell lines from embryonic stem cells: A tool for studying genetically manipulated endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2000;20:1443–51. doi: 10.1161/01.atv.20.6.1443. [DOI] [PubMed] [Google Scholar]
- Blaess S, Graus-Porta D, Belvindrah R, Radakovits R, Pons S, Littlewood-Evans A, Senften M, Guo H, Li Y, Miner JH, et al. Beta1-integrins are critical for cerebellar granule cell precursor proliferation. J Neurosci. 2004;24:3402–12. doi: 10.1523/JNEUROSCI.5241-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch W, Forsberg E, Lentini S, Brakebusch C, Martin K, Krell HW, Weidle UH, Addicks K, Fassler R. Beta 1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol. 1997;139:265–78. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–3. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172:151–62. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau MA, Pietri T, Eder O, Blanche M, Brakebusch C, Fassler R, Thiery JP, Dufour S. Lack of beta1 integrins in enteric neural crest cells leads to a Hirschsprung-like phenotype. Development. 2006;133:1725–34. doi: 10.1242/dev.02346. [DOI] [PubMed] [Google Scholar]
- Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–25. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. doi: 10.1002/gene.10152. [DOI] [PubMed] [Google Scholar]
- Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–31. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. Adhesion events in angiogenesis. Curr Opin Cell Biol. 2001;13:563–8. doi: 10.1016/s0955-0674(00)00252-0. [DOI] [PubMed] [Google Scholar]
- Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fassler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–88. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–33. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–51. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–81. [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, Flitney FW, Jones JC. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Gonzales M, Herron GS, Nagavarapu U, Hopkinson SB, Tsuruta D, Jones JC. Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc Natl Acad Sci U S A. 2002;99:16075–80. doi: 10.1073/pnas.252649399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–79. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci. 2001;114:671–6. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002;8:918–21. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- Iljin K, Petrova TV, Veikkola T, Kumar V, Poutanen M, Alitalo K. A fluorescent Tie1 reporter allows monitoring of vascular development and endothelial cell isolation from transgenic mouse embryos. Faseb J. 2002;16:1764–74. doi: 10.1096/fj.01-1043com. [DOI] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000a;156:1345–62. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Harris M, Varner JA. Regulation of integrin alpha vbeta 3-mediated endothelial cell migration and angiogenesis by integrin alpha5beta1 and protein kinase A. J Biol Chem. 2000b;275:33920–8. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–47. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–75. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- Lei L, Liu D, Huang Y, Jovin I, Shai SY, Kyriakides T, Ross RS, Giordano FJ. Endothelial expression of beta1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol Cell Biol. 2008;28:794–802. doi: 10.1128/MCB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005;24:1942–53. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–83. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–7. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–60. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V, Bhaskar V, Law DA, Wong MH, DuBridge RB, Breinberg D, O’Hara C, Powers DB, Liu G, Grove J, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5:273–86. [PubMed] [Google Scholar]
- Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94:13612–7. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sonne JE, Fitzgerald ML, Damsky CH. Targeted deletion of beta 1 integrins in F9 embryonal carcinoma cells affects morphological differentiation but not tissue-specific gene expression. J Cell Biol. 1993;123:1607–20. doi: 10.1083/jcb.123.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–95. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–86. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- Tanjore H, Zeisberg EM, Gerami-Naini B, Kalluri R. Beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn. 2008;237:75–82. doi: 10.1002/dvdy.21385. [DOI] [PubMed] [Google Scholar]
- Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol. 1999;215:264–77. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol Biol Cell. 1996;7:1737–48. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.