Abstract
Introduction
Cytogenetic heteromorphisms are described as heritable variations at specific chromosomal regions without a proven impact on phenotype.
Materials and methods
We compared the presence of chromosome heteromorphisms in the karyotypes of two patient groups. The first group of patients consisted of 276 individuals of 138 infertile couples. The second group, consisted of 1,130 amniocentesis samples. This group was considered to be a sample of the fertile population, as the fetus being karyotyped is the result of a spontaneous pregnancy. Fetal karyotyping was made due to the standard indications for prenatal diagnosis, such as abnormal maternal serum screening results.
Results and discussion
Eighteen infertile patients (6.52%) and twenty fetuses (1.77%) were found to have chromosome heteromorphisms. The difference between the two groups was statistically significant (p < 0.0001).
Conclusion
These results are consistent with other similar studies that suggest the yet undefined relationship between chromosome heteromorphisms and infertility.
Keywords: Chromosome heteromorphism, Infertility
Introduction
Certain regions in the genome are subject to heteromorphisms due to their repetitive DNA content. Chromosome localizations of these regions may be identified by several methods. Each of the methods reveals typical staining patterns implying constitutional differences in heterochromatin [1]. The term heteromorphism is used synonymously with polymorphism or normal variant. Common cytogenetic polymorphisms detected by G-banding are considered as heteromorphisms and include heterochromatin regions of chromosomes 1, 9, 16 and Y and also prominent acrocentric short arms, satellites and stalks [2]. The role of chromosome heteromorphisms in infertility has been studied previously [3–5]. There seems to be an increased incidence especially in infertile men but the mechanism underlying this association needs to be elucidated.
In the current study, we aimed to detect chromosome heteromorphisms observed in karyotypes of infertile couples and compare their frequencies with the frequencies observed in karyotypes of fetuses of spontaneous pregnancies.
Materials and methods
Patients The first group of patients consisted of 138 infertile couples who consulted the Assisted Reproduction Techniques (ART) Center of our University between January 2005 and January 2007 because of male infertility (due to azoospermia or oligospermia), idiopatic infertility or recurrent failure of assisted reproduction techniques. Our second group of patients were fetuses whose amniotic fluid samples were obtained during the same period (n = 1130). None of the pregnancies was obtained by ART and the reasons for referral were standard indications for amniocentesis such as abnormal serum screening levels or advanced maternal age.
Cytogenetic analysis All studies were performed in our routine cytogenetics laboratory, surveyed annually by the national committee of quality control in cytogenetics laboratories. Peripheral blood samples were obtained from both male and female partners (n = 276) in the infertile group. Chromosomes were harvested from 72 h lymphocyte cultures and Giemsa-trypsin banding (G-banding) was performed.
Amniotic fluid samples were cultured in Amniomed complete medium (Biochrom AG, Germany) and G-banded chromosomes were analyzed after harvesting [6]. When heteromorphisms were detected, the parental peripheral blood samples were also karyotyped. At least 20 metaphases were analyzed for each case and heteromorphisms were reported according to ISCN 2005 after selective banding studies, such as C and NOR banding were performed [7, 8].
Visualized heterochromatic polymorphisms of autosomes 1, 9, 16 and Y chromosome were included, as well as prominent stalks and satellites of D and G-group chromosomes.
The findings were considered as heteromorphic if the chromosome region of interest was greater than the same region on its homolog [9]. As for the Y chromosome, if it was larger than the G-group chromosomes, it was reported as Yqh+, and if smaller, as Yqh− [9]. The common pericentric inversion of chromosome 9; inv(9)(p11q13); was also considered as a heteromorphism.
When heteromorphisms were detected, all karyotypes were examined under light microscope by three independent laboratory technicians (OY, ZY, FIS), at different times in the laboratory to avoid uncertainty and variable results.
Statistical analyses The results of the two groups were compared by two-tailed Fisher’s exact test, calculated online at http://www.graphpad.com/quickcalcs/contingency1.cfm.
Results
In the infertile group, 18 individuals (11 males and seven females; 6.52%) were found to have different kinds of chromosome heteromorphisms (Table 1). In females, the frequency of heteromorphisms was 5% and in males 7.9%. 11 males who had heteromorphisms were oligozoospermic or azoospermic. The seven women with chromosome heteromorphisms had normospermic partners.
Table 1.
The polymorphisms determined in infertile cases according to indications
| Chromosome analysis indication (n = 276) | 1qh+ | 9qh+/− | inv(9) | 16qh+/− | Yqh+/− | Group D cenh+/s+ | Total |
|---|---|---|---|---|---|---|---|
| Azoospermia (n = 13) | 1 | 1 | |||||
| Oligospermia (n = 1) | |||||||
| ART failure (n = 24) | 1 | 1 | |||||
| Pregnancy loss after IVF (n = 2) | |||||||
| Infertility (unclassified) (n = 230) | 3 | 4 | 3 | 2 | 4 | 16 | |
| Secondary Infertility (n = 6) | |||||||
| 18 |
As for the 1,130 amniocentesis samples studied, we detected female karyotype in 543 and male karyotype in 587 fetuses. We observed polymorphisms in nine (1.65%) female and 11 (1.87%) male fetuses. The results of this second group are shown in Table 2. The parents of these fetuses were also karyotyped and all heteromorphisms were found to be inherited from either one of the parents.
Table 2.
The polymorphisms determined in amniocentesis cases according to indications
| Amniocentesis indication (n = 1,130) | 9qh+/− | inv(9) | 16qh+/− | Yqh+/− | Group D cenh+/s+ | Group G cenh+/s+ | Multiple | Total |
|---|---|---|---|---|---|---|---|---|
| Maternal anxiety (n = 42) | ||||||||
| Family history for chromosome abnormalities (n = 42) | 1 | 1 | 2 | |||||
| Abnormal fetal USG (n = 63) | ||||||||
| Advanced maternal age (n = 383) | 4 | 1 | 3 | 8 | ||||
| First trimester screening trisomy 18 risk (n = 6) | ||||||||
| First trimester screening trisomy 21 risk (n = 106) | 3 | 3 | ||||||
| Triple test trisomy 21 risk (n = 463) | 1 | 1 | 3 | 2 | 7 | |||
| Triple test trisomy 18 risk (n = 25) | 20 |
The most frequent types of heteromorphisms in the infertile group were inv(9) and D-group variants, each with a percentage of 1.45%, followed by 9qh+/9ph+/9qh−, 16qh+ and Yqh+/Yqh− variants (1.09% each; Fig. 1). Inherited heteromorphisms were present in 20 fetuses (1.77%), with inv(9) again being the most frequent (0.71%), followed by D-group (0.53%) and G-group variants (0.18%). Other types of heteromorphisms were present in 0.36% of cases. The types of heteromorphisms and their percentages are shown in Table 3.
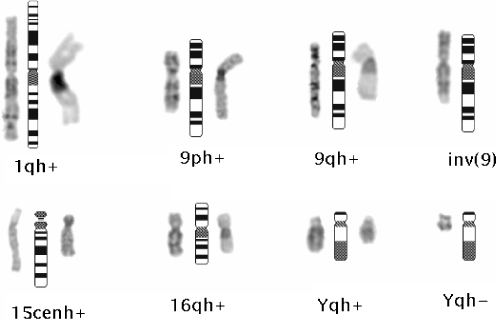
Fig. 1.
Partial karyotypes of the infertile patients showing samples of chromosomal heteromorphisms. Ideograms are shown in the middle; G banded chromosomes are on the left, C banded chromosomes are on the right
Table 3.
Number and percentages of chromosomal heteromorphisms detected in two groups
| No. of individuals with heteromorphisms | Number and Percentage of Variants | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 9qh+/9qh- | inv(9) | Yqh+/Yqh- | D group | G group | 16qh+ | 1qh+ | Multiple | ||
| Infertile Group (n = 276) | 18 (6.52%) | 3 (1.09%) | 4 (1.45%) | 3 (1.09%) | 4 (1.45%) | 0 | 3 (1.09%) | 1 (0.36%) | 0 |
| Amniocentesis Group (n = 1130) | 20 (1.77%) | 1 (0.09%) | 8 (0.71%) | 1 (0.09%) | 6 (0.53%) | 2 (0.18%) | 1 (0.09%) | 0 | 1 (0.09%) |
Discussion
Infertility affects 15% of all couples. The genetic reasons of infertility are complex and have different consequences. The causes can be chromosomal, involve single genes or be multifactorial and they can affect any stage of embryo development [10].
Chromosome analyses have been studied in large groups of infertile patients in recent years [3–5, 11–13]. In some of these studies, chromosome heteromorphisms were reported to have a higher frequency than the normal population and were regarded as abnormalities [4, 5, 13].
Heteromorphisms of chromosomes have been observed from the early studies of cytogenetics and are believed to have no impact on phenotype [2]. They include varying sizes of heterochromatin blocks, satellite or repeat sequence regions and inversions. In our study, our aim was to compare chromosome heteromorphisms detected during routine cytogenetic analyses of infertile couples with the ones detected in amniotic fluid samples of spontaneous pregnancies. We considered this second group as a sample of normal population, as the polymorphisms were all shown to be inherited from one of the parents who had no fertility problems. The indications for fetal karyotyping were abnormal serum screening levels and increased maternal age. There were no findings detected during fetal ultrasound examination. Also, the parents of the fetuses without any phenotypic reflections were karyotyped when polymorphisms were detected. Previously, we reported a relationship between increased risks for trisomy 18 and fetal triploidy in prenatal maternal serum screening [14]. In the current study, it is not easy to find an impact of heteromorphisms and abnormal maternal serum screening results as there were no phenotypic effects on the fetuses detected by ultrasonography. The parental phenotypes were also normal, at least for the evaluated parameter of infertility.
We detected the frequency of heteromorphisms in infertile cases to be significantly higher than the fetuses (p < 0.001). In females, chromosome heteromorphism frequency was 5% and in males 7.9%. This finding was consistent with the previous reports regarding chromosome heteromorphisms as abnormalities in infertile cases [4, 13].
We perform cytogenetic analyses to both partners of infertile couples during routine genetic evaluation. We detected heteromorphisms more frequently in males, however also women had an increased ratio compared to the fetal karyotypes. All men with heteromorphisms had oligo or azoospermia. This could lead us to the hypothesis that heteromorphisms could interfere with male meiosis. Seven women with heteromorphisms had normospermic partners, and these couples had idiopathic infertility. The relationship of idiopathic infertility and female chromosome heteromorphisms needs further investigation and evaluation in larger groups of patients, with more detailed methods.
Regarding the types of heteromorphisms observed, Lissitsina et al, in their study of 90 infertile men observed inv(9)(p11q13) three times more often than controls. On the other hand, they found a similar frequency of 9qh+ and Yqh+ in both groups [12]. In our study, the overall frequency of heteromorphisms were higher in infertile cases, however the most frequent types; inv(9) and D-group variants; were similar in both groups (p = 0.2667 and p = 0.1137 respectively).
In their paper, Brothman et al. report the survey results of The Cytogenetics Committee of the College of American Pathologists and The American College of Medical Genetics and conclude that common cytogenetic variants are considered to be heteromorphic and of no clinical significance. The majority of clinical cytogeneticists would not even mention these variants in their reports except for pericentric inversions and rare variants. They also deduce that there are currently no standards in cytogenetics for reporting heteromorphisms [2].
We mention chromosome variants in our clinical reports and if detected prenatally, although we regard these variants as of no clinical significance, we try to reduce parental anxiety by karyotyping the parents and reporting the origin of inheritance. We regard this as a tool for the clinicians; since they tend to question karyotypes containing heteromorphisms, especially in prenatal diagnosis where it is more difficult to determine the consequences.
Polymorphic heterochromatic regions were found to alter the synapsis of homologous chromosomes during meiosis. These regions are the last to enter synapse, changing the timing of the whole division and leading first to probable meiotic defects, eventually to infertility [15]. As for our cases, although we detected an increased ratio of heteromorphisms, it is not easy to regard the heteromorphisms as the sole reason for infertility and we believe this is rather coexistence than a correlation. We believe analyses at the molecular level will reveal mechanisms in more detail as following molecular genetic studies done in chromosome variants, the heterochromatin has been regarded to have more crucial cellular roles than previously thought. Thus, chromosome variants should not be ignored by cytogeneticists and clinicians, for contributory reasons that may not have been realized yet [12, 13, 16].
As a conclusion, polymorphic chromosome variants will probably be re-evaluated based on their phenotypic reflections as they might not always display normal phenotype. The function of the heterochromatin will be illuminated by both gene expression profiling and epigenetic studies in future.
Footnotes
Capsule Presence of chromosome heteromorphisms in karyotypes of infertile couples and fetuses of spontaneous pregnancies revealed statistically significant difference emphasizing the role of heteromorphisms in infertility.
References
- 1.Babu A, Agarwal AK, Verma S. A new approach in recognition of heterochromatic regions of human chromosomes by means of restriction endonucleases. Am J Hum Genet 1998;42:60–5. [PMC free article] [PubMed]
- 2.Brothman AR, Schneider NR, Saikevych I, Cooley LD, Butler MG, Patil S, et al. Cytogenetics Resource Committee, College of American Pathologists/American College of Medical Genetics. Cytogenetic heteromorphisms: Survey results and reporting practices of Giemsa-band regions that we have pondered for years. Arch Pathol Lab Med 2006;130:947–9. [DOI] [PubMed]
- 3.Cortés-Gutiérrez EI, Cerda-Flores RM, Dávila-Rodríguez MI, Hernández-Herrera R, Vargas-Villarreal J, Leal-Garza CH. Chromosomal abnormalities and polymorphisms in Mexican infertile men. Arch Androl 2004;50:261–5. [DOI] [PubMed]
- 4.Nakamura Y, Kitamura M, Nishimura K, Koga M, Kondoh N, Takeyama M, et al. Chromosomal variants among 1790 infertile men. Int J Urol 2001;8:49–52. [DOI] [PubMed]
- 5.Yakin K, Balaban B, Urman B. Is there a possible correlation between chromosomal variants and spermatogenesis? Int J Urol 2005;12:984–9. [DOI] [PubMed]
- 6.Verma RS, Babu A. Human chromosomes: principles and techniques, Chapter 3. 2nd ed. New York: McGraw Hill; 1995. p. 78–86.
- 7.ISCN. An international system for human cytogenetic nomenclature. Shaffer LG, Tommerup N, editors. Basel: Karger; 2005.
- 8.Babu A, Verma RS. Characterization of human chromosomal constitutive heterochromatin. Can J Genet Cytol 1986 Oct;28(5):631–44. [DOI] [PubMed]
- 9.Wyandt HE, Tonk VS. Atlas of human chromosome heteromorphisms. Dordrecht: Kluwer; 2004. p. 33–44.
- 10.Shah K, Sivapalan G, Gibbons N, Tempet H, Griffin DK. The genetic basis of infertility. Reproduction 2003;126:13–25. [DOI] [PubMed]
- 11.Morel F, Douet Guilbert N, Le Bris MJ, Amice V, Le Martelot MT, Roche S, et al. Chromosomal abnormalities in couples undergoing intracytoplasmic sperm injection. A study of 370 couples and review of the literature. Int J Androl 2004;27(3):178–82. [DOI] [PubMed]
- 12.Lissitsina J, Mikelsaar R, Punab M. Cytogenetic analyses in infertile men. Arch Androl 2006;52(2):91–5. [DOI] [PubMed]
- 13.Madon PF, Athalye AS, Parikh FR. Polymorphic variants on chromosomes probably play a significant role in infertility. Reprod Biomed Online 2005;11(6):726–32. [DOI] [PubMed]
- 14.Yilmaz Z, Sahin FI, Tarim E, Kuscu E. Triploides In first and second trimester pregnancies in Turkey. Balkan J Med Genet 2007;10(2):71–75.
- 15.Codina-Pascual M, Navarro J, Oliver-Bonet M, Kraus J, Speicher MR, Arango O, et al. Behaviour of human heterochromatic regions during the synapsis of homologous chromosomes. Hum Reprod 2006;21(6):1490–7. [DOI] [PubMed]
- 16.Starke H, Seidel J, Henn W, Reichardt S, Volleth M, Stumm M, et al. Homologous sequences at human chromosome 9 bands p12 and q13–21.1 are involved in different patterns of pericentric rearrangements. Eur J Hum Genet 2002;10(12):790–800. [DOI] [PubMed]