Abstract
Human beings differ in their ability to form and retrieve lasting long-term memories. To explore the source of these individual differences, we used functional magnetic resonance imaging to measure blood-oxygen-level-dependent (BOLD) activity in healthy young adults (n = 50) during periods of resting fixation that were interleaved with periods of simple cognitive tasks. We report that medial temporal lobe BOLD activity during periods of rest predicts individual differences in memory ability. Specifically, individuals who exhibited greater magnitudes of task-induced deactivations in medial temporal lobe BOLD signal (as compared to periods of rest) demonstrated superior memory during offline testing. This relationship was independent of differences in general cognitive function and persisted across different control tasks (i.e., number judgment versus checkerboard detection) and experimental designs (i.e., blocked versus event-related). These results offer a neurophysiological basis for the variability in mnemonic ability that is present amongst healthy young adults and may help to guide strategies aimed at early detection and intervention of neurological and mnemonic impairment.
Keywords: default network, fMRI, hippocampus, individual differences, resting-activity
Mnemonic faculty varies as a function of age and mental health, yet also differs across healthy individuals of similar age and neurological status. Neuroscientific investigations of individual differences in memory have predominantly focused on the hippocampus and surrounding medial temporal cortices (i.e., the medial temporal lobe, MTL), regions known to be crucial to long-term memory (1–3). While numerous studies have demonstrated a positive relationship between MTL volume and mnemonic performance in patients with neuropathology to this region (4–7), the search for neuroanatomical markers that may mediate individual differences in memory in healthy individuals has been largely inconclusive (8). Thus, the extent to which individual differences in memory ability are wedded to functional or anatomical differences in brain regions that mediate memory, or simply arise from differences in strategy or behavior during conditions that guide memory formation and retrieval (9, 10) is an outstanding question.
The tendency to search for a relationship between MTL size and individual differences in memory has stemmed, in part, from the practical limitations involved in probing the intrinsic physiological properties of the human brain. However, recent insight gained from functional neuroimaging studies has highlighted a mechanism that allows for explorations of this possibility. Relative to externally cued tasks, a number of regions consistently demonstrate greater levels of activity, indexed by either blood flow (11, 12) or blood-oxygen-level-dependent (BOLD) signal (13) during passive or resting states (e.g., simple fixation or eyes closed). These regions include the medial prefrontal cortex, lateral parietal cortex, posterior cingulate gyrus and retrosplenial cortex, anterior portions of the lateral temporal cortex, and the MTL. Collectively, these regions have been referred to as the “default” network (14). Importantly, during rest, blood flow and metabolic activity are both high in the default network. Transient attenuation occurs during performance of goal-directed tasks, suggesting that these are true deactivations from this physiologic baseline and subserve a “default” mode of brain function that is suspended when subjects are engaged in goal-directed behaviors (15, 16).
Although the functional significance of default network activity has been a topic of considerable recent experimental interest, converging lines of evidence from animal and human work have provided clues regarding the nature of MTL processing during periods of inactivity. Neuroscientific studies using multiunit recording in rats during periods of resting wakefulness (17, 18) and sleep (19–21), and functional neuroimaging in humans (22), have provided evidence that these periods are accompanied by signatures that may reflect hippocampal-mediated consolidation of past experiences. These studies fit in well with the MTL's well-established role in facilitating the establishment of long-term mnemonic traces (1, 23).
As such, measurements of resting period activity may serve as a viable metric with which to investigate the relationship between regionally specific neurophysiological properties and individual differences in cognitive abilities. Interestingly, recent investigations have reported that MTL resting T2* activity is mitigated in elderly individuals with mnemonic deficits (24, 25), while MTL resting-state functional connectivity is reduced in patients with Alzheimer's disease (26). However, whether differences in resting MTL signals are a consequence of the local neurodegenerative changes that accompany aging and disease or reflect differences in the functional properties of the region across individuals is yet to be discerned.
The present set of studies investigates whether resting brain activity indexes a wider range of health and mnemonic faculty. We tested healthy young adults (n = 50) to determine whether there was a relationship between resting MTL BOLD activity and memory ability. Resting period BOLD activity was quantified relative to periods during which subjects were engaged in externally-cued control tasks.
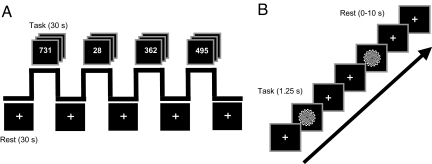
Resting period BOLD activity was measured with functional magnetic resonance imaging (fMRI) using two separate experimental paradigms in subjects who were naïve to the aims of the study. During scanning, subjects engaged in extended periods of a simple number judgment task that alternated with periods of rest, during which subjects simply fixated a crosshair (Fig. 1A, Experiment 1). To assess whether any observed relationship between resting MTL activity and memory would generalize to more rapid transitions between task and rest, all 50 subjects were scanned in a second experiment using an event-related design. Individual task trials were intermixed with shorter, variable periods of rest (0–10 seconds in duration). Task trials during this second experiment required subjects to respond with a button press at both the onset and offset of a large-field 8-Hz counterphase flickering checkerboard (black to white) (Fig. 1B, Experiment 2). Both experimental paradigms were collected in a single scanning session. Of interest to the present report was the activity associated with the periods of rest (treating the scanned control tasks as a reference) in both experiments.
Fig. 1.
fMRI experimental procedure. (A) During Experiment 1, subjects alternated between periods of task (30 s) and periods of rest (30 s). During task periods, subjects made odd/even judgments on a random set of numbers ranging from 1 to 1,000. During the passive rest periods, subjects were instructed to simply maintain fixation on a crosshair that was presented at a central location on the screen. (B) During Experiment 2, subjects alternated between periods of task (1.25 s) and variable periods of rest (0–10 s). During task periods, subjects responded at the onset and offset of a flickering checkerboard stimulus. During the passive rest periods, subjects were instructed to simply maintain fixation on a crosshair that was presented at a central location on the screen.
Following scanning, subjects completed a battery of standardized and nonstandardized cognitive tests outside of the fMRI scanner. These tests provided psychometric measures of general intelligence, vocabulary, executive function, fluency in stimulus-to-response mapping, and long-term memory ability across various domains. Performance scores from each of the psychometric tests were entered into a principle components analysis (PCA) to reduce the data into a set of orthogonal components or “metavariables.” Finally, to explore the relationship between behavioral and BOLD measures, these components were entered in a multiple regression analysis aimed at predicting resting period activity.
Critically, as resting period activity was defined relative to activity during periods of the control task, individual differences in behavioral performance during the scanned control task periods could influence the measure of resting period activity (27). Because the control task was intended as a common reference point for resting period activity, performance measures from the scanned control task (mean response time and mean number of no responses) were also included in the PCA and subsequent brain-behavior regression analysis to serve as indices of inter-subject variability in attention or vigilance that may have been present during scanning.
Results
The data reported here are from 45 young adults (22 males, 23 females; mean age of 20 years old; age range of 18–32 years old) following an initial screen for the presence of outliers on behavioral measures obtained from both within the scanner during number judgment and checkerboard detection, as well as outside of the scanner during offline behavioral testing [see Methods and supporting information (SI) Methods].
Experiment 1–fMRI.
Relative to periods of rest, periods of control task (odd/even number judgment) evoked greater activity in bilateral brain areas, including regions of the occipital cortex, primary motor and somatosensory cortex, thalamus, middle frontal gyrus, and the anterior extent of the cingulate gyrus. By contrast, relative to the control task, periods of rest elicited greater activity in a number of regions, including bilateral regions of the lateral parietal cortex, posterior cingulate gyrus, medial prefrontal cortex, the anterior extent of the lateral middle temporal gyrus, and the medial temporal lobe (i.e., the default network; P < 0.001 uncorrected, minimum cluster size of 5 contiguous voxels) (Fig. S1a). Subsequent analyses focused on this latter default network of brain regions. Activation maps were not used as inclusive masks, but rather served as weighted parameter estimate images to be used in subsequent regression analyses (see SI Methods for details of analysis).
Experiment 1–Behavioral.
A summary of the data from each behavioral measure is reported in Table S1. All behavioral measures were entered into a PCA model to reduce the data into a set of orthogonal metavariables. PCA analysis revealed three components that accounted for over 63.7% of the total variance of the 10 behavioral measures (Table S2). Rotation of the factor loading structure (Table S3) (factor loadings represent Pearson's correlation coefficient, “r”) revealed a component (Component 3) that accounted for 13.7% of the variance and was most highly correlated with performance on two declarative memory tasks (both loading scores: P < 0.001)—the verbal word recognition task and the face-name memory task. One of the remaining two components was most highly correlated with Intelligence Quotient (IQ) and working memory measures (Component 1: verbal-IQ, performance-IQ, full-IQ, and digit-span score; all loading scores: P < 0.001), while the last component was most highly correlated with the two behavioral measures collected during the scanned number judgment task and with the behavioral measures associated with fluency in stimulus-to-response mapping during offline testing (i.e., measures obtained from a serial reaction-time task and a repetition-priming task; Component 2: all loading scores, P < 0.001).
Experiment 1–Brain-Behavior Correlations.
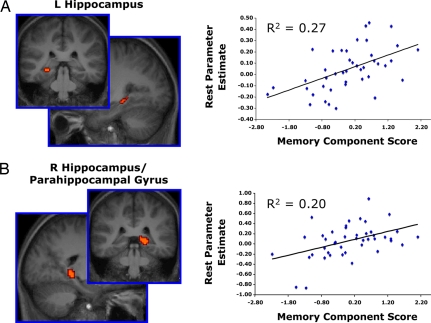
To determine whether resting-state activity was related to any of the behavioral measures, subjects' PCA component scores were entered into a regression analysis as predictors of default network activity. Specifically, each subject's whole-brain resting period weighted parameter estimate image (treating the control task as a reference) was entered into a multiple regression analysis with subjects' component scores for each of the three PCA components serving as covariates of interest. Statistical F-maps were computed to identify brain regions demonstrating correlations with any of the three orthogonal PCA components. Two regions within the MTL demonstrated a significant relationship with PCA memory scores: a region of the left hippocampus (peak xyz Talairach coordinates: −30, −35, −3) and a region of the right hippocampus extending into the right parahippocampal gyrus (peak xyz Talairach coordinates: 21, −35, −1). No other component demonstrated a significant relationship with resting activity within the MTL, and the memory component failed to predict resting activity in other regions within the default network (for additional analyses, see SI Results and Fig. S2).
To explore the nature of the relationship between memory component scores and resting MTL activity, a region-of-interest (ROI) analysis was conducted. Mean resting period weighted parameter estimates in both ROIs were extracted for each subject and entered into a multiple regression model, with the subjects' three PCA component scores as covariates of interest. As expected, the overall model was significant for each region [left MTL: R2 = 0.34, F (3, 39) = 6.75, P < 0.001; right MTL: R2 = 0.24, F (3, 40) = 4.19, P = 0.01]. Resting activity in the left and right MTL correlated positively with the PCA memory component [left MTL: R2 = 0.27, F (1, 41) = 15.31, P < 0.001; right MTL: R2 = 0.20, F (1, 42) = 10.46, P = 0.002]. That is, subjects who demonstrated greater resting period MTL BOLD activity had higher PCA memory component scores—the component onto which the behavioral measures of declarative memory loaded most heavily (Fig. 2). The remaining component coefficients were not significantly related to either MTL region.
Fig. 2.
Individual differences in resting period BOLD activity in regions of the left and right medial temporal lobes predict memory ability (Experiment 1). Subjects were scanned while periods of resting fixation (30 s) were interleaved with periods of a odd/even number judgment task (30 s) in a block-design experiment. Individuals evoking greater resting period MTL BOLD activity demonstrated larger PCA memory component scores during offline behavioral testing. The PCA memory component accounted for the majority of the variance associated with two declarative memory tasks: d' from verbal word recognition and percentage of faces correctly named from the relational memory test. Graphs depict subjects' mean resting period MTL ROI parameter estimates on the y-axis and their memory component scores on the x-axis. Each point represents one subject. (A) Left hippocampus (peak xyz Talairach coordinates: −30, −35, −3); (B) Right hippocampus/parahippocampal gyrus (21, −35, −1).
Experiment 2–fMRI.
During visual checkerboard detection in Experiment 2, task events evoked greater activity in bilateral brain areas, including regions of the occipital cortex, primary motor and somatosensory cortex, thalamus, middle frontal gyrus, the anterior extent of the cingulate gyrus, and subregions of the MTL, when compared to fixation rest. Relative to the checkerboard-detection task, moments of rest evoked greater activity in regions similar to those observed during Experiment 1. When compared to task, moments of rest elicited greater activity in bilateral regions of the default network: lateral parietal cortex, posterior cingulate gyrus, medial prefrontal cortex, the anterior extent of the lateral middle temporal gyrus, and subregions of the MTL (P < 0.001 uncorrected, minimum cluster size of 5 contiguous voxels) (Fig. S1b). Consistent with prior neuroimaging work (11), the present results demonstrate the ubiquity of default network modulation across different experimental paradigms and resting period durations.
Experiment 2–Behavioral.
A second PCA model was computed that included the behavioral scores obtained from the checkerboard-detection task along with the offline standardized and nonstandardized cognitive tasks. This PCA produced four behavioral components that accounted for 73.3% of the total variance (Table S4). As in Experiment 1, rotation of the factor-loading structure revealed a component (Component 4) that accounted for 13.9% of the variance and was most highly correlated with performance on the two declarative memory tasks, whereas the remaining components were most highly correlated with the remaining behavioral measures (Component 1: verbal-IQ, performance-IQ, full-IQ, and digit-span score; Component 2: behavioral measures collected from the serial reaction time task and repetition priming task; Component 3: the two behavioral measures collected from the scanned checkerboard-detection task; all loading scores: P < 0.001) (Table S5).
Experiment 2–Replication Analysis.
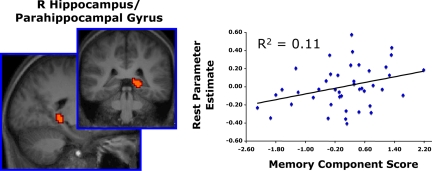
To test the reliability and generality of the resting period BOLD activity-memory relationship across data sets and experimental paradigms, a formal replication analysis was conducted. Specifically, the MTL regions-of-interest that were defined from Experiment 1 were tested for replication on the REST > TASK-weighted parameter estimates derived in Experiment 2. By limiting interrogation to only those regions that demonstrated an effect in the first experiment, this method was both nonbiased in its approach and also highly conservative (see SI Results and Fig. S3 for additional analyses). For each subject, mean resting period parameter estimates representing rest relative to the checkerboard detection task were extracted from each of the two MTL regions and entered into a regression model, with the subjects' PCA memory component scores serving as a covariate of interest. Resting activity in the right MTL was again positively correlated with the memory component scores [R2 = 0.11, F (1, 41) = 5.21, P = 0.03] (Fig. 3). Resting activity in the left MTL region failed to replicate this relationship.
Fig. 3.
Individual differences in resting period BOLD activity within the right medial temporal lobe predict memory ability (replication analysis, Experiment 2). In the same group of subjects as Experiment 1, resting period data were collected in a second fMRI scan that used an event-related checkerboard detection task (1.25 s) that was interleaved with shorter, variable periods of resting fixation (0–10 s). Individuals evoking greater resting period BOLD activity in the right MTL demonstrated larger PCA memory component scores during offline behavioral testing. The MTL ROI was defined from Experiment 1. As in Experiment 1, memory component scores were predictive of performance on the two offline declarative memory tasks: d' from verbal word recognition and percentage of faces correctly named from the relational memory test. Graph depicts subjects' mean resting period MTL parameter estimates on the y-axis and their memory component scores on the x-axis. Each point represents one subject.
Discussion
The present study demonstrates that greater MTL BOLD activity during periods of rest relative to periods of task predicts superior memory ability in healthy young adults*. This relationship is independent of general cognitive functioning, persists across variable resting period durations and variations in experimental design, and is present in a region known to be critical to the formation and retrieval of long-term memories that are declarative and relational in nature (1).
Delineating the functional significance of default network processing during periods of resting wakefulness is a research topic that has received considerable attention (11, 14, 28–30). The observed brain-behavior correlations reported here may shed light on understanding the nature of MTL involvement in the default network.
One possible explanation for resting period MTL activity is that it reflects active encoding of recent experiences or of the immediate external environment during periods of inactivity. Active elaborative rehearsal of recently encountered material facilitates subsequent memory (31), and the differences in MTL activity that were observed across individuals may have revealed a differential propensity to engage in such rehearsal (see also ref. 32). According to this account, subjects in the present study demonstrated greater MTL activity during periods of rest because they were actively encoding certain aspects of the task (i.e., the random numbers in Experiment 1 and flickering checkerboards in Experiment 2) or the scanning environment. Such an account seems unlikely in the present set of experiments, as there was no requirement to explicitly memorize the stimuli, and the rudimentary nature of the stimulus material (simple numbers and repeating checkerboards) likely discouraged any incidental encoding of the stimuli during the intervening rest periods. Similarly, it is not clear what aspect of the scanner environment would serve as a viable stimulus to encode while subjects lay in resting wakefulness.
Alternatively, the observed relationship between resting period MTL activity and memory ability observed here may reflect memory consolidation operations that are passive and do not require subject-initiated operations. For example, the variability in BOLD signal may be capturing differences in basal inter-neuronal firing rate or neurometabolic activity that are not a result of the subjects actively and consciously encoding recent experiences. Functionally, these differences may reflect individual variability in the propensity to spontaneously engage hippocampal-mediated consolidation of recent experiences during periods of inactivity (33, 34). Neuroscientific investigations of animals and humans have provided converging evidence for hippocampal-mediated processes of memory consolidation during periods of both resting wakefulness and sleep. Multiunit recording in the awake rat has been used to demonstrate that neurons in the hippocampus exhibit patterns of resting period activity that are reverse-recapitulations of events that have recently transpired (17, 18). Likewise, periods of slow-wave (19) and rapid-eye movement (20) sleep are accompanied by reactivation of temporally sequenced patterns of hippocampal activity for previous behavioral experiences. Although research investigating the human corollaries of hippocampal-mediated consolidation processes have been sparse because of methodological limitations, the hippocampus has been shown to be critical for the consolidation of declarative memories (33), and there is evidence for this function to be accompanied by periods of sleep (35) and awake behavior (22).
As such, MTL processing and consolidation need not be tied to material encountered in the immediate spatial or temporal vicinity, but may be an obligatory and spontaneous reaction associated with periods of inactivity that serves to stabilize previously encoded information or episodes using otherwise idle MTL-processing resources. This account also fits nicely with animal research demonstrating that the successful storage of recently encoded material can be compromised following damage to MTL regions that has occurred hours following the initial learning experience (1, 23). Perhaps more interestingly, this account suggests that neural signatures of memory consolidation may be revealed using functional neuroimaging in humans during periods of resting wakefulness and that individual differences in these signatures may, in turn, manifest as individual differences in mnemonic ability. An important caveat to this hypothesis is that BOLD activity is an indirect measure of neuronal activity, one that has received considerable debate as of late (36–38). Thus, any direct linkage to memory consolidation will undoubtedly require converging evidence from neuroscientific methodologies that are more sensitive to quantitative measures of cerebral blood flow, glucose metabolism, oxygen consumption, and neuronal activity. Similarly, to determine whether the observed relationship reflects consolidation processes that are passive and obligatory, it will be important to assess whether the relationship persists under reduced levels of consciousness, such as during sleep or under anesthesia.
An additional, possibly complimentary, source of the brain-behavior correlation we consider is that MTL activity during periods of resting wakefulness functions to encode or retrieve the products of default network processing. Default network activity has been linked to internally generated thought processes related to self-reflection (39, 40), mind-wandering, and stimulus-independent thoughts (29, 41).
A subset of default network regions, which includes the posterior cingulate cortex and lateral parietal cortex, has been implicated in the retrieval of past experiences (42). One possibility is that regions of the MTL act in concert with posterior cingulate and lateral parietal regions to recapitulate past experiences, and that individuals who are more likely to recall and reflect on prior experience do so because they were better able to encode such experiences as they initially occurred. However, if resting MTL activity indexes the extent to which individuals engaged active retrieval at rest, other regions of the default network that have been linked to memory retrieval (e.g., the posterior cingulate gyrus and lateral parietal cortex) would also be expected to track with offline memory scores. In the present study, resting period BOLD activity in other default regions failed to exhibit a relationship to memory when indexed along a continuum of mnemonic fluency.
A more parsimonious account is that MTL activity during rest functions to establish memory traces for the kinds of internal thoughts that accompany resting wakefulness. For example, we can readily remember the products of our internal musings. Indeed in many cases, such as when making future goals and plans of action, the ability to do so has considerable adaptive significance (43–45). In much the same way that regions of the MTL bind together the processing outcomes of external experiences, the MTL may facilitate the formation of memory for our internally generated thoughts and feelings. Better memorizers may be more adept at forming memories for information that is externally provided, as well as the product of internally generated thoughts, thus leading to greater activity when comparing periods of rest to periods of task for these individuals. Such an account has intuitive appeal because it offers a putative explanation for the somewhat puzzling functional coupling between regions of the MTL and other regions of the default network; spontaneous fluctuations in MTL activity correlate with spontaneous fluctuations in the other regions of the default network during periods of rest (28, 46). The brain-behavior correlation reported here suggests that the spontaneous fluctuations in MTL activity during moments of rest occur at a higher mean level of activity in individuals who are better memorizers.
These alternative accounts need not be mutually exclusive. Resting period MTL activity may be related to both passive consolidation of recent real world experience as well as the encoding and consolidation of internal thought. Linking these ideas together, distinct subregions of the MTL may function to encode the products of internally generated thoughts and to promote domain-general consolidation of information from the recent past (i.e., information from both external and internal sources). Superior memorizers may be afforded a processing advantage for one or both of these functions, thus leading to the resting period memory performance correlations reported here.
Keeping these points in mind, the results from the present set of studies have a number of additional implications. First, the relationship noted here highlights the need to consider the potential for individual differences in resting period BOLD activity when making comparisons across groups of subjects (also see ref. 47). Second, correlations between offline cognitive measures and resting period BOLD activity may be used as a potentially unique method of parsing out the function of components of the default network, complementing the use of task-based manipulations and resting state intrinsic correlations (46, 48, 49) to further our understanding of both regional and network activity when the brain is at rest and during execution of externally cued tasks.
Resting-state MTL functional connectivity is reduced in patients with Alzheimer's disease (26), and there is evidence that reductions in baseline glucose metabolism in the hippocampal formation differentiates both Alzheimer's patients and patients with mild cognitive impairment from healthy age-matched controls (50). Mitigated T2* resting activity in the MTL has been further implicated in the memory decline that accompanies healthy aging (24, 25). The present study demonstrates that differences in resting MTL activity index a wider spectrum of human mnemonic ability, such that it permeates the subtle variability that is present among healthy young adults. One final hypothesis is that individuals demonstrating significantly reduced levels of MTL resting period activity may be those that are more susceptible to ensuing mnemonic impairment, and that this predisposition may be directly related to the underlying genetic make-up of individuals. Considered in this context, we propose that resting-state MTL activity should be explored as a potentially viable biomarker for early detection of ensuing neurological and mnemonic degeneration, possibly permitting early intervention strategies aimed at both improving memory function and forestalling age-related memory decline.
Materials and Methods
Data Acquisition.
All imaging was performed on a 3.0T Philips Intera Achieva Scanner (Philips Medical Systems, Bothell, WA) equipped with a SENSE (SENSEitivity Encoding) head coil at the Dartmouth College Brain Imaging Center (Hanover, NH). See SI Methods for apparatus and imaging details.
Study Procedure
Functional Imaging.
All subjects were scanned during two separate tasks (experiments). Both experiments were collected in a single scan session and the order of experiments was counterbalanced across individuals. Refer to Fig. 1 and SI Methods for details of functional imaging task procedures.
Behavioral Testing.
Following their scanning session, subjects came back on two separate days for additional behavioral testing. Behavioral testing consisted of a number of standardized and nonstandardized cognitive tests. Tasks were counterbalanced across subjects, with the exception that all standardized testing was administered on one of the two days, while the remaining nonstandardized cognitive tests were administered on the alternate day. See SI Methods for details on standardized and non-standardized cognitive testing.
Data Analysis
Behavioral.
Behavioral data were analyzed using a separate exploratory PCA for each of the two experiments. These analyses produced a set of independent components or metavariables representing the behavioral data set. Factor scores from each of the components were derived for every subject to be used in fMRI regression analyses. These factor scores represented estimations of the actual individual subject values (i.e., the relative contribution of each component to the variance of their behavior both within and across subjects) for each of the components. See SI Methods for details on behavioral data analysis.
Functional Data.
Functional MRI data were analyzed using SPM2. Each experiment was preprocessed and analyzed separately. For each functional run, data were preprocessed to remove sources of noise and artifact. Preprocessing was followed by estimation of parameter estimates and computation of statistical images. Brain-behavior correlations were computed using the PCA component scores obtained from the behavioral data in a series of multiple regression analyses, and subsequently probed using a region of interest analysis. See SI Methods for details on preprocessing and data analysis.
Supplementary Material
Acknowledgments.
We thank Meredith Cashman for assistance with data collection and Jay Hull for helpful discussion. This work was supported by a Natural Sciences and Engineering Research Council of Canada Postgraduate Fellowship (to G.S.W.), a grant from the National Science Foundation (NSF0746220 to W.M.K.), and the Dartmouth Brain Imaging Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804546105/DCSupplemental.
This characterization is based on the correlation between memory ability and task-induced deactivations in MTL activity; better memorizers are those individuals who exhibit greater task-induced deactivation of the MTL.
References
- 1.Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 2.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurochem. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 4.Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. J Clin Exp Neuropsychol. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- 5.Mungas D, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopelman MD, et al. Structural MRI volumetric analysis in patients with organic amnesia, 2: correlations with anterograde memory and executive tests in 40 patients. J Neurol Neurosurg Psychiatry. 2001;71(1):23–28. doi: 10.1136/jnnp.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahn DA, et al. Structural MRI correlates of recognition memory in Alzheimer's disease. J Int Neuropsychol Soc. 1998;4(2):106–114. doi: 10.1017/s1355617798001064. [DOI] [PubMed] [Google Scholar]
- 8.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Maguire EA, Valentine ER, Wilding JM, Kapur N. Routes to remembering: the brains behind superior memory. Nat Neurosci. 2003;6(1):90–95. doi: 10.1038/nn988. [DOI] [PubMed] [Google Scholar]
- 11.Shulman GL, et al. Common blood flow changes across visual tasks: II Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 12.Mazoyer B, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 13.Binder JR, et al. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11(1):80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 14.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 15.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 17.Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 19.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 20.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29(1):145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 21.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 22.Peigneux P, et al. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 24.Small SA, et al. Imaging physiologic dysfunction of individual hippocampal subregions in humans and genetically modified mice. Neuron. 2000;28:653–664. doi: 10.1016/s0896-6273(00)00144-6. [DOI] [PubMed] [Google Scholar]
- 25.Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 26.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 28.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason MF, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol. 2006;32(1):45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- 31.Craik FIM, Watkins MJ. The role of rehearsal in short-term memory. J Verbal Learn Verbal Behav. 1973;12:599–607. [Google Scholar]
- 32.Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. Brain activity before an event predicts later recollection. Nat Neurosci. 2006;9:489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- 33.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 34.McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 35.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 36.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Review Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 37.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 38.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 39.Kelley WM, et al. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 40.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. Neuroreport. 1996;7:2095–2099. [PubMed] [Google Scholar]
- 42.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert DT, Wilson TD. Prospection: experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- 44.Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Transactions R Soc London. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 47.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Santi S, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.