Abstract
17β-Estradiol (E2) binds to the estrogen receptor (ER) to activate gene expression or repression and this involves both genomic (nuclear) and non-genomic (extranuclear pathways). Genomic pathways include the classical interactions of ligand-bound ER dimers with estrogen-responsive elements (EREs) in target gene promoters. ER-dependent activation of gene expression also involves DNA-bound ER which subsequently interacts with other DNA-bound transcriptions factors and direct ER-transcription factor (protein-protein) interactions where ER does not bind promoter DNA. Ligand-induced activation of ER/specificity protein (Sp) and ER/activating protein-1 [(AP-1) consisting of jun/fos] complexes are important pathways for modulating expression of a large number of genes. This review summarizes some of the characteristics of ER/Sp- and ER/AP-1-mediated transactivation which are dependent on ligand structure, cell context, ER-subtype (ERα and ERβ), and Sp protein (Sp1, Sp3 and Sp4) and demonstrates that this non-classical genomic pathway is also functional in vivo.
Keywords: ER, estrogen, ER/Sp, ER/AP-1, genomic signaling
LIGAND DEPENDENT ACTIVATION OF ER/SP
1. Introduction
Specificity protein 1 (Sp1) was the first transcription factor identified in the early 1980s (Dynan & Tjian 1983; Dynan & Tjian 1985; Briggs et al. 1986) and is a member of the Sp/Krüppel-like family (KLF) of at least 25 transcription factors (Suske et al. 2005). Sp/KLF proteins have highly variable modular structures in their N-terminal domains and are characterized by three C2H2-type zinc fingers in their C-terminal domain which are required for their sequence-specific DNA binding (Philipsen & Suske 1999; Bouwman & Philipsen 2002; Black et al. 2001). Sp/KLF family members recognize GC/GT boxes in promoter regions of mammalian and viral genes, and the consensus Sp1 binding site 5′-(G/T)GGGCGG(G/A)(G/A)(C/T)-3′ interacts with most Sp/KLF proteins. Sp2 is the major exception among Sp proteins and this protein binds weakly to consensus GC-rich sites but binds with higher affinity to a 5′-GGGCGGGAC-3′ motif (Moorefield et al. 2004). Interactions of Sp/KLF transcription factors with GC-rich promoters has primarily been investigated with Sp1 and Sp3 proteins which are ubiquitously expressed in normal tissues and highly overexpressed in tumors and cancer cells (Suske et al. 2005; Philipsen & Suske 1999; Bouwman & Philipsen 2002; Black et al. 2001). Both proteins bind the consensus ”Sp1” binding sequence and also a large number of nonconsensus sequences with variable affinities in in vitro binding studies. However, the importance of specific GC-rich sequences in a gene promoter in mediating transactivation is highly variable and dependent on cell context and the relative expression of nuclear coactivators and other cofactors.
Sp proteins play a critical role in several key biological processes including cellular differentiation, proliferation, survival and angiogenesis (Philipsen & Suske 1999; Bouwman & Philipsen 2002; Black et al. 2001) and Sp1, Sp3 and Sp4 knockout animals are either embryolethal or their offspring exhibit severe abnormalities (Marin et al. 1997; Gollner et al. 2001a; Supp et al. 1996; Gollner et al. 2001b). In contrast, expression of Sp1 and possibly other Sp proteins is relatively low in mature animals and decreases with age (Ammendola et al. 1992; Oh et al. 2007). Several reports show that Sp1 and other Sp proteins are overexpressed in tumors and cancer cells (Safe & Abdelrahim 2005; Shi et al. 2001; Zannetti et al. 2000; Chiefari et al. 2002; Hosoi et al. 2004; Kanai et al. 2006; Lou et al. 2005; Wang et al. 2003; Yao et al. 2004) and Sp1 overexpression in gastric tumors is a negative prognostic factor for disease-free survival. For example, Lou and coworkers (Lou et al. 2005) reported that transformation of fibroblasts resulted in an 8- to 18-fold increase in Sp1 protein expression and in xenograft experiments, Sp1 expression was required for tumor growth. Not surprisingly, recent studies showed that Sp1, Sp3 and Sp4 were highly overexpressed in various cancer cell lines including both ER-positive and ER-negative breast cancer cells (Safe & Abdelrahim 2005; Mertens-Talcott et al. 2007), and several reports demonstrate that Sp1 and other Sp proteins play an important role in regulation of E2-dependent genes in breast cancer cell lines and in many other cell-types (Safe & Kim 2004; Safe 2001).
2. Role of Sp Proteins in Hormonal Activation of Genes
(a) ER/Sp Modulation of Genes: DNA-Dependent Interaction of ER and Sp Proteins
Many E2-responsive gene promoters contain both GC-rich, ERE and ERE half sites (ERE½) that cooperatively interact to activate expression of genes. The uteroglobin promoter contains a nonconsensus ERE (-263 to -251) that binds ERα; however, hormonal activation of this gene and related promoter constructs involves interaction with both proximal (-232 to -223; -200 to -199) and more distal (-67 to -60) GC-rich Sp binding sites (Scholz et al. 1998). Although DNA-protein binding studies have not identified an ERα/Sp complex, both Sp1 and ERα bind individually to the uteroglobin gene promoter oligonucleotide fragments containing the GC-rich and ERE sites, respectively. Analysis of the Xenopus vitellogenin A1 promoter has also identified ERE and GC-rich sites separated by 1.8 kb that are required for hormonal activation of this gene (Batistuzzo de Medeiros et al. 1997). Subsequent studies have identified ERE and GC-rich sites in the estrogen-related receptor and the mouse Slo promoter required for hormonal activation (Kundu et al. 2007; Liu et al. 2003).
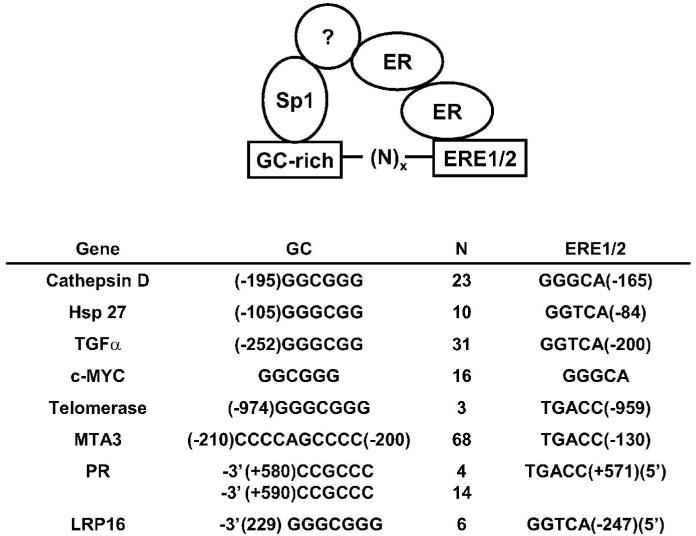
Research in this laboratory initially focused on extensive analysis of the cathepsin D gene promoter which had previously been characterized as an E2-responsive gene (Cavailles et al. 1993; Augereau et al. 1994; Krishnan et al. 1994; Krishnan et al. 1995). We identified an E2-responsive sequence in the -199 to -165 region of the promoter that did not contain ERE motifs. Subsequent analysis identified both a nonconcensus ERE½ and GC-rich elements separated by 23 oligonucleotides (Fig. 1) that was E2-responsive in transfection assays. Mutation of either the GC-rich or ERE½ resulted in loss of hormone activation of this construct. These results coupled with the detection of an ERα/Sp1-DNA complex in gel mobility shift assays clearly demonstrated a novel genomic mechanism of ER-dependent transactivation that required cooperative ERα/Sp1 interactions on the cathepsin D promoter. Subsequent studies in several laboratories confirmed that this DNA-dependent ER/Sp-mediated pathway was important for activation of multiple genes, including transforming growth factor α (TGFα), c-myc, heat shock protein 27 (Hsp27), telomerase, progesterone receptor A, metastasis-associated protein 3 (MTA3) and LPR16 (Vyhlidal et al. 2000; Porter et al. 1996; Petz & Nardulli 2000; Dubik & Shiu 1992; Boggess et al. 2006; Fujita et al. 2004; Zhao et al. 2005). Figure 1 illustrates the variety of the GC-rich and ERE½ motifs, their location in promoters and the number of nucleotides separating the two elements. These motifs and their location are highly variable, making it difficult to predict E2-responsiveness from these sequences. Moreover, cell context will also influence whether these motifs will form hormone-responsive protein-DNA complexes.
Figure 1.
A summary of E2-responsive genes containing functional GC-rich & ERE1/2 motifs. There is evidence that formation of this complex may contain other proteins.
(b) ER/Sp Modulation of Genes Through Interactions of Sp Proteins With GC-Rich Motifs
(i) Identification of ER α/Sp-mediated genes
During analysis of the Hsp27 gene promoter, it was observed that after mutation of the proximal ERE½ site, transfection of the resulting GC-rich construct in MCF-7 cells followed by treatment with E2 resulted in the induction of reporter gene activity (Porter et al. 1997). Subsequent studies with the Hsp27 promoter or a promoter containing one or more consensus GC-rich motifs demonstrated that E2 activated ERα-dependent gene expression through interactions with GC-rich promoter elements (Porter et al. 1997). E2-dependent activation of ERα/Sp through interactions with GC-rich elements in E2-responsive gene promoters is a novel genomic pathway for both inducing and repressing gene expression and exhibits several unique characteristics which include the following.
ERα directly interacts with the C-terminal DNA binding domain of Sp1 which is a region within Sp1 that interacts with a host of other transcription factors including other steroid hormone receptors (Owen et al. 1998; Simmen et al. 1999; Lu et al. 2000; Curtin et al. 2001; Husmann et al. 2000). Interaction of ERα with other Sp proteins have not been well characterized; however, ERα interacts with multiple domains of Sp3 (Stoner et al. 2000). ERβ interacts with the C-terminal domain of Sp1; however, unlike ERα, ERβ interacts with regions outside this domain (Saville et al. 2000). Interactions between ER and Sp1 were ligand-independent.
In gel mobility shift assays, a direct formation of ternary ERα/Sp-DNA complexes was not detected. However, ERα (± E2) enhanced formation of the Sp-DNA complex and kinetic studies showed that ERα also increased the stability of the Sp1-DNA complex.
Results of chromatin immunoprecipitation (ChIP) assays showed that ERα is constitutively associated with E2-responsive GC-rich promoter elements which also bind Sp1, Sp3 and Sp4. Treatment of breast cancer cells with E2 does not appreciably affect ERα and Sp interactions with E2-responsive GC-rich promoter sequences; however, changes in coregulatory proteins may be altered.
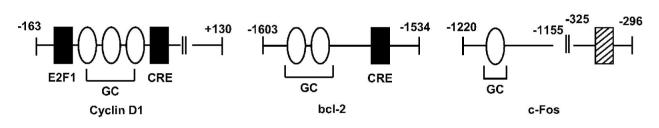
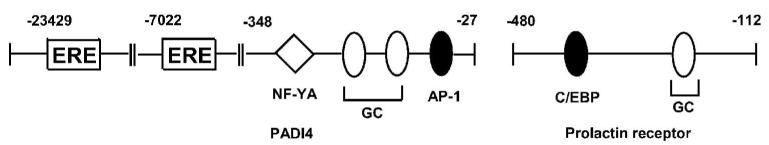
After initial characterization of GC-rich promoter sites as targets for E2-dependent transactivation (Porter et al. 1997), studies in this laboratory focused on identifying other genes in breast cancer cells that are activated by ERα/Sp. Genes activated by this genomic pathway include Hsp27, cathepsin D, c-fos, retinoic acid receptor α1 (RARα1), adenosine deaminase, insulin-like growth factor-binding protein 4, bcl-2, E2F1, thymidylate synthase, vascular endothelial growth factor (VEGF), cyclin D1, creatine kinase B, DNA polymerase α, carbamoylphosphate synthetase/ aspartate carbamyltransferase/dihydroorotase (Cad), ovine oxytocin receptor, and VEGFR2 (Duan et al. 1998; Xie et al. 1999; Sun et al. 1998; Qin et al. 1999; Dong et al. 1999; Xie et al. 2000; Samudio et al. 2001; Castro-Rivera et al. 2001; Wang et al. 2002; Fleming et al. 2006; Stoner et al. 2004; Khan et al. 2003; Wang et al. 1999; Ngwenya & Safe 2003; Higgins et al. 2006; Higgins et al. 2008). Subsequent studies in many other laboratories have extended the list of E2-responsive genes regulated by ERα/Sp interactions with GC-rich sites and these include folate receptor α, estrogen-related receptor α, prothymosin α, progesterone receptor. prolactin receptor, peptidylarginine deiminase type IV (PADI4), epidermal growth factor receptor (EGFR), KiSS1, insulin-like growth factor 1 (IGF-1) receptor, HOXA10, trefoil factor 1, vitamin D receptor, low density lipoprotein receptor, fibulin-1, rat Sk3, and receptor for advanced glycation end products (RAGE) (Salvatori et al. 2000; Tanaka et al. 2000; Briggs et al. 1986; Jacobson et al. 2003; Schultz et al. 2003; Byrne et al. 2000; Martini & Katzenellenbogen 2001; Kelley et al. 2003; Sun et al. 2005; Bardin et al. 2005; Dong et al. 2006; Maor et al. 2006; Dong et al. 2007; Li et al. 2007; Martin et al. 2007). These genes are E2-responsive in several different cell lines and promoter analysis showed that E2 not only induces gene expression through GC-rich motifs but also other sites in the promoters that may be activated by genomic and/or non-genomic pathways. For example, the c-fos, bcl-2 and cyclin D1 genes all contain E2-responsive GC-rich sites; however, induction by E2 also involves non-genomic E2-dependent activation of MAPK/PI3K (fos), and cAMP/PKA (bcl-2 and cyclin D1) (Duan et al. 1998; Dong et al. 1999; Castro-Rivera et al. 2001; Duan et al. 2001; Duan et al. 2002) (Fig. 2). It is also possible that activation of non-genomic pathways also activates ER/Sp-dependent genes. A recent paper on the induction of PADI4 by E2 in MCF-7 cells also demonstrates the role of multiple hormone-dependent pathways for transactivation (Dong et al. 2007). This gene contains two upstream ERE motifs that bind ERα and contribute to induced gene expression; however, the proximal regions of the gene contains GC-rich, AP-1 and NF-YA sites which are important for PADI4 gene expression (Fig. 3). Deletion analysis and RNA interference studies suggest that although ERα/Sp1 (and not ERα/Sp3) contributes to E2-induced gene expression, interactions of ERα and/or Sp1 with NF-YA and AP-1 are also involved in the induction of PADI4 (Dong et al. 2007). Similar results were observed for induction of the human prolactin receptor which involved interactions of ERα/Sp with C-EBPβ (Dong et al. 2006) (Fig. 3).
Figure 2.
Hormonal activation of cyclin D1, bcl-2, and c-Fos in breast cancer cells. In addition to GC-rich sites activated by ERα/Sp, E2-dependent activation of non-genomic cAMP/PKA (cyclin D1 and bcl-2) and MAPK/PI3K(c-Fos) pathways were also identified.
Figure 3.
Multiple E2-responsive elements. The PADI4 and prolactin receptor genes contain E2-responsive GC-rich sites and other functional cis-element that contributed to E2-induced transactivation.
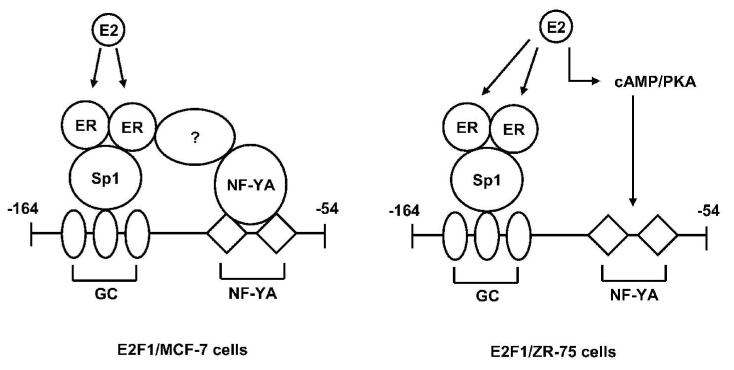
Studies in this laboratory show that cell context plays a critical role in the molecular mechanisms of hormone-dependent transactivation. E2 induced E2F1 gene expression in MCF-7 cells and the minimal E2-responsive region of the E2F1 promoter contained three consecutive GC-rich sites and two CCAAT binding sites (Wang et al. 1999; Ngwenya & Safe 2003). Promoter analysis showed that the GC-rich or CCAAT sites alone were not E2-responsive in transient transfection studies and that hormone-responsiveness required at least one upstream GC-rich site and both CCAAT elements (Fig. 4). DNA binding studies show that ERα and Sp1 enhance NF-YA binding to the CCAAT sites and that hormone-induced transactivation in MCF-7 cells is associated with an ERα/Sp/NF-YA complex. Induction of E2F1 in ZR-75 (ER-positive) breast cancer cells involves the same response elements; however the GC-rich sites are independently activated by ERα/Sp and NF-YA is induced by E2 through non-genomic ER-dependent activation of cAMP/PKA.
Figure 4.
Different mechanisms of hormonal activation of E2F1 in MCF-7 cells and ZR-75 cells.
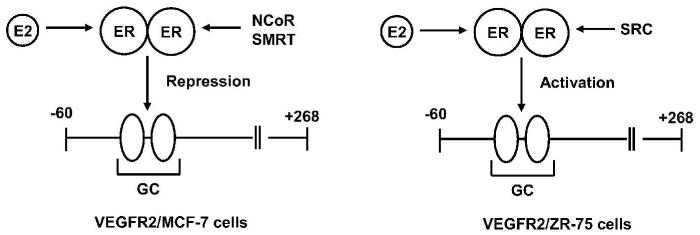
One of the most striking cell context-dependent differences in hormonal modulation of gene expression by ERα/Sp is associated with expression of VEGFR2 in ZR-75 and MCF-7 cells (Higgins et al. 2006; Higgins et al. 2008). VEGF and VEGFR2 are critical angiogenic genes that contain proximal GC-rich sites required for basal and hormone-dependent expression (Fig. 5). Initial studies showed that E2 induced VEGFR2 expression in ZR-75 cells and this involved two proximal GC-rich sites at -58 and -54. In contrast, E2 decreased expression of VEGFR2 in MCF-7 cells and promoter analysis showed that the same promoter elements required for E2-induced transactivation in ZR-75 were required for suppressed expression of VEGFR2 in MCF-7 cells. The major cell context-dependent differences were observed in ChIP assays where E2 induced recruitment of the co-repressors NCoR and SMRT to the VEGFR2 promoter in MCF-7 but not in ZR-75 cells. In the latter cell line, treatment with E2 increased recruitment of the coactivator SRC-3 to the GC-rich promoter, whereas this was not observed in MCF-7 cells. These observations are consistent with the cell context-dependent differences in hormonal regulation of VEGFR2; however, other factors must also be involved and this is currently being investigated.
Figure 5.
Mechanisms of E2-dependent downregulation and induction of VEGFR2 in MCF-7 and ZR-75 cells, respectively.
(ii) Role of ERα and ERβ in activation of ER/Sp
Initial studies investigated the role of ERα and ERβ in activating a GC-rich construct (pSp1) in breast and other cancer cell lines (Saville et al. 2000). The results showed that in these cancer cell lines transected with pSp, ERα or ERβ, E2 activated ERα/Sp but not ERβ/Sp. Subsequent domain swapping experiment with ERα and ERβ demonstrated that the N-terminal AF-1 domain of ERα was important for E2-dependent transactivation, whereas the corresponding A/B domain of ERβ fused to the N- terminal C-F domains of ERβ or ERα was inactive. These results are consistent with the lack of AF-1 activity in the A/B domain of ERβ. Other studies on activation of ERα/Sp vs. ERβ/Sp are variable and depend on promoter and cell context. ERα but not ERβ is involved in activation of PADI4 in HeLa cells (Dong et al. 2007); E2 activates GC-rich EGFR promoter constructs in HeLa cells transfected with ERα or ERβ (Salvatori et al. 2003). Both ERα and ERβ were involved in hormone-dependent activation of GC-rich progesterone receptor promoter constructs; however, the induction response was dependent on cell context (Schultz et al. 2005). Kim and coworkers further investigated ERα-Sp1 interactions using fluorescence resonance energy transfer (FRET) (Kim et al. 2005). Although ERα and Sp1 interactions in in vitro pulldown assays are ligand-independent, results of FRET studies show that in live MCF-7 cells, E2 clearly induces ERα-Sp1 interactions.
(iii) ERα activation of genes through Sp1, Sp3 and Sp4
Most studies on ERα/Sp-mediated transactivation have assumed that Sp1 plays a major role in this response since this protein is overexpressed in cancer cell lines. However, since Sp1, Sp3 and Sp4 proteins are expressed in breast cancer cells, we investigated the effects of individual Sp protein knockdown by RNA interference on the induction of RARα1, E2F1 and CAD gene expression by E2 in MCF-7 cells (Khan et al. 2007). All three genes contain GC-rich promoters; however, the role of individual Sp proteins on hormone-responsiveness had not been determined. The results showed that knockdown of Sp1, Sp3 and Sp4 significantly decreased the fold induction of RARα1, E2F1 and CAD by E2; however, loss of induction was greater in cells where Sp3 or Sp4 was decreased compared to decreased Sp1. This demonstrates, at least for these three genes, that all three Sp proteins play role in ERα/Sp-mediated transactivation in MCF-7 cells.
(iv) Ligand-dependent activation of ER α/Sp
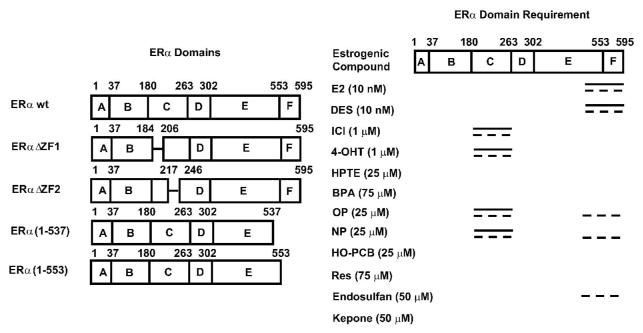
Initial studies showed that in breast cancer cells transfected with pSp1 and wild-type ERα, both E2 and the antiestrogens 4-hydroxytamoxifen (4-OHT) and ICI 182780 (fulvestrant) induced reporter gene [luciferase or chloramphenical acetyltransferase (CAT)] activity (Porter et al. 1997; Saville et al. 2000; Kim et al. 2003). In contrast, E2 but not 4-OHT or ICI 182780 induced luciferase activity in breast cancer cells transfected with GC-rich promoter-reporter constructs from several E2-responsive genes, demonstrating that promoter structure also influences the ER agonist/antagonist activities of E2 and antiestrogens. The structure-dependent activation of ERα/Sp was also investigated in MCF-7 and MDA-MB-231 cells transfected with pSp13 and wild-type ERα, ERαΔZF1, ERαΔZF2, ERα(1-553), and ERα(1-537). These ERα mutants contained deletions of zinc finger 1 (amino acids 185-205), zinc finger 2 (amino acids 218-243), the F domain (amino acids 554-595), and the F domain plus amino acids in helix 12 of the E domain (amino acids 538-595), respectively (Fig. 6) (Wu et al. 2008). The compounds used in this study included E2, diethylstilbestrol (DES), antiestrogens, the phytoestrogen resveratrol, and the xenoestrogens octylphenol (OP), nonylphenol (NP), endosulfan, kepone, 2,3,4,5-tetrachlorobiphenyl-4-ol (HO-PCB-Cl4), bisphenol-A (BPA), and 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE). In MCF-7 cells, all compounds except resveratrol induced luciferase activity in cells transfected with wild-type ERα, whereas in MDA-MB-231 cells, even resveratrol was active. The concentrations of each compound were selected based on their maximal inducing response (with wild-type ERα) that was not accompanied by cytotoxicity. E2 and the xenoestrogens activated wild-type ERα and ERαΔZF1/ ERαΔZF2 in MCF-7 cells, whereas in MDA-MB-231 cells, NP and OP did not activate luciferase activity in cells transfected with ERαΔZF2 and had minimal effects in cells transfected with ERαΔZF1 (Fig. 6). Thus, with the exception of NP and OP, the xenoestrogens resembled E2 and DES but not 4-OHT or ICI 182780. In contrast, 4-OHT and ICI 182780 but not E2 or DES induced transactivation in MCF-7 and MDA-MB-231 cells transfected with ERα(1-537), a mutant form of ERα that has lost part of helix 12 which interacts with coactivators. For this form of ERα, the xenoestrogens all induced luciferase activity and resembled the antiestrogenic drugs 4-OHT and ICI 182780. These results demonstrate that the xenoestrogens can be both “estrogen- and antiestrogen-like”, depending on the expression of ERα variants indicating that these compounds are selective ER modulators (SERMs).
Figure 6.
Wild-type and ERα variants and domains of ERα required for transactivation by E2 and structurally diverse estrogenic compounds in MCF-7 (—) and MDA-MB-231 (---) breast cancer cells.
RNA interference assays using small inhibitory RNAs for Sp1, Sp3 and Sp4 also demonstrated structure-dependent differences in activation of ERα/Sp1, ERα/Sp3 and ERα/Sp4. “Fold induction by estrogens (E2 and DES), xenoestrogens and antiestrogens exhibited three patterns which differentially relied upon ERα/Sp1, ERα/Sp3, ERα/Sp4 or their combinations. For E2, HPTE, DES and HO-PCB-Cl4, ERα/Sp1 > ERα/Sp4, and ERα/Sp3 had minimal to no effect on activation of ERα/Sp by these compounds. The pattern of ERα/Sp activation for BPA, endosulfan, NP and 4-OHT was ERα/Sp1 ≈ ERα/Sp4 with minimal contributions by ERα/Sp3. In contrast, both ERα/Sp1 and ERα/Sp3 play roles in activation of pSp13 by ICI 182780 and kepone, but ERα/Sp4 tends to cause an inhibitory effect since iSp4 enhances the fold induction by these compounds” (Khan et al. 2007). These results illustrate the complexity of activation of ERα/Sp which depends on ligand structure, ERα variant, and individual Sp proteins. Moreover, in this study, cells were transfected with the pSp13 construct which contains three tandem consensus GC-rich motifs and this pattern of induction will undoubtedly vary with different GC-rich promoter constructs.
LIGAND-DEPENDENT ACTIVATION OF ER/AP-1
1. Introduction
Activating protein-1 (AP-1) is a transcription factor complex containing the protooncogenes jun, fos and other family members, and this complex interacts with AP-1 sites in gene promoters to activate genes involved in cell growth, differentiation and development. Early studies showed that fos and jun suppress ER-dependent transactivation from an ERE promoter (Shemshedini et al. 1991; Tzukerman et al. 1991; Doucas et al. 1991), and there are also reports that E2 induces expression of AP-1 transcription factors (Duan et al. 1998; Duan et al. 2001; Duan et al. 2002). AP-1 activity is also increased during the progression of MCF-7 cells to an antiestrogen-resistant phenotype, suggesting a role for this complex in E2-independent and more aggressive breast cancer cells (Dumont et al. 1996). In addition, there was also evidence that hormonal activation of insulin-like growth and ovalbumin involved AP-1 sites and that AP-1 may be important for E2-dependent activation or repression of the progesterone receptor, gonadotropin-releasing hormone receptor, MMP-1, prolactin and pS2 genes (Umayahara et al. 1994; Philips et al. 1993; Gaub et al. 1990; Van der Burg et al. 1990; Savouret et al. 1994; Petz et al. 2002; Barkhem et al. 2002; Cheng et al. 2003; Scafonas et al. 2008; Duan et al. 2008). DeNardo and coworkers identified 20 new E2-induced genes that were AP-1 dependent (DeNardo et al. 2005), and microarrays also identified a subset of 32 ERE-independent differentially expressed genes in breast tumors (Glidewell-Kenney et al. 2005). These data confirm the importance of the non-classical genomic pathways for induction of genes by E2 and other estrogenic compounds. c-Fos is induced by E2 through non-genomic and genomic pathways and the former pathway may also contribute to activation of ER/AP-1.
2. Role of AP-1 Proteins In Hormonal Modulation of Gene Expression
(a) Estrogen/Antiestrogen Activation of ER/AP-1
Webb and coworkers first reported the activation of an AP-1 promoter-reporter construct derived from the human collagenase promoter (Webb et al. 1995). Antiestrogens such as ICI 182780 and 4-OHT and E2 activated the AP-1 construct in cancer cells (HeLa, NIH-3T3, HepG2, SHM, SY5Y, CEF, CV1, CHO and F9) derived from several different tissues. In Ishikawa endometrial cancer cells, E2 and the antiestrogens activated AP-1; however, E2 but not 4-OHT or ICI 182780 activated the AP-1 promoter in ER-positive MCF-7 and ZR-75 cells. These results were interpreted as a mechanism by which antiestrogens such as tamoxifen can exhibit ER agonist activity through the non-classical ER/AP-1 pathway in which ER binds jun but not fos in pulldown assays where the N-terminal AF-1 region of ERα is the major jun-interacting site.
Subsequent studies compared the activation of ERα/AP-1 vs. ERβ/AP-1 by E2 and selected SERMs that are used for breast cancer or other hormonal therapies (Paech et al. 1997). In HeLa cells, the antiestrogens raloxifene, 4-OHT and ICI 182780 but not E2 or DES activated ERβ/AP-1, and similar results were observed in Ishikawa, MCF-7 and ER-negative MDA-MB-453 breast cancer cells. In contrast, both estrogens and antiestrogens activated ERα/AP-1 in HeLa cells, demonstrating that cell context, ER subtype, and ligand structure were important for activation of ER/AP-1. Interestingly, these results clearly distinguish between ER/AP-1 vs. ER/SP where ERβ/Sp is relatively inactive and wild-type ERα/Sp is activated by both estrogens and antiestrogens (Fig. 6).
(b) Effects of ER Deletion Mutants on Activation of ER/AP-1
Several studies have investigated the effects of wild-type and variant ER on activation of ER/AP-1 in different cell lines (Webb et al. 1995; Webb et al. 1999; Jakacka et al. 2001; Bjornstrom & Sjoberg 2002; Weatherman & Scanlan 2001; Kushner et al. 2000). It was initially reported that E2 but not antiestrogens activated HE11/AP-1 where HE11 is a DNA binding domain deletion mutant of ERα (Webb et al. 1995), and this was similar to results obtained for activation of HE11/Sp or the zinc finger deletion mutants ERαΔZF1 and ERαΔZF2 (Fig. 6). The effects of specific zinc finger mutants within the DNA binding domain of ERα have also been investigated (Jakacka et al. 2001; Bjornstrom & Sjoberg 2002). The ERα E207G/G208S and E207A/G208A mutants do not bind DNA and, in ER-negative TSA cells transfected with these ERα mutants and an AP-1 reporter construct, ICI 182780 induced luciferase activity and E2 repressed activity (Jakacka et al. 2001). A more extensive study on a series of DNA binding domain mutants of ERβ further demonstrated the complexity of ERα/AP-1- and ERβ/AP-1-mediated transactivation in COS-7 and HE11 cells (Bjornstrom & Sjoberg 2002). In HC11 cells transfected with wild-type ERβ and an AP-1 reporter construct, E2 repressed and the antiestrogens 4-OHT and ICI 182780 induced activity. However, in the same cell line transfected with ERβ containing L206A, Y210A and Δ122-266 (the entire DBD) mutant and several double mutants, E2 induced activity and the antiestrogens were inactive. Thus, subtle changes in the ERβ DBD completely reversed the ER agonist/antagonist activities of E2, 4-OHT and ICI 182780.
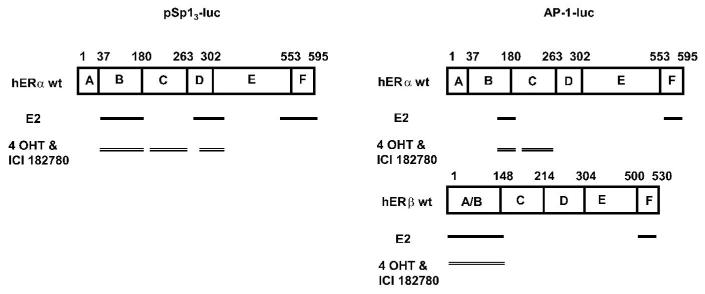
Elegant studies on ERα and ERβ deletion mutants and chimeric ERs with interchangeable domains have shown the importance of the C-terminal and N-terminal AF-2 and AF-1 domains on ligand-dependent activation of ER/AP-1 (Webb et al. 1999; Weatherman & Scanlan 2001). E2 induced transactivation of an AP-1 promoter containing only the LBD of ERα, whereas tamoxifen, raloxifene and ICI 182780 were inactive. Subsequent studies on deletions of the AF-1 domain of ERα showed that E2-induced transactivation was dependent on AF-1. The effects of tamoxifen were AF-1 independent but longer N-terminal deletions (129-178) resulted in loss of activity in HeLa cells. Interestingly, deletion of AF-1 from wild-type ERα enhanced tamoxifen-induced transactivation and the response was similar to that observed in HeLa cells transfected with ERβ which does not contain AF-1-dependent activity. However, deletion of the N-terminal A/B domain of ERβ resulted in loss of tamoxifen-dependent activation of an AP-1 promoter in HeLa cells (Weatherman & Scanlan 2001). Figure 7 compares the domain requirements for E2 and antiestrogens for activation of ERα/Sp and ERα/AP-1. Major differences were observed for E2 which required the AF-1 domain for activation of ERα/Sp, whereas activation of ERα/AP-1 was dependent on AF-1; however, loss of activity was observed after deletion of aa 127-178 in the N-terminal A/B domain.
Figure 7.
Domains of ERα and ERβ required for ligand-dependent activation of ER/AP1 (i.e.deletion results in loss of activity) (104, 107) and comparisons with activation of ERα /Sp.
(c) Structure-Dependent Activation of ER/AP-1
The effects of structurally-diverse pharmacologic SERMs and xenoestrogens on activation of ER/AP-1 has also been reported (Walters et al. 2002; Weatherman et al. 2001; Fujimoto et al. 2004). Differences in activation of ERα/AP-1 and ERβ/AP-1 were observed among several drugs being developed for endocrine therapy; however, EC50 values were generally lower for activation of ERβ/AP-1 (Weatherman et al. 2001). The effects of several estrogenic compounds including xenoestrogens on activation of ERE-luc and AP-1-luc constructs cotransfected with ERα or ERβ were investigated in NIH-3T3 cells (Fujimoto et al. 2004). Activation of ERα/AP-1 was observed in cells treated with most of the compounds including E2, 4-OHT, 17α-estradiol, estriol, dienstrol, bisphenol A, t-methylbutylphenol, kepone, p,p’-biphenol, genistein, o,p’-DDD, zearalenol and p,p’-DDE (but not p,p’-DDT), whereas only 4-OHT activated ERβ/AP-1. Thus, xenoestrogens primarily activate ERα/AP-1 and some of the same compounds also activate ERα/Sp (Fig. 6).
(d) In Vivo Studies
Jameson and coworkers initially developed a transgenic mouse model in which a mutant ERα(E207A/G208A; AA) knockin mouse was generated to investigate promoter DNA-independent estrogenic activity of ERα. The ERα mutant does not bind promoter DNA and therefore a comparison of the effects of E2 in wild-type and knockin mutant ERα mice will provide important insights on the DNA (ERE)-independent effects of ERα which could include interactions with AP-1, Sp and other transcription factors. The knockin mice (ER+/AA) exhibited a gain of function since the females were infertile due to uterine defects and inovulation (Jakacka et al. 2002). These mice have now been bred on an ERα knockout (ER-/-) background to give ERα-/AA mice which can be used in comparative studies with ERα+/+, ERα-/- and ERα-/AA mice to determine ERE-independent responses (O’Brien et al. 2006; Glidewell-Kenney et al. 2007; Syed et al. 2007; McDevitt et al. 2007). In the ER-/AA mouse uterus E2 and tamoxifen induced luminal epithelial cell proliferation but not other prototypical estrogen responses such as hyperemia and fluid retention (O’Brien et al. 2006). The contributions of ERE-independent vs. ERE-dependent pathways were also investigated in the mouse reproductive axis. Estrogen-negative feedback control on luteneizing hormone secretion was primarily ERE-independent, whereas positive feedback and ovulatory cyclicity were ERE-dependent processes (Glidewell-Kenney et al. 2007). Development of the male skeleton and sexual behavior in mice were primarily ERE-dependent, whereas secretion of testosterone was ERE-independent (Syed et al. 2007; McDevitt et al. 2007). These results clearly demonstrate an important role for ER/AP-1, ER/Sp and other DNA-independent estrogenic pathways in mice. However, it is also possible that some of the ERE-dependent/-independent responses may be due to non-genomic pathways and their relative contributions of genomic vs. non-genomic E2-dependent responses in mice and humans requires further research and development of appropriate animal models.
SUMMARY
Ligand-dependent activation of ER is highly complex and dependent on ligand structure, ER subtype and intracellular location, promoter and cell context. The non-classical genomic ER/Sp and ER/AP-1 regulate a large number of genes through both distinct and overlapping pathways. Studies on the identification of genome-wide ER binding sites have confirmed the association of this hormone receptor with other nuclear factors and binding motifs including GC-rich and AP-1 sites (Carroll et al. 2006; Carroll & Brown 2006; Lin et al. 2007; Gao et al. 2008; Kininis et al. 2007; Vega et al. 2006). It has also been reported that many E2-responsive genes are regulated by interactions of ER with cis-elements that are distal to their corresponding transcription start sites. Thus, the molecular biology of E2-dependent activation of ER/Sp and ER/AP-1 and their associated genes may also include contributions of distal binding sites that have not yet been characterized. The ligand structure- and ER subtype-dependent patterns of activation of ER/Sp and ER/AP-1 are different (Figs. 6 and 7), and this may be due, in part, to the relative expression of cofactors required for ER/Sp and ER/AP-1-mediated transactivation. Although coactivator/cofactor requirements for activation of classical ERE promoters have been exhaustively investigated, only a few reports have determined functional coactivator-ER/Sp and ER-AP-1 interactions. Steroid receptor coactivator 2 (or GRIP1) enhances ERα/AP-mediated transcription, and this response requires LXXLL boxes in the coactivator which facilitates interactions with the AF2 domain of ERα (Webb et al. 1999). This is consistent with similar pathways for coactivation of ERα (ERE-dependent) and ERα/AP-1 (Webb et al. 1999). Receptor-interacting protein 140 (RIP140) repressed E2-induced activation of ERα/AP-1 by reversing the effects of SRC2 (Teyssier et al. 2003). In contrast, studies with the vitamin D-interacting protein 150 (DRIP150) coactivator demonstrated that DRIP150 coactivation of ERα/Sp was LXXLL-box-independent and required a novel helical region in DRIP150 (Lee & Safe 2007). Cell context-dependent induction and repression of VEGFR2 in ZR-75 and MCF-7 cells, respectively, is associated with the same GC-rich promoter sequences but there were differences in recruitment of coactivators (induction) and corepressors such as NCoR and SMRT (Higgins et al. 2006; Higgins et al. 2008). Further studies are required on the mechanisms and proteins associated with coactivation and repression of ER/Sp and ER/AP-1, since in vivo studies demonstrate that these ERE-independent pathway play an important role in mediating the effects of estrogens, pharmacologic and other synthetic or naturally-occurring SERMs.
ACKNOWLEDGEMENTS
The financial assistance of the National Institutes of Health (ES04917) and Texas AgriLife is gratefully acknowledged.
LITERATURE CITED
- Ammendola R, Mesuraca M, Russo T, Cimino F. Sp1 DNA binding efficiency is highly reduced in nuclear extracts from aged rat tissues. Journal of Biological Chemistry. 1992;267:17944–17948. [PubMed] [Google Scholar]
- Augereau P, Miralles F, Cavailles V, Gaudelet C, Parker M, Rochefort H. Characterization of the proximal estrogen-responsive element of human cathepsin D gene. Molecular Endocrinology. 1994;8:693–703. doi: 10.1210/mend.8.6.7935485. [DOI] [PubMed] [Google Scholar]
- Bardin A, Moll F, Margueron R, Delfour C, Chu ML, Maudelonde T, Cavailles V, Pujol P. Transcriptional and posttranscriptional regulation of fibulin-1 by estrogens leads to differential induction of messenger ribonucleic acid variants in ovarian and breast cancer cells. Endocrinology. 2005;146:760–768. doi: 10.1210/en.2004-1239. [DOI] [PubMed] [Google Scholar]
- Barkhem T, Haldosen LA, Gustafsson JA, Nilsson S. pS2 Gene expression in HepG2 cells: complex regulation through crosstalk between the estrogen receptor α, an estrogen-responsive element, and the activator protein 1 response element. Molecular Pharmacology. 2002;61:1273–1283. doi: 10.1124/mol.61.6.1273. [DOI] [PubMed] [Google Scholar]
- de Medeiros SR Batistuzzo, Krey G, Hihi AK, Wahli W. Functional interactions between the estrogen receptor and the transcription activator Sp1 regulate the estrogen-dependent transcriptional activity of the vitellogenin A1 io promoter. Journal of Biological Chemistry. 1997;272:18250–18260. doi: 10.1074/jbc.272.29.18250. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mutations in the estrogen receptor DNA-binding domain discriminate between the classical mechanism of action and cross-talk with Stat5b and activating protein 1 (AP-1) Journal of Biological Chemistry. 2002;277:48479–48483. doi: 10.1074/jbc.C200570200. [DOI] [PubMed] [Google Scholar]
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and Krüppel-like factor family of transcription factors in cell growth regulation and cancer. Journal of Cellular Physiology. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- Boggess JF, Zhou C, Bae-Jump VL, Gehrig PA, Whang YE. Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecologic Oncology. 2006;103:417–424. doi: 10.1016/j.ygyno.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Molecular and Cellular Endocrinology. 2002;195:27–38. doi: 10.1016/s0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Briggs MR, Kadonaga JT, Bell SP, Tjian R. Purification and biochemical characterization of the promoter- specific transcription factor, Sp1. Science. 1986;234:47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Byrne IM, Flanagan L, Tenniswood MP, Welsh J. Identification of a hormone-responsive promoter immediately upstream of exon 1c in the human vitamin D receptor gene. Endocrinology. 2000;141:2829–2836. doi: 10.1210/endo.141.8.7618. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Molecular Endocrinology. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Castro-Rivera E, Samudio I, Safe S. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. Journal of Biological Chemistry. 2001;276:30853–30861. doi: 10.1074/jbc.M103339200. [DOI] [PubMed] [Google Scholar]
- Cavailles V, Augereau P, Rochefort H. Cathepsin D gene is controlled by a mixed promoter, and estrogens stimulate only TATA-dependent transcription. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:203–207. doi: 10.1073/pnas.90.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CK, Chow BK, Leung PC. An activator protein 1-like motif mediates 17β-estradiol repression of gonadotropin-releasing hormone receptor promoter via an estrogen receptor α-dependent mechanism in ovarian and breast cancer cells. Molecular Endocrinology. 2003;17:2613–2629. doi: 10.1210/me.2003-0217. [DOI] [PubMed] [Google Scholar]
- Chiefari E, Brunetti A, Arturi F, Bidart JM, Russo D, Schlumberger M, Filetti S. Increased expression of AP2 and Sp1 transcription factors in human thyroid tumors: a role in NIS expression regulation? BMC. Cancer. 2002;2:35. doi: 10.1186/1471-2407-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA. Androgen suppression of GnRH-stimulated rat LHβ gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Molecular Endocrinology. 2001;15:1906–1917. doi: 10.1210/mend.15.11.0723. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogen-induced/activator protein-1-dependent genes. Molecular Endocrinology. 2005;19:362–378. doi: 10.1210/me.2004-0267. [DOI] [PubMed] [Google Scholar]
- Dong J, Tsai-Morris CH, Dufau ML. A novel estradiol/estrogen receptor α-dependent transcriptional mechanism controls expression of the human prolactin receptor. Journal of Biological Chemistry. 2006;281:18825–18836. doi: 10.1074/jbc.M512826200. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Kladde M, Vyhlidal C, Safe S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17β-estradiol in breast cancer cells. Journal of Biological Chemistry. 1999;174:32099–32107. doi: 10.1074/jbc.274.45.32099. [DOI] [PubMed] [Google Scholar]
- Dong S, Zhang Z, Takahara H. Estrogen-enhanced peptidylarginine deiminase type IV gene (PADI4) expression in MCF-7 cells is mediated by estrogen receptor-α-promoted transfactors activator protein-1, nuclear factor-Y, and Sp1. Molecular Endocrinology. 2007;21:1617–1629. doi: 10.1210/me.2006-0550. [DOI] [PubMed] [Google Scholar]
- Doucas V, Spyrou G, Yaniv M. Unregulated expression of c-Jun and c-Fos proteins but not Jun D inhibits oestrogen receptor activity in human breast cancer- derived cells. EMBO Journal. 1991;10:2237–2245. doi: 10.1002/j.1460-2075.1991.tb07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Ginsburg E, Vonderhaar BK. Estrogen stimulates transcription from the human prolactin distal promoter through AP1 and estrogen responsive elements in T47D human breast cancer cells. Molecular and Cellular Endocrinology. 2008;281:9–18. doi: 10.1016/j.mce.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Duan R, Porter W, Safe S. Estrogen-induced c-fos protooncogene expression in MCF-7 human breast cancer cells: role of estrogen receptor Sp1 complex formation. Endocrinology. 1998;139:1981–1990. doi: 10.1210/endo.139.4.5870. [DOI] [PubMed] [Google Scholar]
- Duan R, Xie W, Burghardt R, Safe S. Estrogen receptor-mediated activation of the serum response element in MCF-7 cells through MAPK-dependent phosphorylation of Elk-1. Journal of Biological Chemistry. 2001;276:11590–11598. doi: 10.1074/jbc.M005492200. [DOI] [PubMed] [Google Scholar]
- Duan R, Xie W, Li X, McDougal A, Safe S. Estrogen regulation of c-fos gene expression through phosphatidylinositol-3-kinase-dependent activation of serum response factor in MCF-7 breast cancer cells. Biochemistry and Biophysical Research Communications. 2002;294:384–394. doi: 10.1016/S0006-291X(02)00499-0. [DOI] [PubMed] [Google Scholar]
- Dubik D, Shiu RPC. Mechanism of estrogen activation of c-myc oncogene expression. Oncogene. 1992;7:1587–1594. [PubMed] [Google Scholar]
- Dumont JA, Bitonti AJ, Wallace CD, Baumann RJ, Cashman EA, Cross-Doersen DE. Progression of MCF-7 breast cancer cells to antiestrogen-resistant phenotype is accompanied by elevated levels of AP-1 DNA-binding activity. Cell Growth & Differentiation. 1996;7:351–359. [PubMed] [Google Scholar]
- Dynan WS, Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983;35:79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Dynan WS, Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. Nature. 1985;316:774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Fleming JG, Spencer TE, Safe SH, Bazer FW. Estrogen regulates transcription of the ovine oxytocin receptor gene through GC-rich SP1 promoter elements. Endocrinology. 2006;147:899–911. doi: 10.1210/en.2005-1120. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Honda H, Kitamura S. Effects of environmental estrogenic chemicals on AP1 mediated transcription with estrogen receptors α and α. Journal of Steroid Biochemisty and Molecular Biology. 2004;88:53–59. doi: 10.1016/j.jsbmb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Fujita N, Kajita M, Taysavang P, Wade PA. Hormonal regulation of metastasis-associated protein 3 transcription in breast cancer cells. Molecular Endocrinology. 2004;18:2937–2949. doi: 10.1210/me.2004-0258. [DOI] [PubMed] [Google Scholar]
- Gao H, Falt S, Sandelin A, Gustafsson JA, hlman-Wright K. Genome-wide identification of estrogen receptor α-binding sites in mouse liver. Molecular Endocrinology. 2008;22:10–22. doi: 10.1210/me.2007-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub M-P, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the Fos-Jun complex. Cell. 1990;63:1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Lee EJ, Pillai S, Ishikawa T, Ariazi EA, Jameson JL. ERE-independent ERα target genes differentially expressed in human breast tumors. Molecular and Cellular Endocrinology. 2005;245:53–59. doi: 10.1016/j.mce.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gollner H, Bouwman P, Mangold M, Karis A, Braun H, Rohner I, Del Rey A, Besedovsky HO, Meinhardt A, van den Broek M, Cutforth T, Grosveld F, Philipsen S, Suske G. Complex phenotype of mice homozygous for a null mutation in the Sp4 transcription factor gene. Genes to Cells. 2001a;6:689–697. doi: 10.1046/j.1365-2443.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- Gollner H, Dani C, Phillips B, Philipsen S, Suske G. Impaired ossification in mice lacking the transcription factor Sp3. Mechanisms of Development. 2001b;106:77–83. doi: 10.1016/s0925-4773(01)00420-8. [DOI] [PubMed] [Google Scholar]
- Higgins KJ, Liu S, Abdelrahim M, Vanderlaag K, Liu X, Porter W, Metz R, Safe S. Vascular endothelial growth factor receptor-2 expression is downregulated by 17β-estradiol in MCF-7 breast cancer cells by estrogen receptor α/Sp proteins. Molecular Endocrinology. 2008;22:388–402. doi: 10.1210/me.2007-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins KJ, Liu S, Abdelrahim M, Yoon K, Vanderlaag K, Porter W, Metz RP, Safe S. Vascular endothelial growth factor receptor-2 expression is induced by 17β-estradiol in ZR-75 breast cancer cells by estrogen receptor α/Sp proteins. Endocrinology. 2006;147:3285–3295. doi: 10.1210/en.2006-0081. [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A, Nagawa H, Suzuki N. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. International Journal of Oncology. 2004;25:461–468. [PubMed] [Google Scholar]
- Husmann M, Dragneva Y, Romahn E, Jehnichen P. Nuclear receptors modulate the interaction of Sp1 and GC-rich DNA via ternary complex formation. The BiochemicalJournal. 2000;352:763–772. [PMC free article] [PubMed] [Google Scholar]
- Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochemistry and Biophysical Research Communications. 2003;303:660–668. doi: 10.1016/s0006-291x(03)00408-x. [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Molecular Endocrinology. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien P-Y, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. Journal of Biological Chemistry. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- Kanai M, Wei D, Li Q, Jia Z, Ajani J, Le X, Yao J, Xie K. Loss of Krüppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clinical Cancer Research. 2006;12:6395–6402. doi: 10.1158/1078-0432.CCR-06-1034. [DOI] [PubMed] [Google Scholar]
- Kelley KM, Rowan BG, Ratnam M. Modulation of the folate receptor α gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Research. 2003;63:2820–2828. [PubMed] [Google Scholar]
- Khan S, Abdelrahim M, Samudio I, Safe S. Estrogen receptor/Sp1 complexes are required for induction of cad gene expression by 17β-estradiol in breast cancer cells. Endocrinology. 2003;144:2325–2335. doi: 10.1210/en.2002-0149. [DOI] [PubMed] [Google Scholar]
- Khan S, Wu F, Liu S, Wu Q, Safe S. Role of specificity protein (Sp) transcription factors in estrogen-induced gene expression in MCF-7 breast cancer cells. Journal of Molecular Endocrinology. 2007;39:289–304. doi: 10.1677/JME-07-0043. [DOI] [PubMed] [Google Scholar]
- Kim K, Barhoumi R, Burghardt R, Safe S. Analysis of estrogen receptor α-Sp1 interactions in breast cancer cells by fluorescence resonance energy transfer. Molecular Endocrinology. 2005;19:843–854. doi: 10.1210/me.2004-0326. [DOI] [PubMed] [Google Scholar]
- Kim K, Nguyen T, Saville B, Safe S. Domains of estrogen receptor α (ERα) required for ERα/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells. Molecular Endocrinology. 2003;17:804–817. doi: 10.1210/me.2002-0406. [DOI] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol. Cell Biol. 2007;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Porter W, Santostefano M, Wang X, Safe S. Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Molecular and Cellular Biology. 1995;15:6710–6719. doi: 10.1128/mcb.15.12.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Wang X, Safe S. Estrogen receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. Journal of Biological Chemistry. 1994;269:15912–15917. [PubMed] [Google Scholar]
- Kundu P, Alioua A, Stefani E, Toro L. Regulation of mouse Slo gene expression: multiple promoters, transcription start sites, and genomic action of estrogen. Journal of Biological Chemistry. 2007;282:27478–27492. doi: 10.1074/jbc.M704777200. [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. Journal of Steroid Biochemisty and Molecular Biology. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Lee J, Safe S. Coactivation of estrogen receptor α (ERα)/Sp1 by vitamin D receptor interacting protein 150 (DRIP150) Archives of Biochemistry and Biophysics. 2007;461:200–210. doi: 10.1016/j.abb.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mitchell D, Luo J, Yi Z, Cho SG, Guo J, Li X, Ning G, Wu X, Liu M. Estrogen regulates KiSS1 gene expression through estrogen receptor α and SP protein complexes. Endocrinology. 2007;148:4821–4828. doi: 10.1210/en.2007-0154. [DOI] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET. Whole-genome cartography of estrogen receptor α binding sites. PLoS. Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Gladwell W, Teng CT. Estrogen stimulates estrogen-related receptor α gene expression through conserved hormone response elements. Endocrinology. 2003;144:4894–4904. doi: 10.1210/en.2003-0432. [DOI] [PubMed] [Google Scholar]
- Lou Z, O’Reilly S, Liang H, Maher VM, Sleight SD, Mccormick JJ. Down-regulation of overexpressed Sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Research. 2005;65:1007–1017. [PubMed] [Google Scholar]
- Lu S, Jenster G, Epner DE. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Molecular Endocrinology. 2000;14:753–760. doi: 10.1210/mend.14.5.0461. [DOI] [PubMed] [Google Scholar]
- Maor S, Mayer D, Yarden RI, Lee AV, Sarfstein R, Werner H, Papa MZ. Estrogen receptor regulates insulin-like growth factor-I receptor gene expression in breast tumorcells: involvement of transcription factor Sp1. The Journal of Endocrinology. 2006;191:605–612. doi: 10.1677/joe.1.07016. [DOI] [PubMed] [Google Scholar]
- Marin M, Karis A, Visser P, Grosveld F, Phillipsen S. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell. 1997;89:619–628. doi: 10.1016/s0092-8674(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Martin R, Taylor MB, Krikun G, Lockwood C, Akbas GE, Taylor HS. Differential cell-specific modulation of HOXA10 by estrogen and specificity protein 1 response elements. The Journal of Clinical Endocrinology and Metabolism. 2007;92:1920–1926. doi: 10.1210/jc.2006-1694. [DOI] [PubMed] [Google Scholar]
- Martini PG, Katzenellenbogen BS. Regulation of prothymosin α gene expression by estrogen in estrogen receptor-containing breast cancer cells via upstream half-palindromic estrogen response element motifs. Endocrinology. 2001;142:3493–3501. doi: 10.1210/endo.142.8.8314. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-α signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERα knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein (Sp) transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Research. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- Moorefield KS, Fry SJ, Horowitz JM. Sp2 DNA binding activity and trans-activation are negatively regulated in mammalian cells. Journal of Biological Chemistry. 2004;279:13911–13924. doi: 10.1074/jbc.M313589200. [DOI] [PubMed] [Google Scholar]
- Ngwenya S, Safe S. Cell context-dependent differences in the induction of E2F-1 gene expression by 17β-estradiol in MCF-7 and ZR-75 cells. Endocrinology. 2003;144:1675–1685. doi: 10.1210/en.2002-0009. [DOI] [PubMed] [Google Scholar]
- O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. Journal of Biological Chemistry. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- Oh JE, Han JA, Hwang ES. Downregulation of transcription factor, Sp1, during cellular senescence. Biochemistry and Biophysical Research Communications. 2007;353:86–91. doi: 10.1016/j.bbrc.2006.11.118. [DOI] [PubMed] [Google Scholar]
- Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21WAF1 cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. Journal of Biological Chemistry. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Petz LN, Nardulli AM. Sp1 binding sites and an estrogen response element half-site are involved in regulation of the human progesterone receptor A promoter. Molecular Endocrinology. 2000;14:972–985. doi: 10.1210/mend.14.7.0493. [DOI] [PubMed] [Google Scholar]
- Petz LN, Ziegler YS, Loven MA, Nardulli AM. Estrogen receptor α and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology. 2002;143:4583–4591. doi: 10.1210/en.2002-220369. [DOI] [PubMed] [Google Scholar]
- Philips A, Chalbos D, Rochefort H. Estradiol increases and anti-estrogens antagonize the growth factor-induced activator protein-1 activity in MCF-7 breast cancer cells without affecting c-fos and c-jun synthesis. Journal of Biological Chemistry. 1993;268:14103–14108. [PubMed] [Google Scholar]
- Philipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Research. 1999;27:2991–3000. doi: 10.1093/nar/27.15.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W, Saville B, Hoivik D, Safe S. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Molecular Endocrinology. 1997;11:1569–1580. doi: 10.1210/mend.11.11.9916. [DOI] [PubMed] [Google Scholar]
- Porter W, Wang F, Wang W, Duan R, Safe S. Role of estrogen receptor/Sp1 complexes in estrogen-induced heat shock protein 27 gene expression. Molecular Endocrinology. 1996;10:1371–1378. doi: 10.1210/mend.10.11.8923463. [DOI] [PubMed] [Google Scholar]
- Qin C, Singh P, Safe S. Transcriptional activation of insulin-like growth factor binding protein 4 by 17β-estradiol in MCF-7 cells: role of estrogen receptor-Sp1 complexes. Endocrinology. 1999;140:2501–2508. doi: 10.1210/endo.140.6.6751. [DOI] [PubMed] [Google Scholar]
- Safe S. Transcriptional activation of genes by 17β-estradiol through estrogen receptor-Sp1 interactions. Vitamins and Hormones. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. European Journal of Cancer. 2005;41:2438–2448. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Safe S, Kim K. Nuclear receptor-mediated transactivation through interaction with Sp proteins. Progress in Nucleic Acid Research and Molecular Biology. 2004;77:1–36. doi: 10.1016/S0079-6603(04)77001-4. [DOI] [PubMed] [Google Scholar]
- Salvatori L, Pallante P, Ravenna L, Chinzari P, Frati L, Russo MA, Petrangeli E. Oestrogens and selective oestrogen receptor (ER) modulators regulate EGF receptor gene expression through human ER α and β subtypes via an Sp1 site. Oncogene. 2003;22:4875–4881. doi: 10.1038/sj.onc.1206784. [DOI] [PubMed] [Google Scholar]
- Salvatori L, Ravenna L, Felli MP, Cardillo MR, Russo MA, Frati L, Gulino A, Petrangeli E. Identification of an estrogen-mediated deoxyribonucleic acid-binding independent transactivation pathway on the epidermal growth factor receptor gene promoter. Endocrinology. 2000;141:2266–2274. doi: 10.1210/endo.141.6.7521. [DOI] [PubMed] [Google Scholar]
- Samudio I, Vyhlidal C, Wang F, Stoner M, Chen I, Kladde M, Barhoumi R, Burghardt R, Safe S. Transcriptional activation of DNA polymerase α gene expression in MCF-7 cells by 17β-estradiol. Endocrinology. 2001;142:1000–1008. doi: 10.1210/endo.142.3.8022. [DOI] [PubMed] [Google Scholar]
- Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson J-A, Safe S. Ligand-, cell- and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. Journal of Biological Chemistry. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- Savouret JF, Rauch M, Redeuilh G, Sar S, Chauchereau A, Woodruff K, Parker MG, Milgrom E. Interplay between estrogens, progestins, retinioic acid and AP-1 on a single regulatory site in the progesterone receptor gene. Journal of Biological Chemistry. 1994;269:28955–28962. [PubMed] [Google Scholar]
- Scafonas A, Reszka AA, Kimmel DB, Hou XS, Su Q, Birzin ET, Kim S, Chen HY, Tan Q, Roher SP, Dininno F, Hammond ML, Rodan GA, Towler DA, Schmidt A. Agonist-like SERM effects on ERα-mediated repression of MMP1 promoter activity predict in vivo effects on bone and uterus. Journal of Steroid Biochemisty and Molecular Biology. 2008;110:197–206. doi: 10.1016/j.jsbmb.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Scholz A, Truss M, Beato M. Hormone-induced recruitment of Sp1 mediates estrogen activation of the rabbit uteroglobin gene in endometrial epithelium. Journal of Biological Chemistry. 1998;273:4360–4366. doi: 10.1074/jbc.273.8.4360. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors α and β. Journal of Biological Chemistry. 2005;280:347–354. doi: 10.1074/jbc.M407879200. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM. Estrogen receptor α and Sp1 regulate progesterone receptor gene expression. Molecular and Cellular Endocrinology. 2003;201:165–175. doi: 10.1016/s0303-7207(02)00415-x. [DOI] [PubMed] [Google Scholar]
- Shemshedini L, Knauthe R, Sassone-Corsi P, Pornon A, Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO Journal. 1991;10:3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, Xiong Q, Wang B, Li XC, Xie K. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Research. 2001;61:4143–4154. [PubMed] [Google Scholar]
- Simmen RCM, Chung TE, Imataka H, Michel FJ, Badinga L, Simmen FA. Trans-activation functions of the Sp-related nuclear factor, basic transcription element-binding protein, and progesterone receptor in endometrial epithelial cells. Endocrinology. 1999;140:2517–2525. doi: 10.1210/endo.140.6.6625. [DOI] [PubMed] [Google Scholar]
- Stoner M, Wang F, Wormke M, Nguyen T, Samudio I, Vyhlidal C, Marme D, Finkenzeller G, Safe S. Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor α and Sp3 proteins. Journal of Biological Chemistry. 2000;275:22769–22779. doi: 10.1074/jbc.M002188200. [DOI] [PubMed] [Google Scholar]
- Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor α and Sp proteins. Oncogene. 2004;23:1052–1063. doi: 10.1038/sj.onc.1207201. [DOI] [PubMed] [Google Scholar]
- Sun G, Porter W, Safe S. Estrogen-induced retinoic acid receptor α1 gene expression: role of estrogen receptor-Sp1 complex. Molecular Endocrinology. 1998;12:882–890. doi: 10.1210/mend.12.6.0125. [DOI] [PubMed] [Google Scholar]
- Sun JM, Spencer VA, Li L, Yu CH, Yu J, Davie JR. Estrogen regulation of trefoil factor 1 expression by estrogen receptor α and Sp proteins. Experimental Cell Research. 2005;302:96–107. doi: 10.1016/j.yexcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Branford WW, Smith EP, Potter SS. Sp4, a member of the Sp1-family of zinc finger transcription factors, is required for normal murine growth, viability, and male fertility. Developmental Biology. 1996;176:284–299. doi: 10.1006/dbio.1996.0134. [DOI] [PubMed] [Google Scholar]
- Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Syed FA, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S. Effects of loss of classical estrogen response element signaling on bone in male mice. Endocrinology. 2007;148:1902–1910. doi: 10.1210/en.2006-1165. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-α through nuclear factor-κB, and by 17β-estradiol through Sp-1 in human vascular endothelial cells. Journal of Biological Chemistry. 2000;275:25781–25790. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- Teyssier C, Belguise K, Galtier F, Cavailles V, Chalbos D. Receptor-interacting protein 140 binds c-Jun and inhibits estradiol-induced activator protein-1 activity by reversing glucocorticoid receptor-interacting protein 1 effect. Molecular Endocrinology. 2003;17:287–299. doi: 10.1210/me.2002-0324. [DOI] [PubMed] [Google Scholar]
- Tzukerman M, Zhang X-K, Pfahl M. Inhibition of estrogen receptor activity by the tumor promoter 12-O-tetradecanylphorbol-13-acetate: a molecular analysis. Molecular Endocrinology. 1991;5:1983–1992. doi: 10.1210/mend-5-12-1983. [DOI] [PubMed] [Google Scholar]
- Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. Journal of Biological Chemistry. 1994;269:16433–16442. [PubMed] [Google Scholar]
- Van der Burg B, De Groot RP, Isbrücker L, Kruijer W, De Laat SW. Stimulation of TPA-responsive element activity by a cooperative action of insulin and estrogen in human breast cancer cells. Molecular Endocrinology. 1990;4:1720–1726. doi: 10.1210/mend-4-11-1720. [DOI] [PubMed] [Google Scholar]
- Vega VB, Lin CY, Lai KS, Kong SL, Xie M, Su X, Teh HF, Thomsen JS, Yeo AL, Sung WK, Bourque G, Liu ET. Multiplatform genome-wide identification and modeling of functional human estrogen receptor binding sites. Genome Biol. 2006;7:R82. doi: 10.1186/gb-2006-7-9-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyhlidal C, Samudio I, Kladde M, Safe S. Transcriptional activation of transforming growth factor α by estradiol: requirement for both a GC-rich site and an estrogen response element half-site. Journal of Molecular Endocrinology. 2000;24:329–338. doi: 10.1677/jme.0.0240329. [DOI] [PubMed] [Google Scholar]
- Walters MR, Dutertre M, Smith CL. SKF-82958 is a subtype-selective estrogen receptor-α (ERα) agonist that induces functional interactions between ERα and AP-1. Journal of Biological Chemistry. 2002;277:1669–1679. doi: 10.1074/jbc.M109320200. [DOI] [PubMed] [Google Scholar]
- Wang F, Samudio I, Safe S. Transcriptional activation of rat creatine kinase B by 17β-estradiol in MCF-7 cells involves an estrogen responsive element and GC-rich sites. Journal of Cellular Biochemistry. 2002;84:156–172. doi: 10.1002/jcb.1276. [DOI] [PubMed] [Google Scholar]
- Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clinical Cancer Research. 2003;9:6371–6380. [PubMed] [Google Scholar]
- Wang W, Dong L, Saville B, Safe S. Transcriptional activation of E2F1 gene expression by 17β-estradiol in MCF-7 cells is regulated by NF-Y - Sp1/estrogen receptor interactions. Molecular Endocrinology. 1999;13:1373–1387. doi: 10.1210/mend.13.8.0323. [DOI] [PubMed] [Google Scholar]
- Weatherman RV, Clegg NJ, Scanlan TS. Differential SERM activation of the estrogen receptors (ERα and ERβ) at AP-1 sites. Chem. Biol. 2001;8:427–436. doi: 10.1016/s1074-5521(01)00025-4. [DOI] [PubMed] [Google Scholar]
- Weatherman RV, Scanlan TS. Unique protein determinants of the subtype-selective ligand responses of the estrogen receptors (ERα and ERβ) at AP-2 sites. Journal of Biological Chemistry. 2001;276:3827–3832. doi: 10.1074/jbc.M005414200. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Molecular Endocrinology. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson J-Å, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Molecular Endocrinology. 1999;13:1672–1685. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- Wu F, Khan S, Wu Q, Barhoumi R, Burghardt R, Safe S.Ligand structure-dependent activation of estrogen receptor α/Sp by estrogens and xenoestrogens Journal of Steroid Biochemisty and Molecular Biology 2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Duan R, Chen I, Samudio I, Safe S. Transcriptional activation of thymidylate synthase by 17β-estradiol in MCF-7 human breast cancer cells. Endocrinology. 2000;141:2439–2449. doi: 10.1210/endo.141.7.7538. [DOI] [PubMed] [Google Scholar]
- Xie W, Duan R, Safe S. Estrogen induces adenosine deaminase gene expression in MCF-7 human breast cancer cells: role of estrogen receptor-Sp1 interactions. Endocrinology. 1999;140:219–227. doi: 10.1210/endo.140.1.6394. [DOI] [PubMed] [Google Scholar]
- Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clinical Cancer Research. 2004;10:4109–4117. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- Zannetti A, Del VS, Carriero MV, Fonti R, Franco P, Botti G, D’Aiuto G, Stoppelli MP, Salvatore M. Coordinate up-regulation of Sp1 DNA-binding activity and urokinase receptor expression in breast carcinoma. Cancer Research. 2000;60:1546–1551. [PubMed] [Google Scholar]
- Zhao YL, Han WD, Li Q, Mu YM, Lu XC, Yu L, Song HJ, Li X, Lu JM, Pan CY. Mechanism of transcriptional regulation of LRP16 gene expression by 17-β estradiol in MCF-7 human breast cancer cells. Journal of Molecular Endocrinology. 2005;34:77–89. doi: 10.1677/jme.1.01628. [DOI] [PubMed] [Google Scholar]