One of the promises of the Human Genome Project was that it would provide tools for identifying genetic factors that contribute to common, complex diseases such as cancer and diabetes. Finding these factors would, in turn, suggest possible targets for drug therapy and other forms of treatment. Three papers in this week's issue—by Edwards et al. (1) on page 421, Haines et al. (2) on page 419, and Klein et al. (3) on page 385—deliver on this promise for a debilitating, blinding disease called age-related macular degeneration (AMD). Using several genome-derived tools applied to nonoverlapping groups of AMD patients, the three groups report that a common variant in the complement factor H gene (CFH) on human chromosome 1q31 contributes a substantial fraction of the difference between affected and unaffected individuals. AMD afflicts more than 10 million Americans and is the leading cause of blindness among the elderly. The complement system is the target of a number of modulatory drugs and treatments. Together with the new findings, these facts confirm the broad potential health benefits of “genome science.”
The macula is a circular area at the center of the retina that contains a high density of cone cells—these are photoreceptor cells that are specialized for distinguishing colors, resolving closely spaced objects, and detecting motion. The macula accounts for the central 30% of our visual fields and is critical for the full vision that enriches our lives (see the figure). AMD results in progressive damage to the macula, ultimately leading to patients becoming legally blind. This disorder principally affects individuals over age 50, although in certain inherited forms of macular degeneration symptoms appear at a much younger age. Macular degeneration diminishes the quality of life of those afflicted and is a major burden on society.
A view from the periphery.
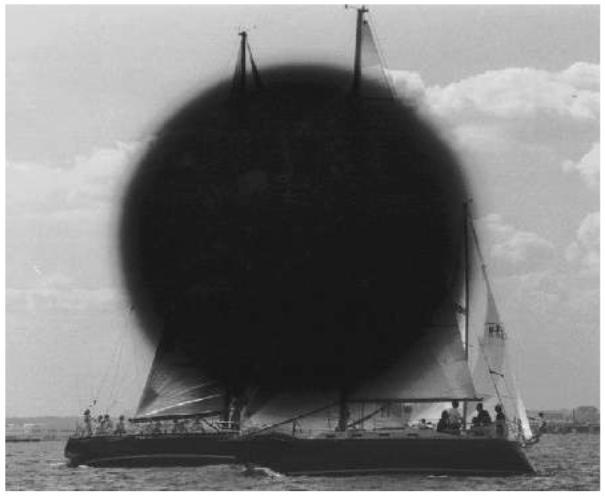
Age-related macular degeneration has a devastating effect on central vision. Peripheral vision is often spared, but objects in the center of vision are blurred or completely blocked. Central vision allows us to read, to recognize faces, and to enjoy our surroundings, as in this sailboat scene.The picture is altered to show the vision lost to the millions of individuals afflicted with age-related macular degeneration.
This complex disease has diverse symptoms and multiple contributing factors. It is a truism that complex diseases are the result of interactions between environmental factors, genetic factors, and stochastic effects. For AMD, smoking and dietary intake of lipids are known environmental risk factors (4); age alone is an additional risk factor. There is also strong evidence indicating a genetic contribution. Concordance between identical twins, family clustering, and increased risk to first-degree relatives all show that genetic differences play a major role in the life-time risk of AMD (5). But how do we find these genetic factors?
Broadly speaking, there are three methods for finding genes contributing to complex diseases: candidate gene screening, linkage mapping, and association (case-control) studies. Candidate gene screening relies on selecting potential disease-causing genes—for example, genes causing inherited forms of disease—and sequencing these genes in patients with complex diseases. Linkage mapping follows the segregation of chromosomal regions marked by random genetic variants in families with complex diseases, in search of regions that cosegregate with the disease trait. For AMD, the “families” range from simple sib-pairs to large extended pedigrees. Finally, case-control association studies look for differences in the frequencies of common genetic variants between ethnically matched cases and controls to find variants that are strongly associated with the disease. The eventual goal of each method is to identify either mutations—that is, rare genetic variants with a strong causal relation to the disease—or polymorphic variants—that is, common, weakly causal variants that affect the life-time risk of disease. These are extremes of a continuum, of course. For AMD, each of these methods and possibilities has been applied and advocated.
Genes causing several inherited forms of macular degeneration have been screened as candidates for AMD. For example, mutations in the ABCA4 gene cause recessive Stargardt disease, an early-onset form of macular degeneration. An earlier Science paper suggested an association between variants in the ABCA4 gene and AMD (6). However, it has been difficult to validate this claim, in part because of the exceptional degree of genetic variation between humans. Although we are 99.9% identical, we differ from each other at millions of nucleotide sites. Many of these differences change an amino acid within a protein, or affect protein function in other ways. For ABCA4, there are at least 11 polymorphic amino acid substitutions within the gene and many more rare variants (7). It is extremely difficult to detect a signal against this noisy background.
Linkage mapping comes to the rescue. The “signal” in this case is summed across families and combines different variants within a gene, if the variants each contribute to the trait. Genetic markers that cover all human chromosomes have been tested for segregation in several independently ascertained sets of AMD families. Several of these genomewide linkage scans implicate a region on chromosome 1q31 (8-12). Suggestively, the gene causing an inherited, late-onset form of macular degeneration, the ARMD1 locus, also maps to this region (13). The problem with linkage mapping, however, is that the delimited regions are large, typically tens of millions of base pairs in length, harboring scores of genes. The haystack is smaller, but still daunting.
The current trio of Science papers (1-3) take off from this point. One recent product of the Human Genome Project is a collection of well-defined, genomewide polymorphic variants called single-nucleotide polymorphisms (SNPs). To date, more than 1 million human SNPs have been identified (14). Klein et al. used gene chips from Affymetrix Corporation to test for association among more than 100,000 SNPs in 96 AMD cases and 50 controls. The strongest signal mapped to chromosome 1q31. They then saturated the region with additional SNPs. Edwards et al. followed up on earlier linkage mapping to the 1q31 region, testing 86 SNPs in 400 cases and 202 controls. Haines et al. conducted additional linkage testing within the 1q31 region and followed up with 61 SNPs in 495 cases and 185 controls. (Cases and controls were Americans of European origin.) All three studies pointed to the CFH gene…but which specific variant or variants within this gene are associated with AMD?
A recent goal of the Human Genome Project has been to determine the haplotype structure of human chromosomes in various ethnic groups, a goal embodied in the International HapMap Project (15). Haplotypes are sets of specific DNA variants that are so close together on a chromosome that they undergo recombination only very rarely. The pattern of nonrecombining variants, known as haplotype blocks, reflects the evolutionary history of humans over the past 50,000 to 100,000 years. Haplotype blocks typically span tens of thousands of base pairs. One immediate benefit of the HapMap Project is that it provides a bridge between linkage mapping at millions of base pairs and SNPs mapped to single base pairs.
Klein et al. made explicit use of HapMap data to disentangle the SNPs in the CFH region. Edwards et al. and Haines et al. developed haplotype data on the basis of their tested samples. In all three cases, the haplotype data point to a specific CFH SNP (rs1061170) that results in replacement of the amino acid tyrosine with histidine at amino acid 402 (Tyr402His). Individuals who carry a single copy of the histidine allele in the Tyr402His polymorphism have a two- to fourfold increased risk of AMD; individuals who carry two copies of the allele have a five- to sevenfold increased risk. Within this retrospective study population, the histidine allele accounts for 20 to 50% of the overall risk of developing AMD.
Does evidence from biology support the statistical findings? The complement system is the innate component of the immune response. Initiation of complement-mediated immunity by a microorganism triggers a cascade of protein interactions in blood that eventually results in opsonization and destruction of the invading organism. Host tissues can also trigger the complement cascade, causing chronic inflammation and tissue damage. Complement factor H modulates the complement cascade by inactivating components of the cascade and by binding initiation factors such as C-reactive protein (16). The CFH protein consists of 20 repeat units of 60 amino acids each, called complement control modules; module 7 contains the Tyr402His polymorphism. This module interacts with cell surface markers such as heparin and sialic acid. A switch from tyrosine to histidine at this site is predicted to weaken this interaction. Thus, the polymorphism is likely to affect complement activity.
A role for complement in AMD has long been suggested (17). Clinical hallmarks of AMD include multiple drusen, geographic atrophy, and choroidal neovascularization. Drusen, the defining feature of AMD, are small, yellowish, extracellular deposits of lipid, protein, and cellular debris, formed beneath the retinal pigment epithelium (RPE), a tissue that underlies the photoreceptor cells. Drusen contain complement proteins. Geographic atrophy is a consequence of the degeneration of the photoreceptor cells and the RPE. Choroidal neovascularization—disruptive growth of capillaries from the choroid, a tissue at the back of the eye that nourishes the retina—occurs in 10% of cases. Atrophic tissue damage and neovascularization are potential consequences of chronic inflammation, mediated by the complement system. Further, the known AMD risk factors—smoking, diet, and age—all correlate with complement activity.
Taken together, the three new studies and preexisting information on complement factor H suggest a direct, causal connection between the polymorphic histidine allele and life-time risk for AMD. What next? These were retrospective studies, so there needs to be more research into prospective risks for individuals who carry one or two copies of the histidine allele. It will be extremely interesting to learn whether the histidine allele is found in other ethnic groups, and whether this allele, or others, contribute to AMD in non-Caucasians. Complement activity is responsive to drug therapy and life-style changes. Will these have differential effects on AMD risks in individuals with one or two copies of the histidine allele? Do other complement factors contribute to AMD? Finally, detecting the CFH Tyr402His type in human DNA is an easy assay to perform. How will this test be incorporated into the diagnosis and treatment of AMD?
As promised, the Human Genome Project provides powerful new insights into human diseases and raises many challenging questions.
References
- 1.Edwards AO, et al. Science. 2005;308:421. published online 10 March 2005 (10.1126/science.1110189) [Google Scholar]
- 2.Haines JL, et al. Science. 2005;308:419. published online 10 March 2005 (10.1126/science.1110359) [Google Scholar]
- 3.Klein RJ, et al. Science. 2005;308:385. published online 10 March 2005 (10.1126/science.1109557) [Google Scholar]
- 4.Hyman L, Neborsky R. Curr. Opin. Ophthalmol. 2002;13:171. doi: 10.1097/00055735-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Stone EM, Sheffield VC, Hageman GS. Hum. Mol. Genet. 2001;10:2285. doi: 10.1093/hmg/10.20.2285. [DOI] [PubMed] [Google Scholar]
- 6.Allikmets R, et al. Science. 1997;277:1805. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 7.Webster AR, et al. Invest. Ophthalmol. Vis. Sci. 2001;42:1179. [PubMed] [Google Scholar]
- 8.Abecais GR, et al. Am. J. Hum. Genet. 2004;74:482. doi: 10.1086/382786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyengar SK, et al. Am. J. Hum. Genet. 2004;74:20. doi: 10.1086/380912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majewski J, et al. Am. J. Hum. Genet. 2003;73:540. doi: 10.1086/378134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon JM, et al. Am. J. Hum. Genet. 2003;73:780. doi: 10.1086/378505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weeks DE, et al. Am. J. Hum. Genet. 2004;75:174. doi: 10.1086/422476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein ML, et al. J. Ophthalmol. 1998;116:1082. doi: 10.1001/archopht.116.8.1082. [DOI] [PubMed] [Google Scholar]
- 14.Sachidanandam R, et al. Nature. 2001;409:928. [Google Scholar]
- 15.The International HapMap Consortium Nature. 2003;426:789. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 16.de Córdoba SR, et al. Mol. Immunol. 2004;41:355. [Google Scholar]
- 17.Hageman GS, et al. Prog. Retinal Eye Res. 2001;20:705. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]


