Abstract
The ability to enter a hypometabolic state upon restriction of caloric intake is pivotal for animal survival: balancing the energy budget in endotherms can be a real struggle when food is not available and/or the demand for heat production to maintain homeothermy becomes excessive. Bouts of torpor, characterized by metabolic rates well below a basal metabolic rate and core body temperatures that may be just a few degrees above the ambient temperature, are utilized among many organisms across the animal kingdom, including those that could be described as typical laboratory animals, like the mouse or hamster. Daily heterotherms, which are the focus of this commentary, enter shallow torpor bouts and do so usually under acute food shortage conditions and a relatively cool environment. Due to their small size, the body temperature of these animals is very responsive to food deprivation, pharmacological inhibition of metabolic rate, and cardiovascular depressants. This commentary examines recent developments concerning the neuroendocrine mechanisms in place that may mediate fasting-induced torpor in daily heterotherms. Further this commentary highlights pharmacological induction of hypothermia in small mammals.
1. Introduction
The idea that all small mammals, including mice, are not true endothermic homeotherms is not well appreciated by either the lay public or among many life scientists. Small mammals such as ground squirrels, marmots, chipmunks, and mice can spend a significant portion of their day, week, or even season at body temperatures well below 37 °C, although rats are not known to enter these torpor bouts [1–6]. Some of these animals obtain cues to enter bouts of torpor from day length and ambient temperature. Many excellent reviews on these obligate hibernators have been published [5, 7–10], and are not the focus of this review. Rather, this review will examine those animals that enter a hypometabolic and hypothermic state in response to cool ambient temperature and calorie restriction. These animals undergo a relatively shallow torpor bout, with a minimum body temperature closer to 22 °C than 2 °C as seen in obligate hibernators [9]. Daily torpor usually lasts less than 24 hours, instead of days or weeks, where it is interrupted with bouts of food-seeking behavior and/or circadian rhythms within the organism. These animals that are daily heterotherms tend to be small, typically 5 – 50 grams, and include hamsters, mice, shrews, gerbils, hummingbirds, numerous marsupials and many others (see Table 1 for an abbreviated list and reference [9] for a complete list of animals that enter torpor).
Table.
| Animal | Typical depth of torpor | Typical length of torpor | Classification | Reference |
|---|---|---|---|---|
| Mouse | 20 °C | 6–10 hours | Daily heterotherm | 1,2 |
| Mus musculus | ||||
| Peromyscus leucopus | ||||
| Hamster | 20 °C | 10 hours | Daily heterotherm | 5 |
| Phodopsus sungorus | ||||
| Golden-mantled ground squirrel | 5 °C | 14 days | Hibernator | 3 |
| Spermophilus lateralis | ||||
| Marmot | 10 °C | 5–6 days | Hibernator | 4 |
| Marmota marmota | ||||
| Rat | Does not enter torpor | N/A | Homeotherm | 6 |
| Rattus rattus | ||||
Upon an acute shortage of food intake, daily heterotherms utilize torpor to help balance their energy budget as most of their fuel comes from ingested food and not stored fat. The metabolic savings approach 70% in animals that undergo shallow torpor bouts. At the onset of the bout of torpor, the metabolic rate drops precipitously before the fall in body temperature (see Figure 1), suggesting an active inhibition of metabolism at least during the entrance into torpor. Energy savings by metabolic reduction as the torpor bout proceeds may stem more from passive processes of the temperature dependence of enzymatic activities [9].
Figure 1. Metabolism falls before body temperature in the fasted mouse.
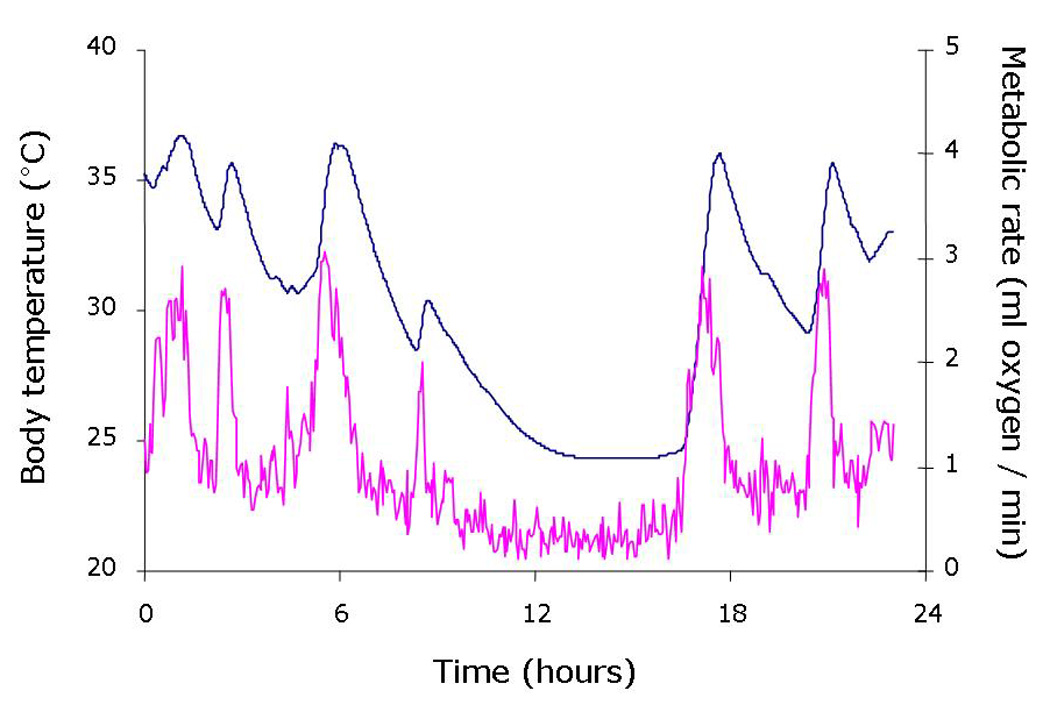
The core body temperature (blue line) and oxygen consumption (pink line) of an ob/ob mouse were measured simultaneously over a 24 hour fasting period. The fast was initiated at time “0”, which was also the beginning of the 12 hour dark cycle. Body temperature was recorded using an abdominal temperature telemeter while oxygen consumption was calculated from the oxygen content leaving the mouse’s metabolic cage. Note the fall in oxygen consumption precedes the fall in body temperature during entrance into this bout of torpor.
Studies on torpor and exogenous compounds used to induce hypothermia may have great therapeutic potential. Hypothermia has been shown to have major protective benefits for bouts of ischemia and exposure to toxic chemicals [11]. Most studies of hypothermia have utilized small mammals that may be inherently tolerant to hypothermia. Because of their small size, the surface area to volume ratio of these animals necessitates a high mass-specific metabolic rate to offset heat loss. Indeed, it has been known for over 100 years that metabolic rate scales with body mass, although the value of the exponent that describes this relationship is still under debate [12]. The high mass-specific metabolic rate and low thermal inertia of these small animals makes their body temperature particularly susceptible to any small disturbance in metabolic rate. Thus, application of some exogenous compound may result in hypothermia simply because the compound poisons the metabolic machinery, as opposed to mimicking a torpor pathway. The difference between these two possibilities can be enormous: a drop in body temperature due to a metabolic poison will invoke mechanisms to counter the fall in temperature. That is, when body temperature is forced below some set point, homeostatic mechanisms engage to re-establish a warmer body temperature. Fasting-induced torpor, or seasonal hibernators for that matter, do not defend a warm body temperature (i.e. 37 °C), but rather experience a fall in the set point for body temperature [13], and allow their body temperature to fall without any mitigating physiological feedbacks. Hence, extrapolation of the use of these compounds in hypothermia-tolerant species like the mouse or hamster to the use of these compounds in larger animals, perhaps humans, should be done so with great caution.
2. Neuroendocrinology of fasting-induced torpor
Daily heterotherms that undergo shallow bouts of torpor in response to food deprivation are likely responding to hormonal cues that reflect short term changes in caloric intake. One such hormone appears to be leptin. This peptide hormone, a product of the ob gene, is derived primarily from white fat and relays nutritional status from the peripheral fat stores to sites within the hypothalamus [14]. In the hypothalamus, leptin binds its receptor, a product of the db gene, to promote satiety and elevate metabolic rate [15]. While leptin was originally thought to reflect long term energy stores, it has become clear that circulating leptin can reflect short term changes in energy intake. In the non-fasting animal, the amount of circulating leptin correlates well with the total amount of WAT [16]. However, fasting causes a fall in circulating leptin on a time scale that is much faster than the disappearance of fat from the animal [17, 18]. Indeed, while fasting causes a near global decline in sympathetic outflow to organs with the net result of a decrease in metabolic rate, the one tissue that receives elevated sympathetic input during food deprivation is WAT [19–21]. Activation of the β3 adrenergic receptor on WAT in response to elevated sympathetic activity results in a decrease in leptin secretion and an elevation in lipolysis with the liberation of free fatty acids to the blood [22, 23]. While the gene for leptin was discovered in 1994 [24], a lack of this circulating “factor” in the ob/ob mouse was known previously [25] to be intimately involved with torpor bouts. The ob/ob mouse, with its prolific stores of WAT that should allow for an ample supply of stored fuel for metabolic function, nonetheless enters deep bouts of torpor when fasted [25–27] and can enter torpor spontaneously while fed (see Figure 2). Leptin replacement in calorically restricted mice [28], ob/ob mice [26] and in the dunnart [29] blunts torpor bouts. Dopamine beta hydroxylase −/− mice that are unable to sympathetically activate WAT due to the lack of epinephrine and norepinephrine do not lower circulating leptin during a fast and do not enter torpor during a fast [18]. The ability to enter torpor is restored in these mice with the selective activation of the β3 adrenergic receptor and subsequent fall in plasma leptin levels [18]. Conversely, the fasted Fxr −/− mouse, missing a functional bile acid receptor, exhibits a greater drop in circulating leptin than that found in fasted wild type mice, which correlates with a deeper level of hypothermia during the fast [30]. This depth of torpor is also reversed by physiological replacement of leptin [30]. However, reduced leptin levels or reduced leptin signaling cannot be the sole signal to induce torpor during a fast for the obvious reason that ob/ob mice as well as db/db mice (missing a functional leptin receptor) are not always in a torpid state. Also, leptin replacement does not blunt bouts of torpor in a genetic model of WAT depletion [26]. Overall, though, it appears that the fall in circulating leptin due to sympathetic activation of WAT during a fast plays a key role in initiation of the torpor bout (see Figure 3).
Figure 2. Spontaneous torpor bouts in leptin-deficient mice.
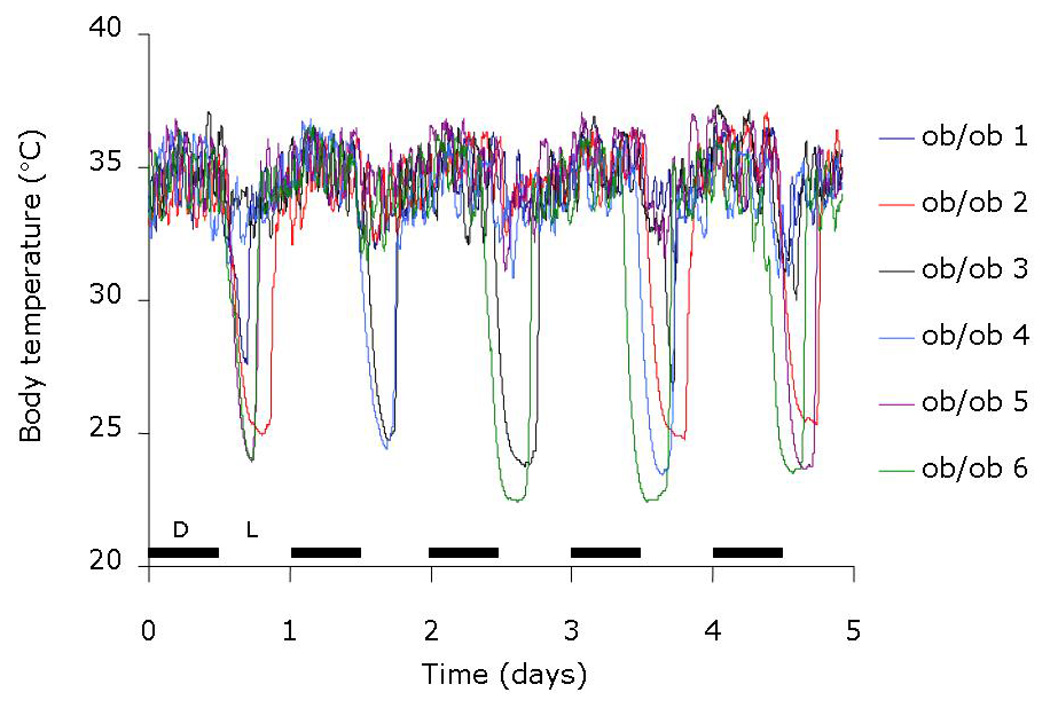
Six different ob/ob mice were implanted with temperature telemeters, allowed to recover for 10 days from the surgery, and housed individually at 20 °C. These mice were on a 12:12 light schedule, with the 12 hour dark cycle (D) commencing at the beginning of each day. These mice had free access to food and water during the duration of the 5 days of data collection. Each mouse entered a torpor bout at least once over the five days. On every day, at least two mice entered a spontaneous bout of torpor. Note the timing of entrance into torpor was typically near the end of the dark cycle, which was consistent across animals and across the different days.
Figure 3. Hypothetical mechanism for the induction of torpor in daily heterotherms.
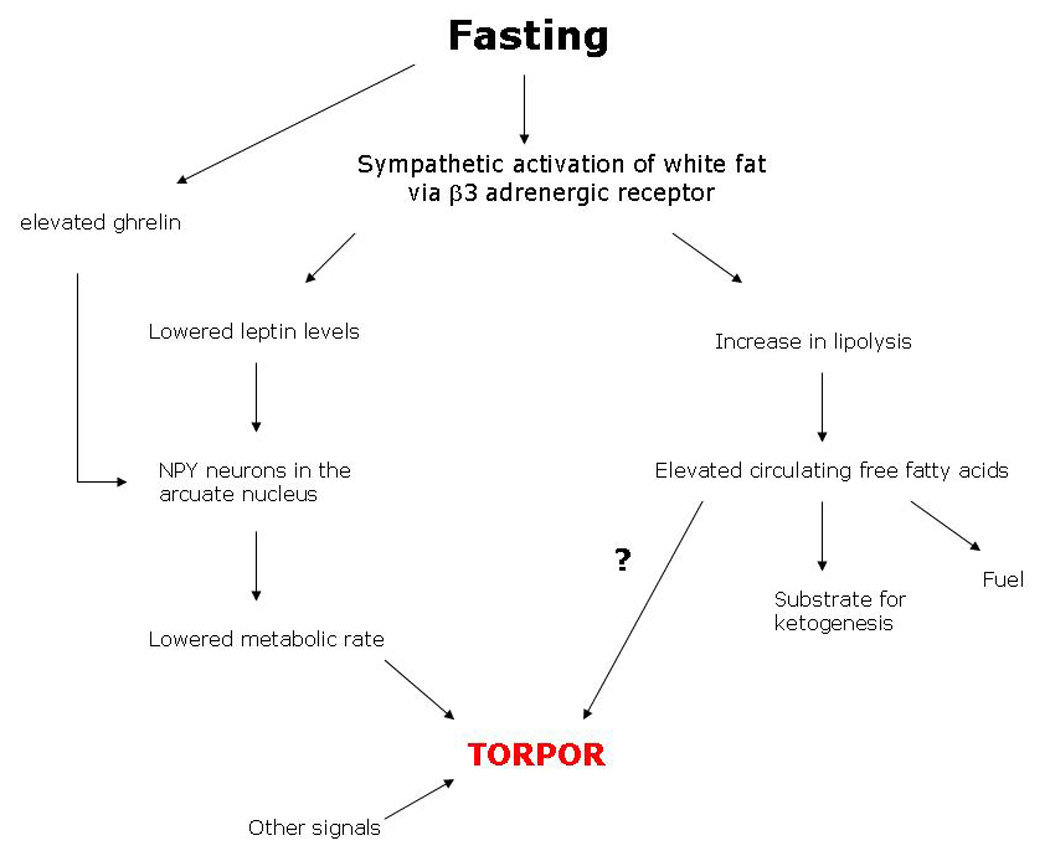
Periods of fasting in mammals are associated with elevated sympathetic discharge to white adipose tissue. This causes a rapid decline in circulating leptin. It is hypothesized that the combination of low leptin and elevated ghrelin from the gut during a fast increase the activity of NPY neurons within the arcuate nucleus of the hypothalamus such to initiate the large fall in metabolic rate. It is unclear whether the increase in lipolysis and resultant release of free fatty acids to the blood also participate in initiation of torpor or serve only as a fuel for oxidation.
Satiety and hunger signals, or lack thereof, from the gut seem to be reasonable candidates for the regulation of torpor in daily heterotherms that are so responsive to lack of food intake. The gut influences food intake and metabolism through a suite of factors that include ghrelin, cholecystokinin, oxyntomodulin, glucagon-like peptides, and PP-fold family of proteins, which includes PYY3–36 [31]. In addition, vagal afferent activity is influenced by gastrointestinal mechanoreceptors, altering appetite, and may be the primary site of action for some gut-derived hormones [31]. One of these hormones has been reported in detail for effects on torpor. Ghrelin is secreted from the stomach during periods of fasting and is repressed after a meal [32]. Ghrelin stimulates food ingestive behavior, possibly via the vagus nerve [33, 34], although ghrelin-producing neurons have been found to reside within the hypothalamus [35]. Administration of ghrelin to a fasted mouse deepens and lengthens a torpor bout, although ghrelin administration in a fed mouse does not influence body temperature [36].
Ghrelin is known to activate neuropeptide Y (NPY)-containing neurons within the arcuate nucleus [37, 38]. Activity of NPY-containing neurons is also repressed during feeding, by high circulating leptin levels, and when leptin is administered directly into the 3rd ventricle of the brain [39]. NPY is a powerful orexigen, stimulating hunger when administered centrally. The ingestive behavior of the Npy −/− mouse is surprisingly normal, and these mice have a normal body weight [40]. However, deletion of NPY neurons in the adult mouse results in lack of food intake with subsequent starvation and death [41], illustrating the development of other mechanisms to stimulate food intake in the Npy −/− mouse. While these Npy −/− mice exhibit normal endocrine and feeding responses to fasting, they do exhibit an altered response in body temperature. The Npy −/− mice are able to initiate torpor bouts when fasted, but they do not sustain those bouts of torpor [36]. Peripheral administration of ghrelin has no impact on these aborted torpor bouts in Npy −/− mice, suggesting that NPY mediates ghrelin’s effects on body temperature [36]. Importantly, central injection of either NPY or an NPY Y1 receptor agonist into the cold-adapted Siberian hamster, another daily heterotherm, leads to torpor-like hypothermia [42, 43]. The central administration of an NPY Y1 receptor antagonist in these hamsters blunts the depth of natural torpor [44]. These data firmly put NPY action as an important player in the mediation of fasting-induced torpor.
Adjacent to the NPY neurons reside anorexigenic neurons that utilize alpha melanocyte stimulating hormone (αMSH) as a neurotransmitter. αMSH partially mediates the anorexigenic and metabolic effects of leptin through the melanocortin 3/4 receptors of secondary neurons [14, 15]. Therefore, this neuron type is a reasonable candidate for mediating the suppression of torpor bouts by leptin. The agouti mouse is a model of adult onset obesity due to the chronic antagonism of the melanocortin receptors within the arcuate nucleus. The juvenile agouti mouse enters torpor bouts normally when fasted. However, the hyperleptinemic adult agouti mouse does not enter torpor [36], likely due to the elevated leptin levels. While the incidence of torpor in another model of hyperleptinemia (the diet-induced obese mouse) has not been examined, mice that are heavier, either strain-dependent or age-dependent, are less likely to enter torpor after a 24 hour fast (unpublished results from SJS). These data suggest that hyperleptinemia blunts torpor in a pathway that is independent of αMSH signaling.
Importantly, when the region of the hypothalamus containing the NPY and POMC neurons, so called the arcuate nucleus, is ablated, the ability for small mammals to enter torpor is severely impacted. Despite the obesity induced by ablation of the arcuate nucleus by neonatal treatment with monosodium glutamate (MSG), treated mice no longer enter torpor bouts [36, 45]; ablation of the arcuate nucleus within suckling aged rats which experience bouts of torpor at such a young age also prevents torpor bouts [46]; and Siberian hamsters treated with MSG also have a blunted torpor response [47]. These data suggest that the arcuate nucleus, and likely the NPY neurons with the arcuate nucleus, play a central role in mediating the torpor response to fasting.
3. Pharmacological induction of hypothermia in small mammals
Adenosine and adenine nucleotides
Adenosine is a logical candidate for involvement with fasting-induced torpor in that its circulating levels are elevated during a fast and it evokes numerous physiological changes consistent with a torpid state. Among the myriad of known functions, adenosine inhibits neural activity, is a potent vasodilator as a particularly efficacious signal for increased blood flow in coronary arteries, and slows heart rate. Administration of adenosine causes profound hypothermia in a dose-dependent fashion in gerbils [48] as well as in mice [45]. Aminophylline, an adenosine A1 receptor antagonist, blocks the hypothermia associated with adenosine in mice [45] and the hypothermia associated with hypoxia in rats [49]. Importantly, adenosine inhibits the hypocretin/orexin neurons within the lateral hypothalamus [50]. These neurons are intimately involved with feeding regulation and energy homeostasis. They receive input from peripheral cues that are known to be involved with torpor regulation, including ghrelin and leptin [51, 52]. Targeted deletion of orexin neurons in mice results in abnormal ambulatory response to fasting, although it is unknown whether these mice entered torpor upon fasting [52]. These same neurons play an important role in wakefulness, and inhibition of these neurons by adenosine promotes sleep [50]. Sleep may be related to torpor bouts, particularly in animals that undergo facultative torpor bouts [53].
The singly phosphorylated form of adenosine, adenosine monophosphate (AMP), is also a molecule that signals cellular energy depletion. The enzyme adenylate kinase catalyzes the reaction
Thus, the AMP:ATP ratio varies roughly as the square of ADP:ATP ratio [54], and as such, AMP is poised to play an important sensor for energy stores. AMP influences the activity of numerous enzymes, and of great importance to energy balance is the enzyme AMP-activated protein kinase. This enzyme is expressed in numerous tissues, including the hypothalamus [55]; its activity is impacted by ghrelin and leptin; and it is a major player in the regulation of both sides of the energy balance equation, feeding and metabolism [56, 57]. While it appears that this enzyme plays little role in torpor in the ground squirrel [58], an obligate hibernator, it may play some role in fasting-induced torpor. Administration of AMP induces a reversible and dose-dependent hypothermia in mice [45, 59], which one group defined as torpor [59]. However, two lines of evidence suggest that the intraperitoneal injection of AMP may be too blunt of a tool to assess whether AMP plays an active role in induction of true torpor. First, AMP-induced hypothermia is prevented using aminophylline [45], an adenosine receptor antagonist, which suggests that AMP must first be dephosphorylated to impact body temperature. Second, other phosphorylated forms of adenosine, namely ATP, induce the same depth of hypothermia [45]. Perhaps the hypothermia induced by AMP will be therapeutically useful. However, the roles of AMP and AMP-activated kinase in fasting-induced torpor have yet to be conclusively tested.
3-iodothyronamine and H2S
A derivative of thyroid hormone has recently been discovered and shown to induce hypothermia in the mouse and Djungarian hamster [60, 61]. 3-iodothyronamine is generated through decarboxylation and deiodination of thyroxine [62] and has been found in the brains of mice, guinea-pigs, and hamsters [60, 61]. Its effects are likely non-genomic, and function through the trace amine associated receptor 1 [63]. Administration of 3-iodothyronamine substantially lowers metabolic rate and body temperature that recover after 6–9 hours. The fall in respiratory quotient to nearly 0.7 with this compound which mimics the increase in lipid oxidation in naturally-occurring torpor bouts, has a recovery period substantially longer than 9 hours [60].
It should be pointed out that both adenosine and 3-iodothyronamine have in common tremendous cardiovascular depression. Both compounds induce a significant fall in heart rate and cardiac output [61, 64, 65]. Breathing hydrogen sulfide gas should also be included in this group. It was first reported that mice breathing H2S fall into a hypothermic state, which was given the “sci-fi” type name of “suspended animation” [66]. However, it was very recently shown that breathing H2S results in a fall in both heart rate and cardiac output of >70% of normal, without impacting stroke volume [67]. It may be that the hypometabolism and hypothermia associated with these compounds (adenosine, 3-iodothyronamine, H2S) is secondary to the cardiovascular effects (see Figure 4). That is, the drop in metabolic rate with a subsequent drop in body temperature results from a lack of O2 delivery to peripheral tissues in these hypothermia-tolerant species. Indeed, it has been shown that mice do experience hypothermia when experiencing hypoxia [68]. Most research suggests that hypoxia is not the signal for torpor entry [5], although hypoxia does have a substantial hypometabolic effect [69]. Alternatively, it may be that these compounds somehow act as a major metabolic inhibitor (see Figure 4). For example, H2S is a known inhibitor of cytochrome c oxidase [70]. The necessary fall in metabolic rate following inhibition of fuel oxidation will have a great impact on a small mammal, lowering its body temperature, which in turn lowers heart rate and cardiac output simply due to the temperature-dependence of the cardiovascular system. Volpato et al. [67] tested these possibilities by administering H2S to mice at an ambient temperature warm enough (35 °C) to prevent a fall in body temperature. They found that both metabolic rate and heart rate still fell even though body temperature did not, demonstrating direct cardiovascular effects of H2S. It remains to be determined whether the metabolic effects of H2S are independent of the cardiovascular effects.
Figure 4. Potential pathways that an exogenous compound might lead to hypothermia.
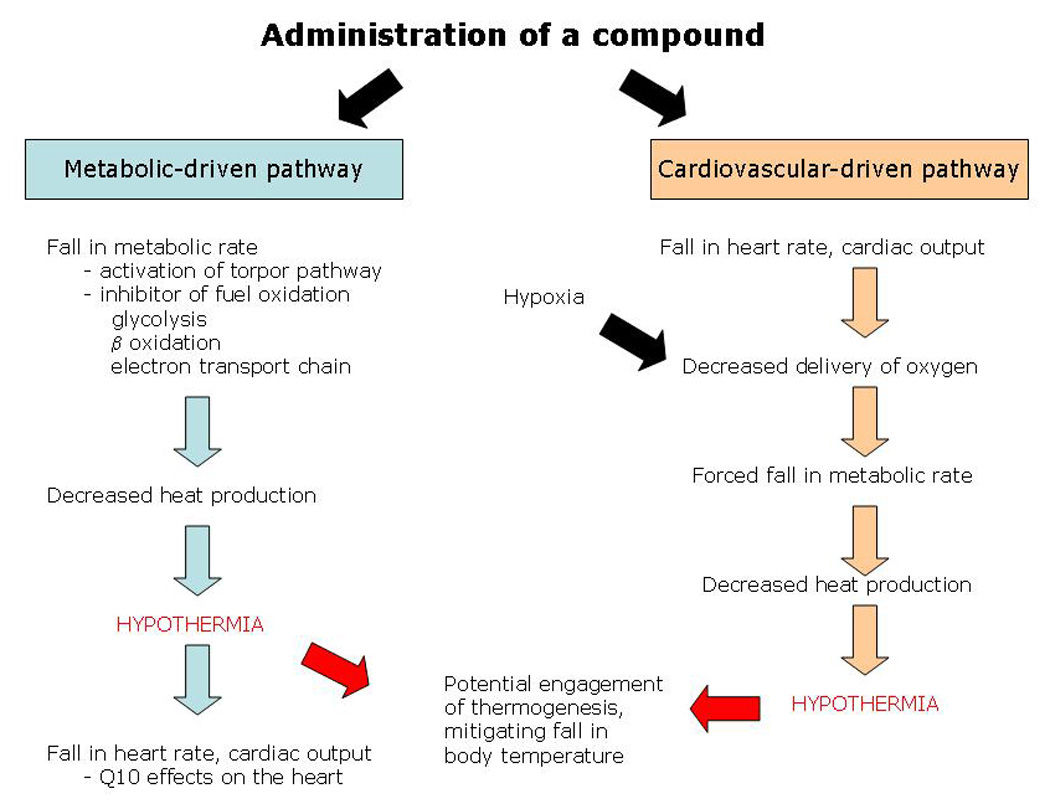
In the “metabolic-driven pathway”, the compound may activate / mimic the natural torpor-inducing pathway or may act simply as a metabolic poison. In either case, one would expect a fall in metabolic rate and therefore heat production, resulting in hypothermia in a small mammal. In the “cardiovascular-driven pathway”, the compound has its primary effect on the heart and/or circulatory system. This impairs the ability of the cardiovascular system to deliver oxygen, resulting in a fall in metabolic rate, followed by hypothermia. Unless the fasting-induced torpor pathway is activated or mimicked, the animal will likely engage homeostatic mechanisms to defend against the fall in body temperature.
4. Conclusion
To sum, hypometabolism and hypothermia is a readily-used tool by small mammals to cope with acute food shortages. These animals clearly have adaptations within multiple organ systems to cope with bouts of hypothermia on a daily basis. Those hormonal and metabolic signals that induce a hypometabolic state in response to food deprivation are likely many of the same signals that govern both sides of the energy balance equation (caloric intake and caloric expenditure) in all mammals, including humans. A valid question to ask is whether fasting-induced torpor or compound-induced hypothermia can be extrapolated from mice to humans. To state the obvious, mice are small with a large surface area to volume ratio. A portion of the metabolic suppression during torpor in a small animal is a result of their small size … as metabolism falls, body temperature falls when heat loss outpaces heat generation from metabolism. Then, as body temperature falls, metabolic rate falls due to the thermal dependence of biochemical reactions. So, even if “torpor-inducing” genes or “hypothermia-tolerant” genes exist within the human genome, one has to consider the thermal inertia and low surface area to volume ratio in a 75,000 gram human relative to a 25 gram mouse. As model organisms like mice are small, great care should be taken to determine whether metabolic inhibition either through inhibition of fuel oxidation or cardiovascular depressant effects results in a natural bout of torpor or achieves hypothermia simply because the animal cannot maintain a high enough metabolic rate to offset heat loss.
Acknowledgement
Supported by NIH grant R15 HL081101-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hudson JW, Scott IM. Daily Torpor in the Laboratory Mouse, Mus-Musculus Var Albino. Physiological Zoology. 1979;52:205–218. [Google Scholar]
- 2.Morhardt JE. Heart Rates, Breathing Rates and Effects of Atropine and Acetylcholine on White-Footed Mice (Peromyscus Sp) During Daily Torpor. Comparative Biochemistry and Physiology. 1970;33:441–457. doi: 10.1016/0010-406x(70)90360-9. [DOI] [PubMed] [Google Scholar]
- 3.Snapp BD, Heller HC. Suppression of Metabolism During Hibernation in Ground-Squirrels (Citellus-Lateralis) Physiological Zoology. 1981;54:297–307. [Google Scholar]
- 4.Ortmann S, Heldmaier G. Regulation of body temperature and energy requirements of hibernating Alpine marmots (Marmota marmota) Am J Physiol Regul Integr Comp Physiol. 2000;278:R698–R704. doi: 10.1152/ajpregu.2000.278.3.R698. [DOI] [PubMed] [Google Scholar]
- 5.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respiratory Physiology & Neurobiology. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Yoda T, Crawshaw LI, Yoshida K, Su L, Hosono T, Shido O, et al. Effects of food deprivation on daily changes in body temperature and behavioral thermoregulation in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R134–R139. doi: 10.1152/ajpregu.2000.278.1.R134. [DOI] [PubMed] [Google Scholar]
- 7.Carey HV, Andrews MT, Martin SL. Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 8.Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. Journal of Neurochemistry. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiser F. Metabolic Rate and Body Temperature Reduction During Hibernation and Daily Torpor. Annual Review of Physiology. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 10.Geiser F, Ruf T. Hibernation Versus Daily Torpor in Mammals and Birds - Physiological Variables and Classification of Torpor Patterns. Physiological Zoology. 1995;68:935–966. [Google Scholar]
- 11.Gordon CJ. The therapeutic potential of regulated hypothermia. Emerg Med J. 2001;18:81–89. doi: 10.1136/emj.18.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoppeler H, Weibel ER. Scaling functions to body size: theories and facts. J Exp Biol. 2005;208:1573–1574. doi: 10.1242/jeb.01630. [DOI] [PubMed] [Google Scholar]
- 13.Heller HC, Hammel HT. Cns Control of Body-Temperature During Hibernation. Comparative Biochemistry and Physiology. 1972;41:349–359. doi: 10.1016/0300-9629(72)90066-7. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 15.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Frontiers in Neuroendocrinology. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 17.Ahima RS. Leptin and the neuroendocrinology of fasting. Neuroendocrinology of Leptin. 2000:42–56. doi: 10.1159/000061014. [DOI] [PubMed] [Google Scholar]
- 18.Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The Full Expression of Fasting-Induced Torpor Requires {beta}3-Adrenergic Receptor Signaling. J Neurosci. 2006;26:241–245. doi: 10.1523/JNEUROSCI.3721-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migliorini RH, Garofalo MA, Kettelhut IC. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am J Physiol Regul Integr Comp Physiol. 1997;272:R656–R661. doi: 10.1152/ajpregu.1997.272.2.R656. [DOI] [PubMed] [Google Scholar]
- 20.Rayner VD. The sympathetic nervous system in white adipose tissue regulation. Proceedings of the Nutrition Society. 2001;60:357–364. doi: 10.1079/pns2001101. [DOI] [PubMed] [Google Scholar]
- 21.Young JB, Landsberg L. Suppression of Sympathetic Nervous-System During Fasting. Science. 1977;196:1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]
- 22.Giacobino JP. Role of the beta(3)-adrenoceptor in the control of leptin expression. Hormone and Metabolic Research. 1996;28:633–637. doi: 10.1055/s-2007-979868. [DOI] [PubMed] [Google Scholar]
- 23.Mantzoros C, Qu D, Frederich R, Susulic V, Lowell B, Maratos-Flier E, et al. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45:909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 25.Himms-Hagen J. Food restriction increases torpor and improves brown adipose tissue thermogenesis in ob/ob mice. Am J Physiol Endocrinol Metab. 1985;248:E531–E539. doi: 10.1152/ajpendo.1985.248.5.E531. [DOI] [PubMed] [Google Scholar]
- 26.Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, et al. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. PNAS. 1999;96:14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swoap SJ. Altered leptin signaling is sufficient, but not required, for hypotension associated with caloric restriction. Am J Physiol Heart Circ Physiol. 2001;281:H2473–H2479. doi: 10.1152/ajpheart.2001.281.6.H2473. [DOI] [PubMed] [Google Scholar]
- 28.Doring H, Schwarzer K, Nuesslein-Hildesheim B, Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. International Journal of Obesity. 1998;22:83–88. doi: 10.1038/sj.ijo.0800547. [DOI] [PubMed] [Google Scholar]
- 29.Geiser F, Kortner G, Schmidt I. Leptin increases energy expenditure of a marsupial by inhibition of daily torpor. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1627–R1632. doi: 10.1152/ajpregu.1998.275.5.R1627. [DOI] [PubMed] [Google Scholar]
- 30.Cariou B, Bouchaert E, Abdelkarim M, Dumont J, Caron S, Fruchart J-C, et al. FXR-deficiency confers increased susceptibility to torpor. FEBS Letters. 2007;581:5191–5198. doi: 10.1016/j.febslet.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal Satiety Signals. Annual Review of Physiology. 2008;70:239–255. doi: 10.1146/annurev.physiol.70.113006.100506. [DOI] [PubMed] [Google Scholar]
- 32.Kojima M, Kangawa K. Ghrelin: Structure and Function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 33.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 34.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and Direction of Ghrelin Transport Across the Blood-Brain Barrier Is Determined by Its Unique Primary Structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 35.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The Distribution and Mechanism of Action of Ghrelin in the CNS Demonstrates a Novel Hypothalamic Circuit Regulating Energy Homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 36.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1303–R1309. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- 37.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, et al. Orexigenic Action of Peripheral Ghrelin Is Mediated by Neuropeptide Y and Agouti-Related Protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neuroscience Letters. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 39.Morton GJ, Schwartz MW. The NPY/AGRP neuron and energy homeostasis. Int J Obes Relat Metab Disord. 2001;25:S56–S62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- 40.Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Progress in Hormone Research. 1998;Vol 53:163–199. [PubMed] [Google Scholar]
- 41.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 42.Paul MJ, Freeman DA, Park JH, Dark J. Neuropeptide Y induces torpor-like hypothermia in Siberian hamsters. Brain Research. 2005;1055:83–92. doi: 10.1016/j.brainres.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 43.Pelz KM, Dark J. ICV NPY Y1 receptor agonist but not Y5 agonist induces torpor-like hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2299–R2311. doi: 10.1152/ajpregu.00790.2006. [DOI] [PubMed] [Google Scholar]
- 44.Dark J, Pelz KM. NPY Y1 receptor antagonist prevents NPY-induced torporlike hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R236–R245. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- 45.Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. Am J Physiol Regul Integr Comp Physiol. 2007;293:R468–R473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
- 46.Schoelch C, Hubschle T, Schmidt I, Nuesslein-Hildesheim B. MSG lesions decrease body mass of suckling-age rats by attenuating circadian decreases of energy expenditure. Am J Physiol Endocrinol Metab. 2002;283:E604–E611. doi: 10.1152/ajpendo.00439.2001. [DOI] [PubMed] [Google Scholar]
- 47.Pelz KM, Routman D, Driscoll JR, Kriegsfeld LJ, Dark J. Monosodium glutamate-induced arcuate nucleus damage affects both natural torpor and 2DG-induced torpor-like hypothermia in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R255–R265. doi: 10.1152/ajpregu.00387.2007. [DOI] [PubMed] [Google Scholar]
- 48.Miller LP, Hsu C. Therapeutic Potential for Adenosine Receptor Activation in Ischemic Brain Injury. Journal of Neurotrauma. 1992;9:S563–S577. [PubMed] [Google Scholar]
- 49.Barros RCH, Branco LGS. Role of central adenosine in the respiratory and thermoregulatory responses to hypoxia. Neuroreport. 2000;11:193–197. doi: 10.1097/00001756-200001170-00038. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z-W, Gao X-B. Adenosine Inhibits Activity of Hypocretin/Orexin Neurons by the A1 Receptor in the Lateral Hypothalamus: A Possible Sleep-Promoting Effect. J Neurophysiol. 2007;97:837–848. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohno K, Sakurai T. Orexin neuronal circuitry: Role in the regulation of sleep and wakefulness. Frontiers in Neuroendocrinology. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 53.Heller HC, Ruby NF. Sleep and Circadian Rhythms in Mammalian Torpor. Annual Review of Physiology. 2004;66:275–289. doi: 10.1146/annurev.physiol.66.032102.115313. [DOI] [PubMed] [Google Scholar]
- 54.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase - development of the energy sensor concept. J Physiol (Lond) 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minokoshi Y, Alquier T, Furukawa N, Kim Y-B, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 57.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, et al. AMP-activated Protein Kinase Plays a Role in the Control of Food Intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 58.Horman S, Hussain N, Dilworth SM, Storey KB, Rider MH. Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2005;142:374–382. doi: 10.1016/j.cbpb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- 60.Braulke LJ, Klingenspor M, DeBarber A, Tobias SC, Grandy DK, Scanlan TS, et al. 3-iodothyronamine: a novel hormone controlling the balance between glucose and lipid utilisation. Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology. 2008;178:167–177. doi: 10.1007/s00360-007-0208-x. [DOI] [PubMed] [Google Scholar]
- 61.Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nature Medicine. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 62.Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18:239–253. doi: 10.1089/thy.2007.0248. [DOI] [PubMed] [Google Scholar]
- 63.Grandy DK. Trace amine-associated receptor 1--Family archetype or iconoclast? Pharmacology & Therapeutics. 2007;116:355–390. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiellini G, Frascarelli S, Ghelardoni S, Carnicelli V, Tobias SC, DeBarber A, et al. Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function. FASEB J. 2007;21:1597–1608. doi: 10.1096/fj.06-7474com. [DOI] [PubMed] [Google Scholar]
- 65.Belardinelli L, Linden J, Berne RM. The cardiac effects of adenosine. Progress in Cardiovascular Diseases. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- 66.Blackstone E, Morrison M, Roth MB. H2S Induces a Suspended Animation-Like State in Mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 67.Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, et al. Inhaled hydrogen sufide - A rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108:659–668. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayden P, Lindberg RG. Hypoxia-Induced Torpor in Pocket Mice (Genus . Perognathus) Comparative Biochemistry and Physiology. 1970;33:167–179. doi: 10.1016/0010-406x(70)90492-5. [DOI] [PubMed] [Google Scholar]
- 69.Gautier H. Interactions among metabolic rate, hypoxia, and control of breathing. J Appl Physiol. 1996;81:521–527. doi: 10.1152/jappl.1996.81.2.521. [DOI] [PubMed] [Google Scholar]
- 70.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of Hydrogen Sulfide. Annual Review of Pharmacology and Toxicology. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]


