Abstract
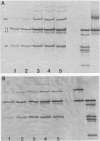
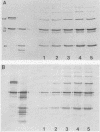
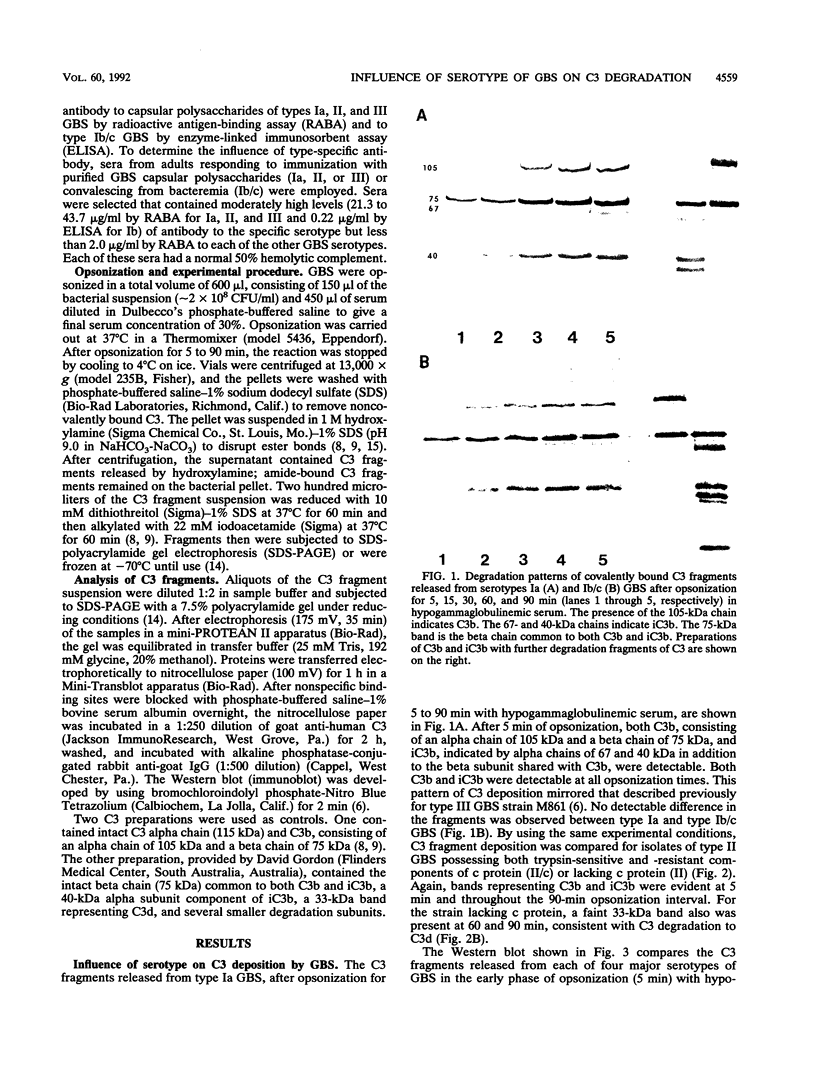
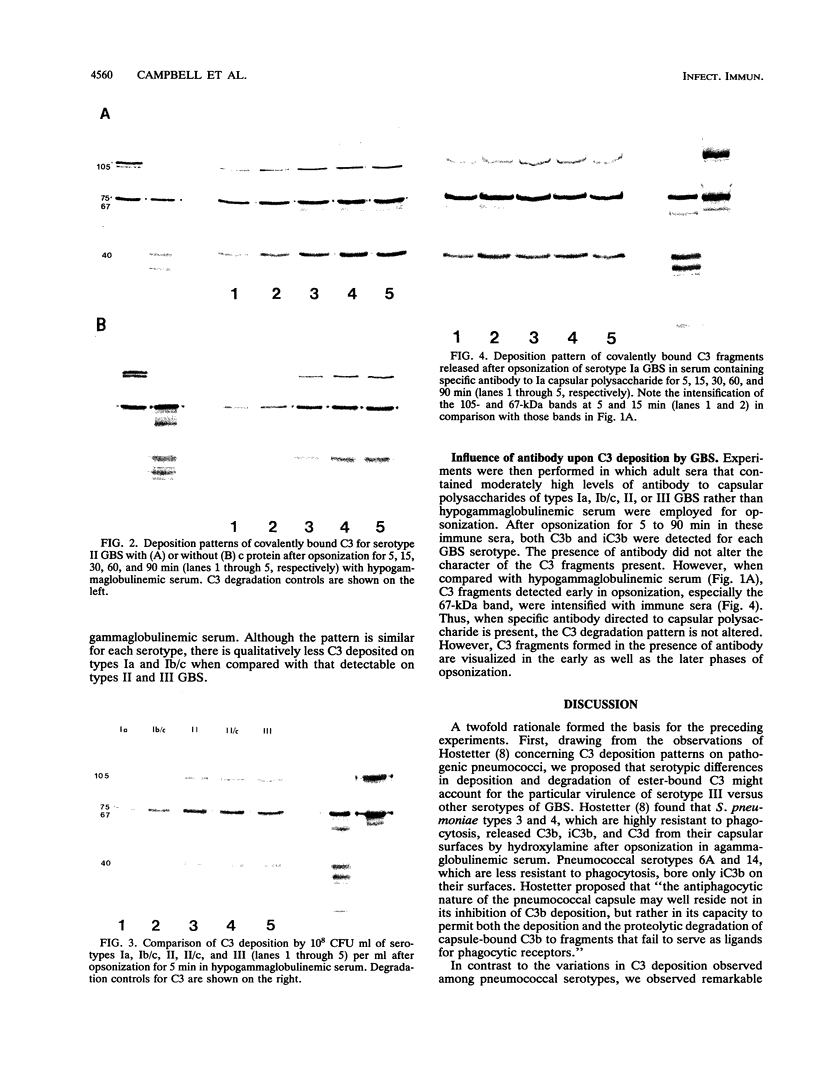
Serotype III strains of group B streptococci (GBS) are isolated from the majority of young infants with bacteremia or meningitis. We hypothesized that serotype-associated differences in structure of the type-specific capsular polysaccharide or the presence of c protein would influence the extent to which C3 degradation occurs on GBS and that type-specific antibody would alter C3 deposition or degradation patterns. When clinical isolates of GBS representing serotypes Ia, Ib/c, II (with or without c protein) and III were employed with hypogammaglobulinemic serum as an opsonic source, a remarkable similarity was observed in patterns of C3 deposition and degradation for each of the four GBS serotypes and between strains with or without c protein. Both C3b and iC3b were detected by 5 min and throughout a 90-min opsonization interval. Less deposition occurred at 5 min on serotypes Ia and Ib/c than on types II and III GBS. Minimal degradation to C3d or smaller fragments was observed. Type-specific antibody facilitated C3b deposition on GBS and C3b degradation to iC3b early in opsonization. Possibly, accessibility of C3 fragments to neutrophil receptors, rather than the extent to which the surface permits C3 degradation, accounts for differential virulence among GBS serotypes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Dana N., Melamed J., Medicus R., Colten H. R. Low ionic strength or chemical cross-linking of monomeric C3b increases its binding affinity to the human complement C3b receptor. Immunology. 1983 Feb;48(2):229–237. [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Edwards M. S., Webb B. J., Kasper D. L. Antibody-independent classical pathway-mediated opsonophagocytosis of type Ia, group B streptococcus. J Clin Invest. 1982 Feb;69(2):394–404. doi: 10.1172/JCI110463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Webb B. J., Kasper D. L., Edwards M. S. The role of complement and antibody in opsonophagocytosis of type II group B streptococci. J Infect Dis. 1986 Jul;154(1):47–54. doi: 10.1093/infdis/154.1.47. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Berger M., Joiner K. A., Frank M. M. Classical complement pathway activation by antipneumococcal antibodies leads to covalent binding of C3b to antibody molecules. Infect Immun. 1983 Nov;42(2):594–598. doi: 10.1128/iai.42.2.594-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. R., Baker C. J., Edwards M. S. Deposition and degradation of C3 on type III group B streptococci. Infect Immun. 1991 Jun;59(6):1978–1983. doi: 10.1128/iai.59.6.1978-1983.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Gaither T. A., Hammer C. H., Frank M. M. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984 Dec 1;160(6):1640–1655. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter M. K., Krueger R. A., Schmeling D. J. The biochemistry of opsonization: central role of the reactive thiolester of the third component of complement. J Infect Dis. 1984 Nov;150(5):653–661. doi: 10.1093/infdis/150.5.653. [DOI] [PubMed] [Google Scholar]
- Hostetter M. K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986 Apr;153(4):682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Katzenellenbogen E., Lugowski C., Kasper D. L. Structure of native polysaccharide antigens of type Ia and type Ib group B Streptococcus. Biochemistry. 1983 Mar 1;22(5):1258–1264. doi: 10.1021/bi00274a042. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Rosell K. G., Kasper D. L. Structural determination and serology of the native polysaccharide antigen of type-III group B Streptococcus. Can J Biochem. 1980 Feb;58(2):112–120. doi: 10.1139/o80-016. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Rosell K. G., Katzenellenbogen E., Kasper D. L. Structural determination of the capsular polysaccharide antigen of type II group B Streptococcus. J Biol Chem. 1983 Feb 10;258(3):1793–1798. [PubMed] [Google Scholar]
- Johnson D. R., Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984 Apr;19(4):506–510. doi: 10.1128/jcm.19.4.506-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Noya F. J., Rench M. A., Metzger T. G., Colman G., Naidoo J., Baker C. J. Unusual occurrence of an epidemic of type Ib/c group B streptococcal sepsis in a neonatal intensive care unit. J Infect Dis. 1987 Jun;155(6):1135–1144. doi: 10.1093/infdis/155.6.1135. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Ferrieri P. The relation of the Ibc protein antigen to the opsonization differences between strains of type II group B streptococci. J Infect Dis. 1985 Apr;151(4):672–681. doi: 10.1093/infdis/151.4.672. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli J. A., Ng Y. C., Peters D. K. The role of complement and its receptor in the elimination of immune complexes. N Engl J Med. 1986 Aug 21;315(8):488–495. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- Wessels M. R., Pozsgay V., Kasper D. L., Jennings H. J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987 Jun 15;262(17):8262–8267. [PubMed] [Google Scholar]