Abstract
The only causative treatment for IgE-mediated allergies is allergen-specific immunotherapy. However, fewer than 5% of allergy patients receive immunotherapy because of its long duration and risk of allergic side effects. We aimed at enhancing s.c. immunotherapy by direct administration of allergen into s.c. lymph nodes. The objective was to evaluate safety and efficacy compared with conventional s.c. immunotherapy. In a monocentric open-label trial, 165 patients with grass pollen-induced rhinoconjunctivitis were randomized to receive either 54 s.c. injections with pollen extract over 3 years [cumulative allergen dose 4,031,540 standardized quality units (SQ-U)] or 3 intralymphatic injections over 2 months (cumulative allergen dose 3,000 SQ-U). Patients were evaluated after 4 months, 1 year, and 3 years by nasal provocation, skin prick testing, IgE measurements, and symptom scores. Three low-dose intralymphatic allergen administrations increased tolerance to nasal provocation with pollen already within 4 months (P < 0.001). Tolerance was long lasting and equivalent to that achievable after standard s.c. immunotherapy (P = 0.291 after 3 years). Intralymphatic immunotherapy ameliorated hay fever symptoms (P < 0.001), reduced skin prick test reactivity (P < 0.001), decreased specific serum IgE (P < 0.001), caused fewer adverse events than s.c. immunotherapy (P = 0.001), enhanced compliance (P < 0.001), and was less painful than venous puncture (P = 0.018). In conclusion, intralymphatic allergen administration enhanced safety and efficacy of immunotherapy and reduced treatment time from 3 years to 8 weeks.
Keywords: allergy, pollen, rhinoconjunctivitis
IgE-mediated allergies, such as allergic rhinoconjunctivitis and asthma, have become highly prevalent, affecting up to 35% of the population in westernized countries (1). Allergen-specific immunotherapy, usually the s.c. administration of an allergen extract, is an effective treatment for allergic rhinitis, conjunctivitis, and allergy to insect venoms (2–4), conferring long-term benefit (5). Subcutaneous immunotherapy also interrupts progression of allergic sensitization from single to multiple allergens (6) and from rhinitis to asthma (7).
Despite these benefits over symptomatic treatment, only a few allergy patients choose immunotherapy (8), mainly because it involves 30–70 doctor visits over 3–5 years. Such immunotherapy also has a risk of allergic side effects, including anaphylaxis (9), requiring supervision by emergency-trained personnel (2).
We have previously shown in mice that direct intralymphatic injection, compared with s.c. or intramuscular injection, enhances the efficiency of class I-binding peptide vaccines (10), naked DNA vaccines (11), protein allergens (in preparation), and adjuvants (12). Moreover, the feasibility of intralymphatic vaccination has been demonstrated in clinical studies (13, 14), but no clinical study has compared these 2 administration routes.
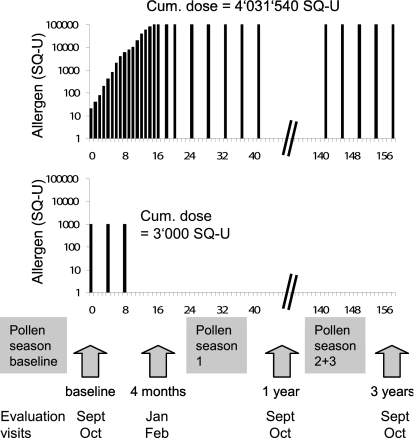
Our hypothesis was that immunotherapy is improved by direct administration of allergen into a lymph node, and the objectives were to evaluate the clinical feasibility and safety of allergen administration directly into lymph nodes, as well as to compare its efficacy with conventional immunotherapy. Patients with hay fever to grass pollen were injected 3 times directly into inguinal lymph nodes, whereas the control group was treated with conventional immunotherapy during 3 years (Fig. 1).
Fig. 1.
Trial design. Patients treated by s.c. immunotherapy received a cumulative allergen dose of 4,031,540 SQ-U in 54 injections over 3 years. Patients treated by intralymphatic immunotherapy received a cumulative dose of 3,000 SQ-U within 8 weeks. All patients were evaluated at baseline and after 4 months, 1 year, and 3 years.
Results
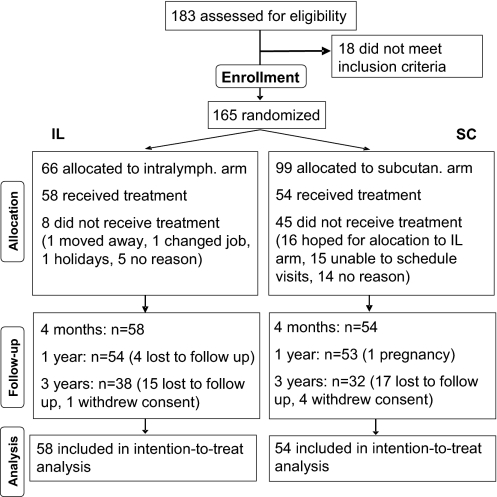
A total of 183 patients with hay fever due to grass pollen allergy were recruited June to August 2001.* Eighteen patients did not fulfill inclusion criteria (Fig. 2). Expecting a higher dropout rate in the s.c. arm, patients were asymmetrically randomized s.c./intralymphatic = 3:2. Hence, 66 patients were randomized to the intralymphatic group, and 58 started the treatment in January 2002. A total of 99 patients were randomized to the conventional s.c. immunotherapy group, and 54 started the treatment. In the intralymphatic group, 19 patients were lost to follow-up, and 1 patient withdrew consent. In the s.c. group, 17 patients were lost to follow-up, 4 patients withdrew consent, and 1 patient became pregnant.
Fig. 2.
Flow of patients. A total of 183 patients suffering from hay fever during the summer months May to July were assessed for eligibility. Eighteen did not meet inclusion criteria. A total of 165 patients with allergy to grass pollen were randomized 3:2 into s.c. and intralymphatic immunotherapies, respectively. Of the 99 patients randomized into the s.c. arm, 54 showed up for the first injection at the baseline visit. Of the 66 patients assigned to the intralymphatic arm, 58 showed up for the first injection at the baseline visit.
Baseline characteristics of the 2 groups were similar with regard to age, sex, body mass, and nasal sensitivity to grass pollen (Table 1). Although more patients with asthma had been randomized into the intralymphatic arm, nasal sensitivity to pollen was balanced, as evidenced by nasal provocation testing (P = 0.373) and total subjective symptom score (P = 0.124). All cases of seasonal asthma were mild, requiring only occasional therapy. A total of 19 of the 58 intralymphatically treated patients showed additional springtime hay fever with sensitization to trees, as well as 19 of the 54 s.c.-treated patients.
Table 1.
Baseline characteristics by treatment group
| Characteristics | Treatment group |
|
|---|---|---|
| Intralymphatic (n = 58) | Subcutaneous (n = 54) | |
| Age, mean (±SD), y | 32 (±8.7) | 36 (±12) |
| Female (%) | 20 (34.5) | 19 (35.2) |
| Weight, mean (±SD), kg | 70.8 (±11.7) | 70.8 (±11.5) |
| Seasonal asthma (%) | 32 (55.2) | 16 (29.6) |
| Spring time hay fever (%) | 19 (32.7) | 19 (35.2) |
| Sensitization to cat dander* (%) | 10 (17.2) | 7 (13.0) |
| Sensitization to dog dander* (%) | 3 (5.2) | 2 (3.7) |
| Sensitization to dust mite* (%) | 7 (12.1) | 6 (11.1) |
| Sensitization to mold* (%) | 0 (0) | 0 (0) |
| NPT threshold conc. (%) | ||
| 1,000 SQ-U | 0 (0) | 2 (3.7) |
| 10,000 SQ-U | 14 (24.1) | 8 (14.8) |
| 100,000 SQ-U | 43 (74.1) | 41 (75.9) |
| >100,000 SQ-U | 1 (1.7) | 3 (5.6) |
*Positive skin prick tests without clinical symptoms.
Intralymphatic Administration Was Practically Painless.
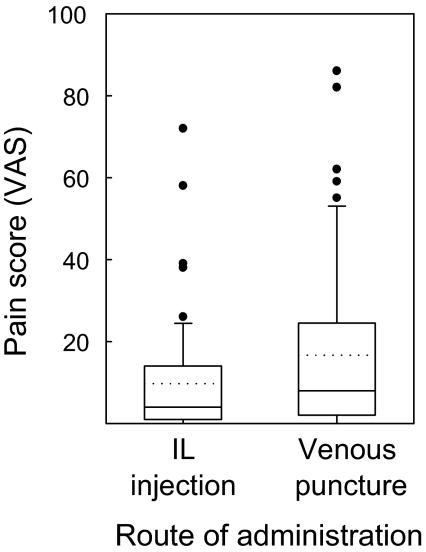
Patients receiving intralymphatic injections were asked to compare the pain of an injection into a lymph node to the venous puncture during the same visit (Fig. 3). On visual analogue scales ranging from 0 to 100 mm, the pain of intralymphatic injections averaged 9.7 ± 14.4, significantly less (P = 0.018; paired-samples t test) than the pain of the venous puncture (16.7 ± 21.2).
Fig. 3.
Pain of intralymphatic (IL) immunotherapy. Patients were asked to compare the pain of injection into a lymph node to a venous puncture during the same visit on a visual analogue scale ranging from 0 to 100 mm (n = 53). Box plots show mean (dotted line), median (continuous line), 25th and 75th percentiles (box), 5th and 95th percentiles (whiskers), and outliers. The paired-samples t test showed a significant difference between intralymphatic injection and venous puncture (P = 0.018).
Intralymphatic Was Safer than s.c. Immunotherapy.
Intralymphatic allergen injections caused fewer allergic adverse events than s.c. injections (P = 0.001, Mann–Whitney test; Table 2). During the first 4 months of treatment, 18 mild [urticaria, flush, and angioedema, grades 1 and 2 according to Mueller (15)], as well as 2 severe (asthma requiring hospitalization, grade 3; ref. 15) allergic reactions were observed in the s.c. group. In the latter 2 patients, uptitration had to be continued form the last tolerated dose. In contrast, only 6 mild allergic reactions (urticaria and angioedema) were seen after intralymphatic allergen administration. There was no premedication with antihistamines before injections.
Table 2.
Safety of intralymphatic and subcutaneous immunotherapy: Patients with adverse events
| Adverse events | Subcutaneous | Intralymphatic |
|---|---|---|
| None | 34 | 52 |
| Mild (grade 1* and 2*) | 18 | 6 |
| Severe (grade 3*) | 2 | 0 |
| Anaphylactic (grade 4*) | 0 | 0 |
| Total | 54 | 58 |
*Mueller grading of allergic reactions.
Intralymphatic Immunotherapy Induced Allergen Tolerance Faster Than s.c. Immunotherapy.
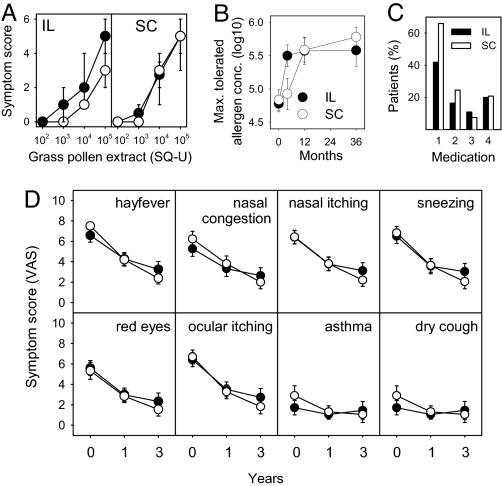
At baseline, both groups showed similar sensitivities to grass pollen in nasal provocation testing (P = 0.373). After 4 months, patients in the intralymphatic group showed an approximately 10-fold increase of the pollen concentration required to provoke nasal symptoms (P < 0.001, Wilcoxon signed-rank test) (Fig. 4A). In contrast, patients in the s.c. group did not show significant improvement at this early time point (P = 0.425) (Fig. 4 A and B). A significant increase in allergen tolerance was observed only 1 year after continuous therapy (P < 0.001) (Fig. 4B).
Fig. 4.
Efficacy of intralymphatic (IL) vs. s.c. (SC) immunotherapy. (A) Intralymphatic therapy rapidly induced tolerance to grass pollen: Symptom scores during nasal provocation testing with grass pollen extract at baseline (●) and 4 months after initiation of treatment (○). Symbols show median and error bars for the 25th and 75th percentiles. The threshold shift in the intralymphatic group was significant (P < 0.001), whereas the shift in the s.c. group was not (P = 0.425). (B) Intralymphatic therapy induced long-term allergen tolerance: maximal tolerated pollen concentrations (pollen concentrations inducing scores ≥4) were evaluated in nasal provocation tests with grass pollen at baseline and after 4 months, 1 year, and 3 years. Symbols show means, and error bars 95% C.I.. A statistically significant increase of the maximal tolerated pollen concentration (P < 0.001) was already observed after 4 months and was long lasting. In contrast, s.c. immunotherapy did not significantly increase allergen tolerance within 4 months, but required 1 year of treatment. After 1-year (P = 0.856) and 3-year (P = 0.291) time points there was no significant difference between the 2 test groups (Mann–Whitney U test). (C) Patients in the intralymphatic group used less rescue medication. Rescue medication during the first year: antihistamine tablets (medication 1; desloratidine), nasal corticosteroid sprays (medication 2; mometasone furoate), asthma inhaler (medication 3; budesonide with formoterol), and antihistamine eye drops (medication 4; emadistinum). The percentage of patients using antihistamine tablets was significantly lower in intralymphatic than in s.c. patients (P = 0.020, χ2 test with continuity correction). No significant differences were seen for other medications (nasal sprays P = 0.416; asthma inhaler P = 0.787; and antihistamine eye drops P = 1.000). (D) Intralymphatic and s.c. therapies comparably ameliorated hay fever symptoms. Patients were asked to score the symptoms of hay fever, congested nose, itchy nose, sneezing, red eyes, itchy eyes, asthma, and cough, at baseline, after 1 year, and after 3 years on a visual analogue scale ranging from 0 to 10. Symbols show means, and error bars 95% C.I.. Both intralymphatic (●) and s.c. (○) therapies significantly ameliorated subjective symptoms (within-subjects effect P < 0.001). Amelioration in both patient groups was not significantly different (hay fever P = 0.597; congested nose P = 0.503; itchy nose P = 0.926; sneezing P = 0.739; red eyes P = 0.328; itchy eyes P = 0.678; asthma P = 0.727; and coughing P = 0.485).
Allergen Tolerance Induced by Intralymphatic Immunotherapy Was Long Lasting.
Amelioration of allergy symptoms after 3 intralymphatic injections within 8 weeks was long lasting and comparable to that achieved after 3 years of conventional immunotherapy (Fig. 4B). After 1 year (P = 0.856, Mann–Whitney test) and 3 years (P = 0.291), the intralymphatic groups did not differ significantly from the subcutaneous group.
Patients in the Intralymphatic Group Used Less Rescue Medication.
All patients were provided with the same rescue medication in case of symptoms during the pollen season: antihistamine tablets (desloratidine; Aerius), nasal sprays (mometasone furoate; Nasonex), asthma inhalers (budesonide combined with formoterol; Symbicort), and antihistamine eye drops (emadistinum; Emadine). Whether patients used rescue medications was recorded during the first and third pollen seasons. The percentage of patients using antihistamine tablets was significantly lower in the intralymphatic group (23 of 54) than in the s.c. group (35 of 53) during the first pollen season (P = 0.020; χ2 test) (Fig. 4C), which is consistent with the faster induction of allergen tolerance observed in the nasal provocation test. Other rescue medications were rarely used, and no significant differences were observed between the 2 patient groups. During the pollen season of the third year, no significant differences were observed between intralymphatic and s.c. patients (data not shown).
Intralymphatic and s.c. Immunotherapies Induced Comparable Subjective Symptom Amelioration.
Patients recorded summertime hay fever symptoms from May to July on visual analog scales at baseline (pollen baseline season), after 1 year (pollen season 1), and after 3 years (pollen season 3). The grass pollen seasons in these 3 years were not significantly different (data not shown). At baseline, both groups recorded similar symptom severities with no significant difference (P = 0.124). Both intralymphatic and s.c. immunotherapies significantly ameliorated subjective symptoms (within-subjects effect for all symptoms P < 0.001, general linear model repeated-measures analysis; Fig. 4D), the 2 groups being not significantly different (between-subjects effect hay fever P = 0.597; congested nose P = 0.503; itchy nose P = 0.926; sneezing P = 0.739; red eyes P = 0.328; itchy eyes P = 0.678; asthma P = 0.727; and coughing P = 0.485). There was no recording of symptoms at the 4-month time point, as there was no pollen season between trial entry and this early time point.
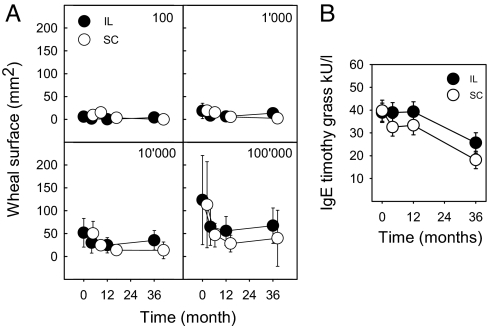
Intralymphatic and s.c. Immunotherapies Reduced Skin Prick Test Reactivity and Specific Serum IgE Comparably.
Titrated skin prick tests were performed at baseline and after 4 months, 1 year, and 3 years (Fig. 5A). The wheal surface analysis showed that both intralymphatic and s.c. therapies reduced reactivity (P < 0.001 for all allergen doses; general linear model repeated-measures analysis). Consistent with reduced skin prick reactivity, timothy grass-specific serum IgE decreased significantly in both intralymphatic and s.c. patients (within-subjects effect P < 0.001; general linear model repeated-measures analysis) (Fig. 5B). The decrease in serum IgE was not significantly different in the intralymphatic and s.c. patient groups (between-subjects effect P = 0.204).
Fig. 5.
Skin prick test reactivity and serum IgE. (A) Reduction of reactivity to allergen in skin prick tests. Wheal areas in titrated skin prick tests at baseline and after 4 months, 1 year, and 3 years. Both the intralymphatic (●) and s.c. (○) patients showed significantly reduced reactivity (P < 0.001 for all allergen doses; general linear model repeated-measures analysis). Symbols show means, and error bars show SD. (B) Decrease of allergen-specific serum IgE. Timothy grass-specific serum IgE (kU/l) decreased significantly both in patients receiving intralymphatic (●) and s.c. (○) immunotherapies (within-subjects effect P < 0.001; general linear model repeated-measures analysis). Symbols show means, and error bars show SE. The difference between intralymphatic and s.c. immunotherapies was not statistically significant (between-subjects effect P = 0.204).
Discussion
We demonstrated that intralymphatic allergen administration enhanced safety, efficacy, and compliance of s.c. immunotherapy and allowed reduction of the number of injections from 54 to 3, and reduction of the cumulative allergen dose by more than 1000-fold. This is in line with our preclinical observations in mice (10, 16) in terms of efficacy, and also required lower vaccine doses. Enhanced efficacy of targeted lymph node immunization has also been found for immunostimulatory complexes (17), bacteriophages (18), and a recombinant simian immunodeficiency virus vaccine (19). The same appears to be true for the allergen extracts used here. Thus, intralymphatic injection appears to generally enhance the efficacy of vaccination and immunotherapy. The explanation is that only a small fraction of s.c.-injected biomolecules reaches the draining lymph nodes to stimulate the immune response, whereas direct injection into a lymph node delivers all of the vaccine to the lymphatic organ.
Intralymphatic injections are technically not difficult and not painful. Subcutaneous lymph nodes can be found readily by ultrasound, as their paracortical area is hypoechoic compared with the s.c. fat. Superficial inguinal lymph nodes are ≈1.5 cm long and only a few millimeters under the skin surface, even in obese patients. The pain of intralymphatic injection arose solely from penetrating the skin, as the sensory innervation of lymph nodes is sparse (20). The pain of an intralymphatic injection is comparable with s.c. injections, because the same small size (28 gauge) needles are used. Patients in the present study rated intralymphatic injection as less painful than venous puncture.
The short intralymphatic treatment enhanced patient compliance. Only 32 of 54 patients finished the 3 years of s.c. immunotherapy (Fig. 2). As all of the 58 intralymphatic patients received the full treatment consisting of 3 injections, the percentage of patients finishing the treatment was significantly higher (Pearson χ2 test P < 0.001). Another interesting observation was that 45 of the 99 patients allocated to s.c. immunotherapy did not show up for the first treatment visit. Of these, 16 patients reported that they had participated at randomization only because they hoped to be allocated to the intralymphatic arm, and another 15 said that they could not possibly fit the 54 treatment visits into their schedules. As we had randomized more patients into the s.c. arm, originally expecting a higher dropout rate, this did not affect our results. In contrast, only 8 of the 66 patients allocated to intralymphatic arm did not return for the first injection. Evidently, intralymphatic immunotherapy is a more attractive treatment alternative.
The 2 treatment groups showed similar baseline characteristics with regard to age, sex, and body mass. Although more patients with asthma had been randomized into the intralymphatic arm, nasal sensitivity to grass pollen was similar in the 2 treatment groups, as evidenced by nasal provocation testing (P = 0.373) and total subjective symptom score (P = 0.124). If anything, one would expect the presence of asthma to correlate with more severe allergic rhinoconjuncitivits, and to therefore negatively bias the outcome in the intralymphatic group.
Intralymphatic allergen injections were well tolerated and caused fewer allergic adverse events than s.c. injections. As allergen immunotherapy often causes severe allergic adverse events (9, 21), 1 hour of medical supervision is recommended after each s.c. allergen injection, and rapid initial uptitration regimens with insect venom or cat dander require hospitalization. The enhanced safety and efficacy observed with intralymphatic therapy could solve these problems and make immunotherapy more convenient, shorter, and less costly.
Intralymphatic immunotherapy reduced nasal reactivity to grass pollen already after 4 months, and patients in the intralymphatic group used significantly less rescue medication during the first pollen season than the s.c. group. Despite the short treatment period, amelioration in the intralymphatic patients was long lasting and not significantly different from the amelioration achieved after 3 years of s.c. immunotherapy.
In conclusion, intralymphatic immunotherapy allowed a marked reduction of both number and dose of allergen injections necessary to induce allergen tolerance, making the treatment shorter and safer. The practically painless procedure also enhanced patient compliance, thus making intralymphatic immunotherapy an interesting alternative to conventional s.c. treatment.
Methods
Statistical Considerations.
Sample size calculations were based on the study objective efficacy. As patients receiving s.c. immunotherapy were injected with a ≈1000-fold lower cumulative allergen dose, the objective safety comparison was anticipated to require lower patient numbers than efficacy comparison. Nasal provocation testing was planned to detect significant differences (β = 20%, 2-sided α = 0.05) if change from baseline scores differed by 0.7 or greater (variance = 0.9) across treatment groups. A total sample size of 165 allowed for loss to follow-up. Expecting a higher dropout rate during 3 years of s.c. immunotherapy, randomization of s.c. vs. intralymphatic immunotherapy was performed at a 3:2 proportion by having the patients draw lots (99 s.c. and 66 intralymphatic identical-looking folded lots were mixed together in a blinding bag). Drawing a lot and starting the corresponding treatment constituted admission to the trial, and intention-to-treat analysis was conducted on the basis of that allocation. Parametric data were evaluated using a Student t test or the general linear model. Independent nonparametric data were compared using the Mann–Whitney U test or χ2 test. The Wilcoxon signed-rank test or Friedman test was applied for paired data.
Eligibility.
Study participants were recruited by newspaper ads searching for patients with seasonal rhinoconjunctivitis but without perennial allergies. At screening, skin prick tests with all frequent aeroallergens were performed. Inclusion criteria were history of allergic rhinoconjunctivitis in summer, age 18 to 65 years, positive skin prick test to grass pollen, and written informed consent. Springtime hay fever and skin prick test positivity to tree pollen (birch, hazel, and alder) were not exclusion criteria. Skin prick test reactivity to cat dander or house dust mite with no clinically significant symptoms were not exclusion criteria. Exclusion criteria were blood donation or surgery within the previous 30 days; use of investigational drugs within the previous 90 days; pregnancy or nursing; mastocytosis; significant cardiovascular, hepatic, renal, autoimmune, hematological, or active infectious disease; and history of malignancy, hypertension, immunosuppressive agents, β blockers, ACE inhibitors, and tricyclic antidepressants. Pulmonary disease, including perennial asthma and perennial use of inhalative corticosteroids, was an exclusion criterion, whereas seasonal allergic asthma was not.
Treatment and Follow-up Procedures.
One group of patients received three 0.1-ml injections with 1,000 standardized quality units (SQ-U) of aluminum hydroxide-adsorbed grass pollen extract at day 0 and after 4 and 8 weeks (Alutard SQ; ALK-Abelló). A superficial inguinal lymph node was aseptically and slowly injected under ultrasound guidance. Aspirations were made before the injection to avoid inadvertent intravascular administration.
The other group of patients received a total of 54 s.c. injections over 3 years (cumulative dose of 4,031,540 SQ-U). The doses of aluminum-adsorbed grass pollen extracts (Alutard SQ) were increased weekly for 16 weeks (Fig. 1). After the maintenance dose (100,000 SQ-U) was reached, injections were given monthly.
Nasal provocation tests were performed according to standard procedures (22). After adaptation to room temperature for 10 min, an anterior rhinoscopy was performed to determine baseline values. Then, 50 μl of isotonic test solutions was administered to the middle nose conch using a pipette. Patients then were challenged with allergen diluent—100, 1,000, 10,000, or 100,000 SQ-U aqueous grass pollen extract (Aquagen SQ) in 10-min intervals. A symptom score ranging from 0–6 points was recorded (22): nasal secretion (none = 0 points, mild = 1 point, severe = 2 points), sneezing (0–2 times = 0 points, 3–5 times = 1 point, >5 times = 2 points), remote symptoms (none = 0 points, lacrimation or pruritus of the ear or palate = 1 point, conjunctivitis, chemosis, urticaria, coughing, or shortness of breath = 2 points).
The pollen concentration inducing a score ≥4 was defined as the maximal tolerated pollen concentration. In these patients, the pollen dose was not further escalated for safety reasons, and patients were assumed to have the maximal score of 6 at the next higher concentration. If patients did not reach a score ≥4 with the highest test concentration of 100,000 SQ-U, the maximum tolerated dose was 1,000,000 SQ-U. Skin prick tests were performed according to the European Academy of Allergology and Clinical Immunology recommendations (23). A total of 30 μl serially diluted of grass pollen allergens (Soluprick Alutard SQ; ALK-Abelló) was applied on the volar forearm. Tests were evaluated after 15 min. Wheals were outlined on the skin with a pen, blotted onto a cellophane tape, and the areas of wheals and flares were assessed by planimetry (24). On a 100-mm scale, patients were asked to record the severity of hay fever, asthma, nasal congestion, nasal itching, sneezing, red eyes, ocular itching, and dry cough. Serum IgE was measured by UniCAP (Amersham Pharmacia) for grass–pollen mix and timothy grass (g6).
The study was performed from June 2001 to March 2005 at the University Hospital Zurich, Switzerland, according to International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. It was approved by the local ethics committee and Swissmedic was notified. Written informed consent was obtained from all patients before enrolment.
Acknowledgments.
We thank S. Marthy, I. Cuhat, M. C. Weber, E. Schulthess, and P. Schmid for assistance, and M. Gerber for statistics. Work at the Swiss Institute for Asthma and Allergy Research was supported by Swiss National Science Foundation Grant 310000-114634.
Footnotes
Conflict of interest statement: The corresponding author, T.M.K., is named as the inventor on a patent on intralymphatic immunotherapy. The patent is owned by the University of Zurich.
This article is a PNAS Direct Submission. A.F. is a guest editor invited by the Editorial Board.
Trial Registration: www.clinicaltrials.gov (NCT00470457).
References
- 1.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 3.Lockey RF. “ARIA”: Global guidelines and new forms of allergen immunotherapy. J Allergy Clin Immunol. 2001;108:497–499. doi: 10.1067/mai.2001.118638. [DOI] [PubMed] [Google Scholar]
- 4.Varney VA, et al. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302:265–269. doi: 10.1136/bmj.302.6771.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durham SR, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 6.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–1397. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 7.Moller C, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Epidemiology Surveys and Danske Equities. Hørsholm, Denmark: ALK-Abelló; 2005. [Google Scholar]
- 9.Stewart GE, 2nd, Lockey RF. Systemic reactions from allergen immunotherapy. J Allergy Clin Immunol. 1992;90:567–578. doi: 10.1016/0091-6749(92)90129-p. [DOI] [PubMed] [Google Scholar]
- 10.Johansen P, et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol. 2005;35:568–574. doi: 10.1002/eji.200425599. [DOI] [PubMed] [Google Scholar]
- 11.Maloy KJ, et al. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci USA. 2001;98:3299–3303. doi: 10.1073/pnas.051630798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Beust BR, et al. Improving the therapeutic index of CpG oligodeoxynucleotides by intralymphatic administration. Eur J Immunol. 2005;35:1869–1876. doi: 10.1002/eji.200526124. [DOI] [PubMed] [Google Scholar]
- 13.Finerty S, et al. Targeted lymph node immunization can protect cats from a mucosal challenge with feline immunodeficiency virus. Vaccine. 2001;20:49–58. doi: 10.1016/s0264-410x(01)00323-1. [DOI] [PubMed] [Google Scholar]
- 14.Lehner T, et al. Protective mucosal immunity elicited by targeted lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Dev Biol Stand. 1998;92:225–235. [PubMed] [Google Scholar]
- 15.Mueller UR. Insect Sting Allergy. Stuttgart: Gustav Fischer; 1990. [Google Scholar]
- 16.Kundig TM, et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 17.Koopman G, et al. Comparison of intranasal with targeted lymph node immunization using PR8-Flu ISCOM adjuvanted HIV antigens in macaques. J Med Virol. 2007;79:474–482. doi: 10.1002/jmv.20860. [DOI] [PubMed] [Google Scholar]
- 18.Trepel M, Arap W, Pasqualini R. Modulation of the immune response by systemic targeting of antigens to lymph nodes. Cancer Res. 2001;61:8110–8112. [PubMed] [Google Scholar]
- 19.Lehner T, et al. The effect of route of immunization on mucosal immunity and protection. J Infect Dis. 1999;179(Suppl 3):S489–S492. doi: 10.1086/314809. [DOI] [PubMed] [Google Scholar]
- 20.Popper P, Mantyh CR, Vigna SR, Maggio JE, Mantyh PW. The localization of sensory nerve fibers and receptor binding sites for sensory neuropeptides in canine mesenteric lymph nodes. Peptides. 1988;9:257–267. doi: 10.1016/0196-9781(88)90258-6. [DOI] [PubMed] [Google Scholar]
- 21.Lockey RF, et al. The Hymenoptera venom study. III. Safety of venom immunotherapy. J Allergy Clin Immunol. 1990;86:775–780. doi: 10.1016/s0091-6749(05)80182-4. [DOI] [PubMed] [Google Scholar]
- 22.Riechelmann H, et al. Application of the nasal provocation test on diseases of the upper airways. Position paper of the German Society for Allergology and Clinical Immunology (ENT Section) in cooperation with the Working Team for Clinical Immunology. Laryngorhinootologie. 2003;82:183–188. doi: 10.1055/s-2003-38411. in German. [DOI] [PubMed] [Google Scholar]
- 23.Position paper: Allergen standardization and skin tests. The European Academy of Allergology and Clinical Immunology. Allergy. 1993;48:48–82. [PubMed] [Google Scholar]
- 24.Sussman GL, Harvey RP, Schocket AL. Evaluation of skin test response using two techniques of measurement. Ann Allergy. 1982;48:75–77. [PubMed] [Google Scholar]