Abstract
Background and Aims
Despite many studies of rectal cancer outcomes, no clear relationship between hospital or surgeon volume and patient outcomes has emerged for rectal cancer. We aimed to characterize the effect of hospital and surgical volume on surgery type and surgical outcomes in rectal cancer through a systematic review of the literature.
Methods
We conducted a systematic review of studies evaluating the association between hospital or surgeon volume and rectal cancer outcomes. We searched PubMed for relevant articles and reviewed 23 articles. We describe each study and report outcomes in terms of the effect of hospital or surgeon volume on the type of surgery performed, surgical complications, postoperative mortality, survival, and recurrence.
Results
Hospitals and surgeons with higher caseloads appear to perform more sphincter-preserving surgeries and have lower post-operative mortality rates. Hospital and surgeon volume appear to have no effect or a small beneficial effect on the rate of leaks, complication rate, local recurrence, overall survival, and cancer-specific survival.
Conclusions
For rectal cancer, the effects of hospital volume may be stronger for more short-term outcomes. Beyond the immediate recovery period, the effect of hospital and surgeon volume may be minimal. As more technically challenging operations, such as total mesorectal resection, become more widespread it will be important to evaluate the impact of hospital and surgeon volume on outcomes.
Introduction
Rectal cancer will affect over 41,000 people in the U.S. in 2007, the majority of whom will undergo surgery.1-3 There have been important advances in the surgery for rectal cancer that have favorably impacted quality of life and survival. More widespread use of low anterior resection has obviated the need for a colostomy for most patients. Local therapy, often accompanied by neo-adjuvant therapy has further enhanced the chances for sphincter-sparing surgery. Mesorectal resection, although not widely practiced, has been shown to improve survival.4 As is the case for other operations there is reason to suspect that the quality of rectal cancer care depends on hospital and surgeon caseloads.
Hospital and surgeon volume have been demonstrated to affect outcomes for patients with cancers that require high-risk procedures, such as pancreatic and esophageal cancers.5 When the risk is lower, as is the case for colorectal surgery, it may be more difficult to demonstrate a difference with increased surgical volume because most patients survive and few have complications. Several studies have addressed colorectal cancer surgery and found inconsistent evidence for a volume outcome relationship.6 There may be more variation in outcomes from rectal cancer surgery compared to colon cancer surgery because the operation is more difficult technically and because there are likely to be greater differences in performance across surgeons and hospitals. Despite many studies of rectal cancer outcomes, no clear relationship between hospital or surgeon volume and patient outcomes has emerged for rectal cancer alone.
The purpose of this systematic review of the literature was to determine whether hospital and surgeon volume influence the type of surgery performed and outcomes of surgery for rectal cancer. A rigorous qualitative approach was optimal considering the heterogeneity of the outcomes measures. Systematic reviews can elucidate clear patterns of results in the absence of more quantitative data analysis. We hypothesized that hospitals and surgeons with higher volumes, and therefore more experience, would have higher rates of sphincter-sparing surgery, fewer local complications and better survival than lower volume hospitals.
Methods
Search strategy
We searched the MEDLINE database through April 2007 for all English-language articles using the following search strategy:
((colorectal cancer) OR (cancer AND (rectum OR colorectal OR rectal))) AND surgery AND (treatment outcome OR outcome* OR quality OR adverse OR treatment failure OR length of stay OR mortality OR survival OR recurrence OR intraoperative complications OR postoperative complications)
AND (caseload* OR workload OR ((hospital OR surgeon OR surgery) AND volume))
One authors (TS) reviewed abstracts of possibly relevant titles, and then reviewed possibly relevant manuscripts along with the reference list of included articles or review articles for additional relevant articles. The type of outcome was not limited in advance.
Inclusion criteria
We required that studies include results for rectal cancer patients and that all studies report original data for which bivariate or multivariate results were reported. Studies reporting results without showing effect sizes were included.
Exclusion criteria
We excluded articles for which results for rectal cancer could not be distinguished from larger patient groups, such as articles where cancers of the colon and rectum were aggregated.
Exposures
The number of cases per hospital (hospital volume) or the number of cases per surgeon (surgeon volume) had to be stated.
Measures and outcomes
Because hospital and surgeon volume may be associated with sphincter-sparing surgery, we sought information on type of surgery. Similarly, we included data on use of radiation therapy. The outcomes of interest were short- and long-term surgical outcomes including complications, length of stay, mortality, survival, and recurrence rates.
Quality assessment
Although we did not formally rate the quality of reports, we recorded and present information on variables that may reflect the quality of reporting. The variables included study design (retrospective or prospective), recency of data collection, data source, sample size, and inclusion of important prognostic factors in multivariate analyses.
Analysis
We constructed an evidence table (Table) that described each study in terms of volume (hospital volume, surgeon volume, or both), treatment type, and short- and long-term outcomes. Statistical significance was specified at a level of α = 0.05. We included summary statistics for significant effect sizes, if they were reported in the study. Statistical significance was reported from each study, even if significance was for a trend rather than a reported effect size. In studies where hospital or surgeon volume was measured categorically, the table shows the mean number of cases per year that define high and low volumes. Comparisons between the highest and lowest volume groups were reported. If both bivariate and multivariate results were reported, only multivariate results were included in this review. Outcomes for which only bivariate analyses were reported are noted in the summary table. Because neoadjuvant and adjuvant therapies are potentially important prognostic factors, outcomes for which these therapies were controlled are noted in the summary table.
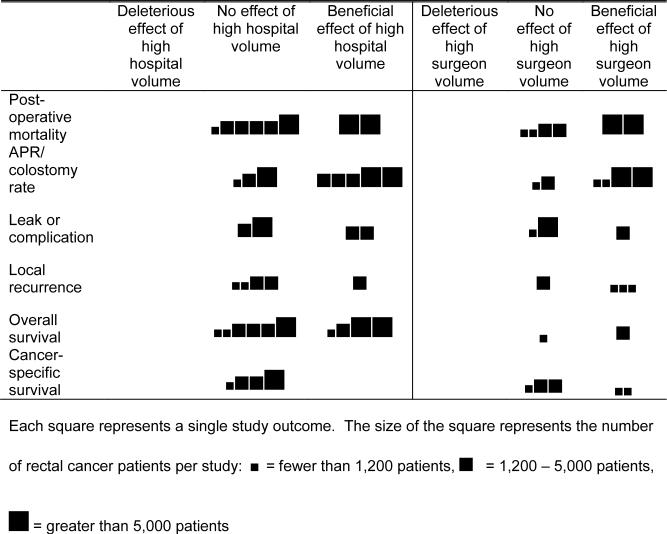
We constructed a figure in order to provide a graphical representation of the effect of hospital and surgeon volume on outcome. Individual studies were categorized into tertiles based on the number of rectal cancer patients included in each study. Similar outcomes were aggregated to simplify the results. For example, post-operative mortality and in-hospital mortality were grouped as post-operative mortality. Cancer-specific survival, relative survival, and disease-specific mortality were grouped as cancer-specific survival. Each finding was categorized as a negative, neutral, or beneficial effect of volume on that outcome,
Figure.
Effect of hospital and surgeon volume on various outcome measures, arranged by study size tertiles.
The original search yielded 526 articles, of which 41 addressed colon or rectal cancer. We found thirteen additional articles from the reference sections of other studies or review articles. After removing articles that did not include rectal cancer outcomes separate from colon cancer outcomes, 22 studies remained. Eleven studies addressed the influence of hospital volume, six studies addressed the influence of surgeon volume, and five studies addressed both.
Results
This review included 5,984,195 patients from eight North American and European countries over the years 1979 − 2002. The definition of rectal cancer varied. In most cases rectal cancer was defined by ICD code. In six studies rectal cancer was defined as cancers located a maximum of 12 to 16 cm from the anal verge.
Type of surgery performed
Twelve studies reported the type of surgery performed.7-18 The operations were categorized as either sphincter-sparing procedures (SSP), which included low anterior resection and local excision, or abdominoperineal resections (APR), which included permanent colostomy or ostomy. Low anterior resections yielding temporary colostomies were considered SSPs.
Of the nine studies that measured hospital volume and surgery type, five found a positive association between higher hospital volume and higher rate of sphincter-sparing procedures.9-12, 16 The significant odds ratios were 0.44, 0.55, and 0.73.9, 10, 12 Significant differences between APR rates for high and low volume hospitals were reported as 26.4% vs. 29.8% for high and low volume, respectively, in one study, and 47% vs. 49% for high and low volume hospitals, respectively, in another study.11, 16 Three studies showed no effect of hospital volume on type of surgery performed.8, 15, 17 A final study showed a decreasing trend of APR rates for lower volume hospitals, but no analyses were done on the data to establish an effect size or statistical significance. It is counted as no effect for this review.7
Four of the six studies that measured surgeon volume found a positive relationship between higher surgeon volume and higher rate of sphincter-sparing procedures.13-15, 19 Significant odds ratios ranged from 0.22 to 0.70, and one study demonstrated APR rates of 38.9% for low volume surgeons and 49.2% for high volume surgeons13-15, 19 The remaining two studies of surgeon volume showed no effect of surgeon volume on the type of surgery performed.8, 18
A tenth study reported the effect of hospital volume and number of anterior resections, abdominoperineal resections, and Hartmann's procedures, and while a significant trend was found, no post-hoc analyses identified the direction of this effect.20
The ten positive associations found between volume (either hospital or surgeon) and rate of sphincter-sparing procedures, and the lack of negative associations, suggest that higher volume hospitals and higher volume surgeons perform more sphincter-sparing procedures.
Surgical complications
Surgical complications were measured in six studies, and the type of complications varied by study.9, 11, 18-21 Anastomotic leak was identified as a complication in all six studies.9, 11, 18-21 Other types of complications were included in some studies, as described below.
Four studies of surgical complications investigated the influence of hospital volume. 9, 11, 20, 21 One study described multiple intraoperative complications, including iatrogenic perforation; hemorrhage; ureteral injury; injury to spleen, bladder, or neighboring organs; and complications associated with pneumoperitoneum.11 In the same study, general and specific complications were measured, but not defined. This study found an association of higher hospital volume and fewer complications on all outcomes except general complications; this was counted as a single overall beneficial effect of higher hospital volume for this analysis.11 Two studies showed an association between higher hospital volume and lower complication rate. 11, 20 Both studies used bivariate analyses. The percentages of complication rates in high vs. low hospitals that were significant for trend were 13.6% vs. 18.5% for intraoperative complications, 29.8% vs. 34.5% for postoperative complications, 13.0% vs. 11.2% for anastomotic leaks in one study, and 7.3% vs. 8.3% for anastomotic leaks in the other study. 11, 20 The two other studies, which used multivariate analyses, showed no effect of hospital volume on complication, where complications included wound infection in one study 21 and anastomotic leak in both studies 9, 21. Because these findings were mixed, it is difficult to conclude any consistent effect. However, the better conducted studies using multivariate analyses found no effect, suggesting that there is no effect of hospital volume on complication rates.
One of the three studies measuring the effect of surgical volume on complication rates reported a relationship between higher surgeon volume and lower complication rate (OR = 0.7).21 The other two studies found no effect.18, 19
Recurrence risk
Local recurrence rate was assessed in seven studies.12, 13, 18, 20-23 The actual definition of local recurrence (recurrence in the pelvis) was only stated in three studies.13, 20, 22 One study measured cancer recurrence risk in any site.12 Another study measured distant metastasis.18
Of the five studies measuring the association of hospital volume with local recurrence rate, three studies found no effect 21-23. Two studies found that higher hospital volume was associated with lower local recurrence risk, with an odds ratio of 0.91 in one study 12 and a hazard ratio of 0.5 in the other.20 Any effect of hospital volume on local recurrence rate, if an effect exists, appears small.
Four studies assessed the role of surgeon volume on local recurrence rates.13, 18, 21, 23 One study reported no association between surgeon volume and local recurrence 21, and the other three studies found that higher volume surgeons had lower local recurrence 13, 18, 23. The significant hazard ratios for local recurrence were 0.42 18 and 0.56 13. In the studies showing only percentages, the statistically significant rates of local recurrence were 11% vs. 17% for high and low volume surgeons, respectively.23
The study that measured risk for recurrence at any site and the study that measured distant metastasis found no effect of hospital volume.12, 18
Mortality
Thirty-day post-operative mortality was identified in eight studies.8-10, 13, 18-21, 24 Three additional studies measured in-hospital mortality.11, 17, 25 One study measured two-year mortality.8
For this analysis, thirty-day and in-hospital mortality are grouped together as postoperative mortality to reflect short-term mortality after surgery. Eight studies measured the relationship between hospital volume and postoperative mortality. Two studies found that high volume was associated with low postoperative mortality, with odds ratios of 0.92 25 and 0.38 10. The remaining six studies showed no effect of hospital volume.8, 9, 11, 17, 20, 21 Taken together, the eight studies showed a positive effect for the two larger studies, suggesting that a small protective effect on mortality may be conferred on patients at higher volume hospitals that may only be detected in larger studies.
Six studies evaluated the effect of surgeon volume on post-operative mortality.8, 13, 18, 19, 21, 25 Four studies found no effect of surgeon volume 8, 13, 18, 21, and two studies found that higher volume surgeons had lower mortality rates, with odds ratios of 0.87 25 and 0.58 19.
The study measuring two-year mortality found no effect of hospital volume, but found that higher surgeon volume resulted in lower two-year mortality (24% in high volume hospitals vs. 34% in high volume hospitals).8
Survival
Fifteen studies reported survival using proportional hazards models.8-10, 12, 13, 16-18, 20-23, 26-29 The time period of survival analysis outcomes was assessed for two years in one study 22, and at least five years in the remaining studies.
Overall survival, independent of cause of death, was measured in ten studies.8-10, 12, 16, 17, 20, 22, 23, 27 Of the ten studies of overall survival, an association between higher hospital volume and higher overall survival was found in four studies, with odds ratios ranging from 1.09 to 1.28. 10, 17, 20, 27 The remaining six studies found no association 8, 9, 12, 16, 22, 23. The small effect sizes and the preponderance of null results suggests no effect of hospital volume on overall survival. For the two studies of surgeon volume, one study found a positive relationship between higher surgeon volume and longer survival (RR = 1.35) 8, and one study found no significant relationship 23.
Two studies calculated relative survival, comparing observed survival to expected survival of people with similar demographic characteristics.12, 22, 28 One study measured cancer-specific survival, with an endpoint of local recurrence or death from rectal cancer 13, while three others measured disease-specific survival, with an endpoint of death from rectal cancer 18, 21, 29. One study measured disease-free survival with an endpoint of local tumor recurrence, a second primary colorectal cancer, or death from any cause. Although relative survival, cancer-specific survival, disease-specific survival, and disease-free survival, are defined somewhat differently in the included studies, they were evaluated as relative survival in this analysis, because they all generally reflect survival with death from cancer as the outcome. The four studies of hospital volume showed no effect of hospital volume on relative survival.12, 21, 22, 28
Two of the five studies of surgeon volume found a positive effect of volume and relative survival, with hazard ratios for survival of 1.4 and 1.89. 13, 18 Three studies of surgeon volume and relative survival showed no effect 21, 26, 29. This pattern suggests that neither hospital nor surgeon volume influences relative survival.
Other findings
Adjuvant therapy use was reported as an outcome in only one study, which investigated the association between surgeon volume and rates of radiation therapy utilization and found no effect of volume on use of adjuvant therapy.15 One study measured length of stay after surgery for rectal cancer and found that higher volume hospitals were associated with shorter lengths of stay.11 A study investigated surgeon caseload and rates of reoperation and found no effect.18
Possible moderators
Some study-level variables may affect the influence of hospital and surgeon volume on surgery type and surgical outcomes. We collected data on location of study, recency of data collection completion, size of the study, and reporting of only bivariate effects. We found no apparent pattern between the location of the study and any of the associations found in this review. We also found no pattern with regard to recency of data collection completion.
Across all studies, studies in the largest tertile (>6,000 patients) generally showed stronger associations between hospital volume and various outcomes. The same pattern appeared for studies of surgeon volume, although the studies of surgeon volume are fewer and smaller. Bivariate analyses generally showed a more beneficial effect of both hospital and surgeon volume than did multivariate analyses.
Discussion
Hospital volume and surgeon volume have been shown to influence patient care in some cancers. For rectal cancer, the volume-outcomes relationship appears to be small but positive. This systematic review of 22 published studies of rectal cancer surgery type and outcomes found that across all studies, high hospital volume and high surgeon volume have either a beneficial or neutral effect on patient care and outcomes.
Because the larger studies more often showed a beneficial effect of high hospital or surgeon volume than the smaller studies, especially for short-term outcomes such as post-operative mortality and colostomy rate, the positive effects of high hospital and surgeon volume may be too small to detect in all but the larger studies. The studies also may have lacked sufficient power to demonstrate clinically important differences, particularly because many of the outcomes we investigated were uncommon. None of the studies found a detrimental effect of high hospital or surgeon volume. If there were truly no effect of hospital or surgeon volume, one would expect some studies to demonstrate a detrimental effect of hospital or surgeon volume as part of a normal variation of findings.
For hospital volume, the evidence suggests that high volume hospitals perform more sphincter-sparing procedures and have lower post-operative mortality, even if these effects are small. It is less clear that higher hospital volume has any effect on anastomotic leaks, complications, or overall survival, for which findings were mixed between neutral and beneficial. Hospital volume does not appear to influence cancer-specific survival. Most of the studies were performed before total mesorectal excision was developed. The impact of this surgery on short and long term outcomes is not reflected in this review.
Studies of surgeon volume demonstrate the same effects. There are fewer studies of surgeon volume, and the studies are generally smaller than those of hospital volume, but higher surgeon volume is always associated with either no effect or a positive effect on patient care and outcomes.
Halm et al. proposed that hospital and surgeon volume have little effect on procedures that are less risky.5 Rectal cancer surgery is more complicated than colon cancer surgery, where minimal effects for hospital and surgeon volume have been found.6 However, rectal cancer surgery is less complicated than pancreatic or esophageal cancer surgery, where dramatic effects of hospital and surgeon volume are seen. For less technically complicated cancer surgeries, such as those required for rectal cancer, perhaps any effect of hospital volume is stronger for more short-term outcomes, as we found in this review. Beyond the immediate recovery period, the effect of the success of surgery may be minimal. However, surgeries for rectal cancer are becoming more technically complicated, and with these more complicated procedures, such as total mesorectal excision, we may see more dramatic effects of surgeon and hospital volume on surgical outcomes. Although total mesorectal excision may improve outcomes, we hypothesize that the benefits may be greater when performed in higher volume hospitals or by surgeons with larger caseloads.
This systematic review of the literature has limitations. First, because of the heterogeneous outcomes and measures, we were unable to perform a more quantitative meta-analysis. However, this systematic review presents a qualitative analysis that describes a general pattern of the volume-outcomes relationship. The studies in this review include diverse definitions of high and low hospital and surgeon volume. They also represent different parts of the world and widely varying data sources. Data were collected over different time periods, starting from as far back as 1979 to as recently as 1999, and surgical procedures have changed significantly over this time period.
The quality of these studies also varied greatly. Six studies had sample sizes below 1000 patients, which may limit the power to detect small effects. Many findings arose from bivariate analyses, not adjusting for clinical and demographic factors in the analyses. Sixteen of the studies looked at surgeon or hospital volume alone, without investigating the interaction between surgeons and hospitals. Only five studies controlled for clustering between surgeons or between hospitals.
Despite the variation in study design and quality, a clear pattern of the effect of hospital and surgeon volume on rectal cancer treatment and outcomes emerges from this systematic review. Hospitals and surgeons with higher caseloads appear to perform more sphincter-preserving surgeries and have lower post-operative mortality rates. Hospital and surgeon volume appear to have no effect or a small beneficial effect on the rate of leaks, complication rate, local recurrence, overall survival, and cancer-specific survival. As more technically challenging operations, such as mesorectal resection, become more widespread it will be important to evaluate the impact of hospital and surgeon volume on outcomes.
Source of support
Supported in part by a grant from the National Institutes of Health U01 CA93326
Footnotes
The authors declare no conflict of interest.
Contributor Information
Talya Salz, Department of Health Policy and Administration University of North Carolina Chapel Hill, NC 27599−7411 Phone: 919−824−2643 Fax: none email: talya@unc.edu.
Robert S. Sandler, Division of Gastroenterology and Hepatology CB# 7555, 4157 Bioinformatics Building University of North Carolina Chapel Hill, NC 27599−7555.
References
- 1.National Cancer Data Base. American College of Surgeons; 2007. [Google Scholar]
- 2. [2007];Colon and rectal cancer. 2007 Available from: http://www.cancer.gov/cancertopics/types/colon-and-rectal.
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Bulow S, Christensen IJ, Harling H, et al. Recurrence and survival after mesorectal excision for rectal cancer. Br J Surg. 2003;90(8):974–80. doi: 10.1002/bjs.4137. [DOI] [PubMed] [Google Scholar]
- 5.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93(7):501–15. doi: 10.1093/jnci/93.7.501. [DOI] [PubMed] [Google Scholar]
- 7.Beart RW, Steele GD, Jr., Menck HR, et al. Management and survival of patients with adenocarcinoma of the colon and rectum: a national survey of the Commission on Cancer. J Am Coll Surg. 1995;181(3):225–36. [PubMed] [Google Scholar]
- 8.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236(5):583–92. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harling H, Bulow S, Moller LN, et al. Hospital volume and outcome of rectal cancer surgery in Denmark 1994−99. Colorectal Dis. 2005;7(1):90–5. doi: 10.1111/j.1463-1318.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson DC, Zhang W, Zaslavsky AM, et al. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst. 2003;95(10):708–16. doi: 10.1093/jnci/95.10.708. [DOI] [PubMed] [Google Scholar]
- 11.Marusch F, Koch A, Schmidt U, et al. Hospital caseload and the results achieved in patients with rectal cancer. Br J Surg. 2001;88(10):1397–402. doi: 10.1046/j.0007-1323.2001.01873.x. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: findings from the Intergroup 0114 Study. J Clin Oncol. 2004;22(1):166–74. doi: 10.1200/JCO.2004.04.172. [DOI] [PubMed] [Google Scholar]
- 13.Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg. 1998;227(2):157–67. doi: 10.1097/00000658-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purves H, Pietrobon R, Hervey S, et al. Relationship between surgeon caseload and sphincter preservation in patients with rectal cancer. Dis Colon Rectum. 2005;48(2):195–202. doi: 10.1007/s10350-004-0793-7. discussion 202−4. [DOI] [PubMed] [Google Scholar]
- 15.Rogers SO, Jr., Wolf RE, Zaslavsky AM, et al. Relation of surgeon and hospital volume to processes and outcomes of colorectal cancer surgery. Ann Surg. 2006;244(6):1003–11. doi: 10.1097/01.sla.0000231759.10432.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons AJ, Ker R, Groshen S, et al. Variations in treatment of rectal cancer: the influence of hospital type and caseload. Dis Colon Rectum. 1997;40(6):641–6. doi: 10.1007/BF02140891. [DOI] [PubMed] [Google Scholar]
- 17.Simunovic M, To T, Baxter N, et al. Hospital procedure volume and teaching status do not influence treatment and outcome measures of rectal cancer surgery in a large general population. J Gastrointest Surg. 2000;4(3):324–30. doi: 10.1016/s1091-255x(00)80083-9. [DOI] [PubMed] [Google Scholar]
- 18.Martling A, Cedermark B, Johansson H, et al. The surgeon as a prognostic factor after the introduction of total mesorectal excision in the treatment of rectal cancer. Br J Surg. 2002;89(8):1008–13. doi: 10.1046/j.1365-2168.2002.02151.x. [DOI] [PubMed] [Google Scholar]
- 19.Borowski DW, Kelly SB, Bradburn DM, et al. Impact of surgeon volume and specialization on short-term outcomes in colorectal cancer surgery. Br J Surg. 2007;94(7):880–9. doi: 10.1002/bjs.5721. [DOI] [PubMed] [Google Scholar]
- 20.Wibe A, Eriksen MT, Syse A, et al. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg. 2005;92(2):217–24. doi: 10.1002/bjs.4821. [DOI] [PubMed] [Google Scholar]
- 21.Holm T, Johansson H, Cedermark B, et al. Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg. 1997;84(5):657–63. [PubMed] [Google Scholar]
- 22.Engel J, Kerr J, Eckel R, et al. Influence of hospital volume on local recurrence and survival in a population sample of rectal cancer patients. Eur J Surg Oncol. 2005;31(5):512–20. doi: 10.1016/j.ejso.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Stocchi L, Nelson H, Sargent DJ, et al. Impact of surgical and pathologic variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol. 2001;19(18):3895–902. doi: 10.1200/JCO.2001.19.18.3895. [DOI] [PubMed] [Google Scholar]
- 24.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 25.Ho V, Heslin MJ, Yun H, et al. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13(6):851–8. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 26.McArdle CS, Hole DJ. Influence of volume and specialization on survival following surgery for colorectal cancer. Br J Surg. 2004;91(5):610–7. doi: 10.1002/bjs.4476. [DOI] [PubMed] [Google Scholar]
- 27.Rabeneck L, Davila JA, Thompson M, et al. Surgical volume and long-term survival following surgery for colorectal cancer in the Veterans Affairs Health-Care System. Am J Gastroenterol. 2004;99(4):668–75. doi: 10.1111/j.1572-0241.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- 28.Jessup JM, Stewart AK, Menck HR. The National Cancer Data Base report on patterns of care for adenocarcinoma of the rectum, 1985−95. Cancer. 1998;83(11):2408–18. doi: 10.1002/(sici)1097-0142(19981201)83:11<2408::aid-cncr22>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Hermanek P, Mansmann U, Staimmer DS, et al. The German experience: the surgeon as a prognostic factor in colon and rectal cancer surgery. Surg Oncol Clin N Am. 2000;9(1):33–49. vi. [PubMed] [Google Scholar]