1. INTRODUCTION
Inherited retinal diseases such as autosomal dominant retinitis pigmentosa (adRP) are strikingly complex, with mutations in many different genes causing the same disease, with many different mutations in each gene, and with different clinical consequences resulting from the same mutation, even within the same family. For example, mutations in sixteen genes are known to cause adRP and an additional two adRP genes have been mapped but not identified yet (Table 1). This raises two questions: what fraction of adRP cases are accounted for by mutations in known genes, and what accounts for the remaining cases?
Table 1.
Genes known to cause dominant retinitis pigmentosa (in chromosomal order) 7
| Symbol | Protein | Location | |
|---|---|---|---|
| a. Known adRP genes |
|||
| 1. | PRPF3 (RP18) | pre-mRNA splicing factor 3 | 1q21.2 |
| 2. | SEMA4A | semaphorin 4A | 1q22 |
| 3. | RHO | rhodopsin | 3q22.1 |
| 4. | GUCA1B | guanylate cyclase activating protein 1B | 6p21.1 |
| 5. | RDS | peripherin 2 | 6p21.2 |
| 6. | RP9 (PAP1) | pim-1 kinase associated protein | 7p14.3 |
| 7. | IMPDH1 (RP10) | inosine monophosphate dehydrogenase 1 | 7q32.1 |
| 8. | RP1 | RP1 protein | 8q12.1 |
| 9. | ROM1 | rod outer membrane protein 1 | 11q12.3 |
| 10. | NRL | neural retina leucine zipper | 14q11.2 |
| 11. | NR2E3 | nuclear receptor subfamily 2 group E3 | 15q23 |
| 12. | PRPF8 (RP13) | pre-mRNA splicing factor 8 | 17p13.3 |
| 13. | CA4 (RP17) | carbonic anhydrase 4 | 17q23.2 |
| 14. | FSCN2 | retinal fascin homolog 2 | 17q25 |
| 15. | CRX | cone-rod homeobox transcription factor | 19q13.32 |
| 16. | PRPF31 (RP11) | pre-mRNA splicing factor 31 | 19q13.42 |
|
b. Mapped autosomal genes |
|||
| 1. | RP33 | 2cen-q12.1 | |
| 2. | RP31 | 9p22-p13 | |
|
c. Dominant-acting X-linked gene |
|||
| 1. | RPGR (RP3) | retinitis pigmentosa GTPase regulator | Xp11.4 |
To answer these questions we applied a step-wise screening process to a cohort of well-characterized adRP families, now numbering 215.1 Methods included sequencing of known genes, detection of deletions using MLPA (multiplex ligation-dependent probe amplification),2 linkage mapping against known loci, and genome-wide linkage mapping. By this combination of approaches we detected mutations in 58% of the families (largely Americans of European origin). Approximately 3% of these families have large deletions that cannot be detected by conventional PCR-based methods, and linkage testing against known loci revealed several additional mutations that were not detected earlier. Thus some of the remaining families are likely to have large deletions or other “hidden” mutations in known genes. However, linkage testing also confirms the existence of new adRP genes.
Being able to find the cause of adRP in all or nearly all affected individuals is a difficult but achievable goal, perhaps within the next decade.3 This information is of immediate value to patients and families, and is a necessary precursor to gene and mutation-specific therapies.
2. METHODS
We tested a panel of affected individuals from 215 adRP families for mutations in most of the known dominant RP genes (Table 1) [Sullivan et al., 20061 and unpublished]. To be included in the study a family had to have a diagnosis of adRP by a knowledgeable clinical specialist, and either a) three affected generations with affected females, or b) two affected generations with male-to-male transmission. The latter requirement was to reduce the likelihood of including families with X-linked RP. This possibility arises because some mutations in the X-linked gene RPGR affect female “carriers”, thus the disease in these families may be misinterpreted as adRP.4-6
The cohort of adRP patients was screened (largely by DNA sequencing) for mutations in the protein coding regions and intron-exon junctions of all adRP genes or gene regions causing at least 1% of cases. ORF15, the “hot spot” for mutations in RPGR, was also tested in families without male-to-male transmission. Determining whether a novel, rare variant is pathogenic was done using several computational and genetic tools.8-11
In subsequent studies, we tested several of the remaining families for linkage to genetic markers within or close to the known adRP genes and to RPGR [Sullivan et al.,2 2006 and unpublished]. This was done to uncover mutations that might have been missed by sequencing or to locate genes that have been mapped but not identified yet. In one large family we found linkage to the PRPF31 gene, even though careful re-sequencing failed to disclose a DNA change. Further testing revealed that affected members of the family have a complex deletion and insertion in PRPF31. This rearrangement was not detected earlier because only the non-deleted, homologous chromosome was sequenced, that is, the deletion is “invisible” to sequencing. We then tested the remaining families for deletions in PRPF31 using multiplex ligation-dependent probe amplification (MLPA).2,12
Finally, two large families without mutations in known genes were tested for genome-wide linkage using the ABI 5 cM microsatellite panel (n = 811) [Sullivan et al., 200513 and unpublished]. Multipoint linkage analysis was done using the LINKAGE package.14
3. RESULTS
3.1. Mutations in known dominant RP genes account for 58.6% of adRP families
To determine the genes and mutations causing retinopathy in the 215 families in the adRP cohort we tested affected probands for mutations predicted to cause at least 1% of adRP cases. Subsequently, families without mutations detected by sequencing were subjected to linkage testing against STRP (short tandem repeat polymorphism) marker sets within or contiguous to known adRP loci, if sufficient family members were present.2 In those cases were linkage testing indicated a known gene, that gene was tested more extensively in affected family members.
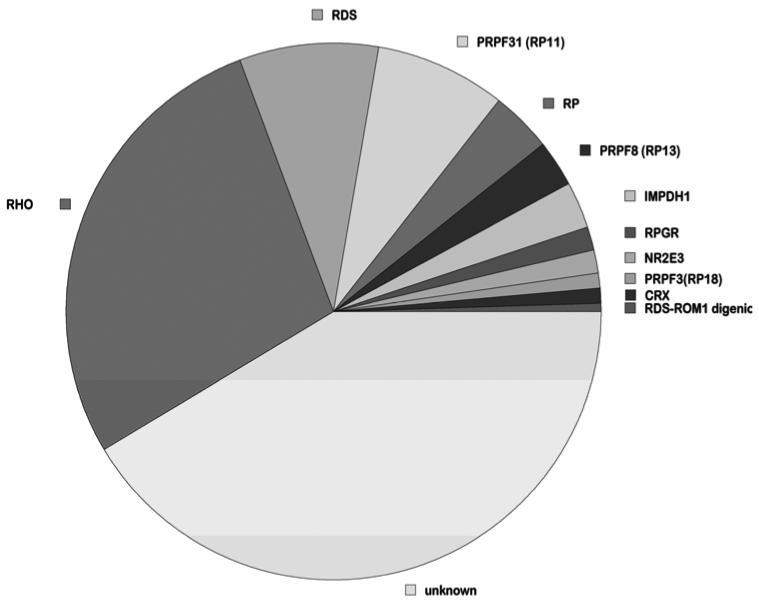
By these approaches, we identified single-nucleotide substitutions and small indels as the likely cause of adRP in 55.8% of the cohort families (Table 2 and Figure 1). Pathogenicity of novel variants was confirmed by segregation within families and bioinformatic analyses [Sullivan et al., 20061 and unpublished].
Table 2.
| Gene | No. families |
% total | Gene | No. families |
% total |
|---|---|---|---|---|---|
| CA4 | 0 | 0.0 | PRPF31 (RP11) | 17 | 7.9 |
| CRX | 2 | 0.9 | RDS | 18 | 8.4 |
| FSCN2 | 0 | 0.0 | RDS-ROM1 digenic | 1 | 0.5 |
| IMPDH1 | 6 | 2.8 | RHO | 60 | 27.9 |
| NR2E3 | 3 | 1.4 | ROM1 | 0 | 0.0 |
| NRL | 0 | 0.0 | RP1 | 8 | 3.7 |
| PRPF3 (RP18) | 2 | 0.9 | RP9 | 0 | 0.0 |
| PRPF8 (RP13) | 6 | 2.8 | RPGR | 3 | 1.4 |
| TOTALS | 126 | 58.6 | |||
Figure 1.
Percent of mutations per gene in adRP families.
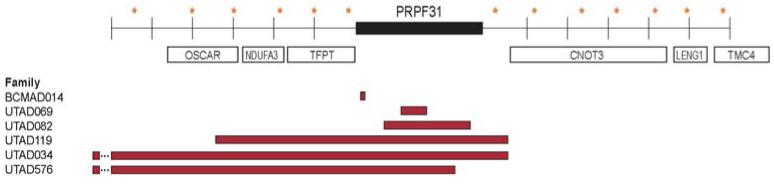
In addition, linkage mapping and SNP (single-nucleotide polymorphism) exclusion in one large adRP family revealed a complex chromosomal rearrangement in the PRPF31 (RP11) gene not detectable by sequencing. Based on this finding we designed MLPA probe sets spanning the PRPF31 locus and tested for deletions and copy number variants in other families in the cohort. In total, we identified deletions and rearrangements in thePRPF31 gene in six (2.8%) additional families (Figure 2) [Sullivan et al., 20062 and unpublished].
Figure 2.
PRPF31 deletions in six adRP families. Stars indicate SNPs, bars indicate deleted regions.
3.2. Several adRP genes are rare causes of adRP or are misidentified
We found no mutations in four of the genes, CA4, FSCN2, NRL and RP9 (PAP1). Based on published evidence, mutations in CA4, FSCN2 and NRL are real but rare causes of adRP. In contrast, we believe that the gene associated with the RP9 locus, PAP1, is not the cause of this disease. We and others failed to find mutations; in addition, we discovered that one of the reported disease-causing mutations is probably a paralogus variant, that is, the result of PCR amplification of two nearly identical gene copies. For this reason, we believe that the gene at the RP9 locus has not been identified yet.
3.3. Family history and phenotypes are useful for prioritizing genes to test
We considered whether the pedigree and phenotype are useful predictors of the underlying gene. In three circumstances they are. First, families in which females are consistently less severely affected than males, and without male-to-male transmission, are more likely to have an RPGR mutation. Second, skipped generations are more common with mutations in PRPF31 than in other adRP genes. Third, symptoms of RDS mutations are much more varied than mutations in other genes, ranging from RP, to choroidal atrophy, to complex maculopathies.15-17 Otherwise, there are numerous phenotypic differences among individuals with different mutations, but there is so much clinical variability that these differences are not pathognomonic.
3.4 Linkage mapping indicates additional adRP loci
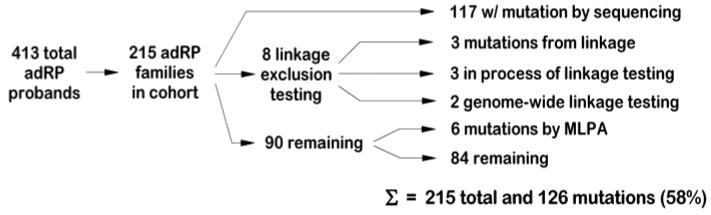
Of the 215 adRP families enrolled in these studies, we identified disease-causing mutations in 126 using a variety of methods (Figure 3). Of the remaining families, two are large enough for genome-wide linkage mapping and were tested for linkage to the ABI 5 cM STR marker set. Linkage mapping in these families suggests the existence of novel adRP loci [Sullivan et al., 200513 and unpublished].
Figure 3.
Summary of results of genetic testing of probands and families with adRP.
4. CONCLUSIONS
Mutations in known adRP genes account for at least 58% of adRP cases. “Common” mutations among the total account for at least 35% of cases, but novel mutations are found in the remainder. Thus screening adRP patients for known mutations and for mutations in selected regions of adRP genes can detect a large fraction of disease-causing variants, but additional methods, including MPLA and linkage, are also required. These prevalences are based largely on Americans of European origin and Europeans; other populations have different “common” mutations and different prevalences.
Deletions and copy number variants in PRPF31, not detectable by sequencing, account for 2.8 % of cases. Some of these deletions are very large, encompassing flanking genes. Deletions in other genes may also cause adRP.
Digenic RDS-ROM1 and X-linked dominant mutations in RPGR affect 0.5% and 1.5%, respectively, of “dominant” RP families. For diagnostic and counseling purposes it is very important to consider alternate modes of inheritance in adRP families.
Identifying the underlying disease-causing mutation in families with adRP is an essential step in diagnosis, counseling and, eventually, treatment.3
5. ACKNOWLEDGEMENTS
Supported by grants from the Foundation Fighting Blindness, the William Stamps Farish Fund, the Gustavus and Louise Pfeiffer Research Foundation, and the Hermann Eye Fund; and by NIH grants EY007142, EY014170, and EY005235.
6. REFERENCES
- 1.Sullivan LS, Bowne SJ, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, Lewis RA, Garcia CA, Ruiz RS, Blanton SH, Northrup H, Gire AI, Seaman R, Duzkale H, Spellicy CJ, Zhu J, Shankar SP, Daiger SP. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa (adRP): a screen of known genes in 200 families. Invest. Ophthalmol. Vis. Sci. 2006;47:3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan LS, Bowne SJ, Seaman CR, Blanton SH, Lewis RA, Heckenlively JR, Birch DG, Hughbanks-Wheaton D, Daiger SP. Genomic rearrangements of the PRPF31 gene account for 2.5% of autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2006;47:4579–4588. doi: 10.1167/iovs.06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mears AJ, Hiriyanna S, Vervoort R, Yashar B, Gieser L, Fahrner S, Daiger SP, Heckenlively JR, Sieving PA, Wright AF, Swaroop A. Remapping of the RP15 locus for X-linked cone-rod degeneration to Xp11.4-p21.1, and identification of a de novo insertion in the RPGR exon ORF15. Am. J. Hum. Genet. 2000;67:1000–100.3. doi: 10.1086/303091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozet JM, Perrault I, Gigarel N, Souied E, Ghazi I, Gerber S, Dufier JL, Munnich A, Kaplan J. Dominant X linked retinitis pigmentosa is frequently accounted for by truncating mutations in exon ORF15 of the RPGR gene. J. Med. Genet. 2002;39:284–285. doi: 10.1136/jmg.39.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vervoort R, Lennon A, Bird AC, Tulloch B, Axton R, Miano MG, Meindl A, Meitinger T, Ciccodicola A, Wright AF. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat. Genet. 2000;25:462–466. doi: 10.1038/78182. [DOI] [PubMed] [Google Scholar]
- 7.RetNet. Daiger Stephen P. The Retinal Information Network, http://www.sph.uth.tmc.edu/RetNet/ Administrator; The Univ. of Texas Health Science Center at Houston: http://www.sph.uth.tmc.edu/RetNet/ PhD. 1996-present. [Google Scholar]
- 8.Grantham R. Amino acid difference formula to help explain protein evolution. Science. 1974;185:862–864. doi: 10.1126/science.185.4154.862. [DOI] [PubMed] [Google Scholar]
- 9.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in Genie. J. Comput. Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 12.Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan LS, Bowne SJ, Shankar SP, Blanton SH, Heckenlively JR, Birch DG, Wheaton DH, Pelias MZ, Daiger SP. Linkage mapping in families with autosomal dominant retinitis pigmentosa (adRP) Invest. Ophthalmol. Vis. Sci. 2005;46 E-Abstract 2293. [Google Scholar]
- 14.Lathrop GM, Lalouel JM, Julier C, Ott J. Strategies for multilocus linkage analysis in humans. Proc. Natl. Acad. Sci. USA. 1984;81:3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felbor U, Schilling H, Weber BH. Adult vitelliform macular dystrophy is frequently associated with mutations in the peripherin/RDS gene. Hum. Mutat. 1997;10:301–309. doi: 10.1002/(SICI)1098-1004(1997)10:4<301::AID-HUMU6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Kajiwara K, Sandberg MA, Berson EL, Dryja TP. A null mutation in the human peripherin/RDS gene in a family with autosomal dominant retinitis punctata albescens. Nat. Genet. 1993;3:208–212. doi: 10.1038/ng0393-208. [DOI] [PubMed] [Google Scholar]
- 17.Sears JE, Aaberg TA, Sr., Daiger SP, Moshfeghi DM. Splice site mutation in the peripherin/RDS gene associated with pattern dystrophy of the retina. Am. J. Ophthalmol. 2001;132:693–9. doi: 10.1016/s0002-9394(01)01179-5. [DOI] [PubMed] [Google Scholar]