Abstract
Background
It has become increasingly clear that the follicular microenvironment of the maturing human oocyte is a determining factor for the implantation potential of an embryo deriving from that oocyte. Indeed the quality and maturity of an oocyte are influenced by the level of intrafollicular oxygen content which, in turn, is proportional to the degree of follicular vascularity. The aim of the study was to establish whether there is a relationship between follicular fluid VEGF concentrations, perifollicular vascularity and reproductive outcome in normal responders under the age of 35 undergoing IVF.
Materials and methods
Sixty-one consecutive patients, all at their first IVF cycle, were included in the study. All patients had primary infertility due to male factor or tubal factor. At oocyte retrieval, the perifollicular vascularity of two follicles per ovary was estimated qualitatively through power Doppler blood flow, for a total of two hundred forty-four follicles. The follicular fluid from the identified follicles was centrifuged and stored until VEGF assay. The maturity and fertilization rate of the corresponding oocytes as well as embryo quality and pregnancy rate were recorded.
Results
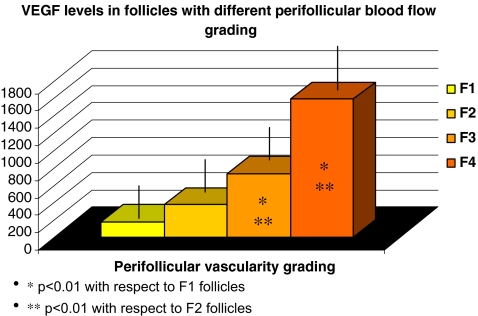
In our study, we found VEGF levels to be significantly correlated with grade of perifollicular vascularity. Oocytes obtained from follicles with the higher grade of vascularization also showed a higher rate of fertilization, embryos, a better quality and higher pregnancy rates were obtained in women with highly vascularized follicles. Perifollicular blood flow doppler indices seem to predict oocyte viability and quality. Moreover, VEGF may play a potential role in the development of the perifollicular capillary network.
Discussion
The ability of a given follicle to express VEGF and develop an adequate vascular network may be inter-related in patients under the age of 35. An adequate blood supply may be fundamental important in the regulation of intrafollicular oxygen levels and the determination of oocyte quality.
Keywords: VEGF, Perifollicular blood flow, IVF
Introduction
The quality of an oocyte is one of the determining factors of embryo quality. It has become increasingly clear that the follicular microenvironment of a human oocyte is a crucial factor for its developmental competence [1]. Indeed the quality and maturity of an oocyte is influenced by the intrafollicular level of oxygen content which, in turn, is proportional to the degree of follicular vascularity [2]. The development of an adequate capillary network seems to depend at least in part on the action of vascular endothelial growth factor (VEGF). VEGF is produced by follicular granulosa, thecal cells [3]. This growth factor plays a central role in the regulation of angiogenetic processes in the ovary and is critical for the growth of the ovarian follicle [4]. In particular, during folliculogenesis, VEGF secretion, which is induced by gonadotropins, determines the formation of a vascular network in the thecal cell layer of the follicle [4, 5]. Indeed, VEGF is detectable in ovarian follicular fluid [6]. VEGF also increases vascular permeability, thus allowing the delivery of cholesterol for steroid synthesis. Indeed, in growing follicles, VEGF and estradiol levels increase in a parallel manner [7].
Van Blerkom et al. [1] reported the occurrence of significant defects in spindle organization, cytoplasmic structure and chromosome number more frequently in oocytes that developed in conditions of hypoxia (<3% follicular fluid dissolved oxygen content) than in oocytes exposed to follicular O2 levels ≥3%. Oocytes originating from poorly vascularized follicles, once fertilized, showed a reduced capacity to progress to the 6–8 cell embryo stage. Although the author found no direct correlation between follicular fluid VEGF and oxygen content, VEGF levels were consistently higher in follicles with a percentage of dissolved oxygen >3%.
On the basis of these findings, we aimed at establishing whether there is a relationship between follicular fluid VEGF concentrations, perifollicular vascularity and reproductive outcome in normal responders undergoing IVF.
Materials and methods
The present study was approved by the Ethical Committee of the University of Pisa and was carried out according to rules of good clinical practice. Informed consent was obtained from each patient.
Subjects
In a prospective observational study, we enrolled sixty-one consecutive patients under 35 years of age between January 2006 and January 2007 at the Centre of Reproductive Pathophysiology of the Pisa University Hospital. All patients were at their first IVF or IVF with intracytoplasmic sperm injection (ICSI) and embryo transfer cycle. All patients had primary infertility due to male factor or tubal factor. Only normal responders to controlled ovarian hyperstimulation, i.e. presenting a number of follicles ≥3 were enrolled in the study. Patients with endometriosis or polycystic ovary syndrome were excluded because it is well known that these patients have higher intrafollicular VEGF levels than other infertile patients [8, 9]. Patients aged more than 35 years were excluded as it has been reported that older patients may have higher intrafollicular VEGF levels than the younger patients [10].
Treatment protocol
Controlled ovarian stimulation was carried out with 2 to 6 ampoules/day, according to basal FSH levels and age, of recombinant FSH (Gonal F®, Serono, Italy) after a pre-treatment with oral contraceptives. All patients were administered cetrorelix (Cetrotide®, Serono, Italy), a GnRH antagonist, according to a personalized regimen, i.e. when the lead follicle reached 14 mm in diameter, to prevent premature ovulation. Recombinant HCG (Ovitrelle®, Serono, Italy) was administered when at least 2 follicles reached a mean diameter of 18 mm. After approximately 36 hours, transvaginal follicular aspiration was performed for oocyte retrieval.
The follicular fluid from the studied follicles was centrifuged and stored at-20°C until VEGF assay. The maturity and eventual fertilization of the individual corresponding oocytes were recorded as well as the quality of the embryos deriving from those oocytes according to the method described by Veeck LL [11] and pregnancy rates in women with a given perifollicular blood flow grading. IVF or IVF-ICSI and embryo transfer were performed as appropriate.
Evaluation of perifollicular blood flow
The perifollicular vascularity of two follicles per ovary was estimated immediately prior to oocyte retrieval through power Doppler blood flow analysis (GE medical system Logic). Follicles were graded according to the percentage of follicular circumference in which most flow was identified from a single cross-sectional slice as described by Chui DKC et al [12]. The grading system was as follows: <25% follicular circumference in which blood flow was identified (F1), 26–50% (F2), 51–75% (F3), 76–100% (F4).
VEGF assay
VEGF follicular fluid levels were measured by ELISA (Endogen® Human VEGF ELISA Kit, Pierce Biotechnology, Inc., Rockford). Sensitivity was <8.0 pg/ml, intraassay and interassay coefficients of variation were 8.9% and 9.8%, respectively.
Statistical analysis
Results are expressed as mean ± SD. Between-group differences were evaluated by means of Student T-test, while between-group comparison of percentage values was carried by χ2-test. The correlation between perifollicular vascularity and VEGF levels was verified by means of Pearson's method. A p value of <0.05 was considered statistically significant.
Results
A total of 244 follicles were studied in 61 patients. Of the 244 evaluated follicles, 46 were graded as F1, 74 as F2, 60 as F3 and 64 as F4. There was no significant difference in follicular size. In our study, we found VEGF levels to be significantly correlated with grade of perifollicular vascularity (Pearson correlation index 0.85). There was a significant difference in VEGF levels between groups of follicles with different grading (Fig. 1). All patients showed a homogeneous vascularity grading, i.e. either F1 and F2 follicles or F3 and F4 follicles upon power Doppler evaluation. For this reason and to interpret clinical data (Table 1), follicles were classified as low grade (F1/F2) or high grade (F3/F4). There was no statistically significant mean age difference between women presenting a prevalence of high grade follicles and those with low grade follicles (33.3 ± 0.65 vs 33.3 ± 1.67, respectively). IVF was performed on 154 oocytes and IVF-ICSI was performed on 90 oocytes. Statistical analysis revealed no difference in ICSI percentage rate between the two groups. However, interestingly, oocytes obtained from follicles with the higher grade of vascularization (F3/F4), and with the higher levels of VEGF, also showed a higher rate of fertilization. Moreover, the percentage of grade A embryos was greater when oocytes deriving from highly vascularized follicles (F3/F4) were used. No triploid embryos were recorded. Finally, the pregnancy rate per embryo transfer was higher in women with embryos deriving from follicles with a high grading (F3/F4). See Table 1.
Fig. 1.
VEGF levels in follicles with different perifollicular blood flow grading
Table 1.
| F1/F2 follicles | F3/F4 follicles | |
|---|---|---|
| Age | 33.3 ± 0,65 | 33,3 ± 1,67 |
| Number of evaluated follicles | 120 | 124 |
| Mature oocytes | 80,0% | 84,2% |
| Fertilization rate | 73,6% | 90,0%* |
| Grade A embryos | 41,0% | 64,7%* |
| Pregnancy rate per embryo transfer | 21,1% | 37.5%* |
| ICSI | 30.0% | 37.5% |
* p < 0.05 with respect to F1/F2 follicles
Discussion
It is known that the most common factors responsible for implantation failure of embryos in patients undergoing IVF are oocyte cytoplasmic or chromosomal defects [13]. Oocytes are very sensitive to hypoxic damage [1]. A sufficient supply of oxygen seems to be mandatory for the formation and stability of the meiotic spindle and the development of a given embryo [1]. In light of the fact that the oocyte depends on the diffusion of oxygen particles from the thecal microvasculature through the follicular basement membrane granulosa cells, follicular fluid and zona pellucida, the ability of a given follicle to develop an adequate vascular network is crucial. An adequate blood supply is of fundamental importance in the regulation of intrafollicular oxygen levels, provision of growth factors, nutritional factors and gonadotropins and the determination of oocyte quality.
Chui and Bhal have demonstrated a strong association between development of perifollicular vascularity and ability of an embryo to implant [12, 14]. Our data confirm these findings in that we found that, in age-matched women, oocytes deriving from highly vascularized follicles presented a higher fecundibility. Moreover, a significantly higher percentage of grade A embryos was obtained in patients with highly vascularized (F3/F4). No difference in oocyte maturity was found between F1/2 follicles and F3/4 follicles. Indeed, as reported by other authors [15], morphological criteria are not always predicitve of oocyte quality. It is likely that a greater perifollicular vascularity determines better cytoplasmic biochemical conditions, that are not evaluable with routine parameters of oocyte quality. Indeed Van Blerkom et al. observed differences in ATP content in morphologically equivalent mature oocytes obtained from the same ovary in IVF cycles [16]. The aim of our study study was not to establish a cause-effect but high VEGF levels could be a surrogate for better blood flow. In our study a direct correlation emerged between follicular fluid VEGF concentrations and perifollcilaur vascularity grading, that is the higher the grading, the higher the VEGF levels. We believe that this correlation is highly specific because, for the first time, follicular fluid VEGF measurements were performed on follicles analysed individually by power Doppler blood flow quantitation. Since VEGF is secreted from granulosa and thecal cells, it is likely that higher VEGF levels reflect a greater ability of the follicle to create its own vascular network and therefore guarantee a better follicular microenvironment for the developing oocyte. Therefore the ability of a given follicle to express VEGF, on the one hand, and to develop a vascular network, on the other hand, seem strongly correlated. There are evidences, not specific to VEGF, that the oocyte can actively influence follicular development and control its environmental conditions [17]. At least hypothetically, the quality of an oocyte, a follicle and its vascular network are correlated and therefore perifollicular vascularization and follicular VEGF levels may be valid indirect markers of oocyte quality.
In conclusion, the present study confirms that perifollicular blood flow power Doppler analysis seems to predict oocyte viability and fecundibility and, consequently, embryo quality. Moreover, VEGF may play a potential role in the development of the perifollicular capillary network and may be a marker of the quality of the follicular microenvironment.
References
- 1.Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–55. [DOI] [PubMed]
- 2.Van Blerkom J. The influence of intrinsic and extrinsic factors on the developmental potential of chromosomal normality of the human oocyte. J Soc Gyn Invest. 1996;3:3–11. [DOI] [PubMed]
- 3.Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol. 1995;146:157–65. [PMC free article] [PubMed]
- 4.Lam PM, Haines C. Vascular endothelial growth factor plays more than an angiogenic role in the female reproductive system. Fertil Steril. 2005;84:1775–8. [DOI] [PubMed]
- 5.Gordon JD, Mesiano S, Zaloudek CJ, Jaffe RB. Vascular endothelial growth factor localization in human ovary and fallopian tubes: possible role in reproductive function and ovarian cyst formation. J Clin Endocrinol Metab. 1996;81:353–9. [DOI] [PubMed]
- 6.Moncayo HE, Penz-Koza A, Marth C, Gastl G, Herold M, Moncayo R. Vascular endothelial growth factor serum and in the follicular fluid of patients undergoing hormonal stimulation for in-vitro fertilization. Hum Reprod. 1998;13:3310–4. [DOI] [PubMed]
- 7.Mattioli M, Barboni B, Turriani M, Galeati G, Zannoni A, Castellani G, Berardinelli P, Scapolo PA. Follicle activation involves vascular endothelial growth factor production and increased blood vessel extension. Biol Reprod. 2001;65:1014–9. [DOI] [PubMed]
- 8.Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the female reproductive organs: pathological implications. Int J Exp Pathol. 2002;83:151–63. [DOI] [PMC free article] [PubMed]
- 9.Artini PG, Monti M, Matteucci C, Valentino V, Cristello F, Genazzani AR. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol Endocrinol. 2006;22:465–70. [DOI] [PubMed]
- 10.Friedman CI, Danforth DR, Herbosa-Encarnacion C, Arbogast L, Alak BM, Seifer DB. Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril. 1997;68(4):607–12. Oct. [DOI] [PubMed]
- 11.Veeck LL. The morphological estimation of mature oocytes and their preparation for insemination. In: Jones HW Jr, Jones GS, Hodgen GD, Rosenwaks Z, editors. In vitro fertilization-Norfolk. Baltimore: Williams and Wilkins; 1986.
- 12.Chui DKC, Phugh ND, Walzer SM, Gregory L, Shaw R. Follicular vascularity-the predictive value of transvaginal power Doppler ultrasonography in an in-vitro fertilization programme: a preliminary study. Hum Reprod. 1997;12:191–6. [DOI] [PubMed]
- 13.Gaulden ME. Maternal age effect: the enigma of Down syndrome and other trisomic conditions. Mutat Res. 1992;296:69–88. [DOI] [PubMed]
- 14.Bhal PS, Pugh ND, Chui DK, Gregory L, Walzer SM, Shaw RW. The use of transvaginal power Doppler ultrasonography to evaluate the relationship between perifollicular vascularity and outcome in in-vitro fertilization treatment cycles. Hum Reprod. 1999;14:939–45. [DOI] [PubMed]
- 15.Coticchio G, Sereni E, Serrao L, Mazzone S, Iadarola I, Borini A. What criteria for the definition of oocyte qualità? Ann NY Acad Sci. 2004;1034:132–44. [DOI] [PubMed]
- 16.Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24. [DOI] [PubMed]
- 17.Gosden RG. Oogenesis as a foundation for embryogenesis. Mol Cell Endocrinol. 2002;186:149–53. [DOI] [PubMed]