Abstract
Purpose
Identification of the unique genes playing critical roles in human embryo cleavage.
Methods
Isolation of human ePAB cDNA using human ovary cDNA libraries and mouse ePAB amino acid sequences, followed by analysis of its expression pattern in various adult tissues and stages during early oocyte development excluding ePABP2.
Results
Human ePAB encodes a 330-aa protein and is located on chromosome 20q12-q13.1. The amino acid sequence is 72% homologous with that of mouse ePab. Human ePAB has only three RRMs and lacks a PABP domain; the expression pattern is nonspecific in adult tissues and detected in all stages, from oocyte to blastocyst. Human ePABP2 encodes a 282-aa protein and is located on chromosome 16q24.3. The amino acid sequence is 68% homologous with mouse ePabp2.
Conclusions
We identified human ePAB and ePABP2 cDNA. Human ePAB cDNA is not expressed specific to the ovary. Biological discrepancies exist between the human and the mouse.
Keywords: Cleavage, Embryo, ePAB, ePABP2
Introduction
Infertility is a common problem faced by the clinician; approximately 10% of couples have difficulty conceiving a child [1]. Regardless of the cause of infertility, the procedure that leads to the highest pregnancy rate per cycle is in vitro fertilization (IVF). Since its inception in 1978 [2], there has been a remarkable world-wide increase in the number of IVF procedures. For 2003 in the United States, the clinical pregnancy rate per cycle for IVF cycles with fresh, non-donor eggs was 34% [1]. However, there are several risk factors associated with IVF; the most common complication of IVF is multiple gestation. In general, the transfer of more than one embryo results in a higher pregnancy rate than single-embryo transfer; however, this technique is also associated with a high risk of multiple gestation. An obvious way to reduce multiple gestations is to transfer a single embryo. Normally, the transferred embryo is at the cleavage stage (2-day embryo). However, recent studies indicate that transfer of a single blastocyst (5-day embryo) may result in an even higher rate of pregnancy while dramatically lowering the rate of multiple gestations [3, 4]. However, not all embryos grow to the blastocyst-stage. Moreover, no eggs grow to the blastocyst-stage in some patients.
It has been demonstrated that several genes may play critical roles in cleavage in both mice and humans [5]. However, most genes that play a role in cleavage are still unknown, especially in humans. Oocyte maturation is accompanied by a complex network of translational activation and repression of dormant maternal mRNAs [6–9]. These maternal mRNAs drive the oocyte’s reentry into meiosis and control the rate of mitosis during the early cleavage divisions of the embryo [9–12]. The first step in translational activation of stored maternal mRNAs is cytoplasmic extension of their poly(A) tails. This process, called cytoplasmic polyadenylation, was initially thought to be confined to gametes and embryos [13]. It has been reported that a poly(A)-binding protein (PABP) can bind to the newly elongated poly(A) tail [14]. Two structurally distinct groups of PABPs have been identified in vertebrates. PABP1 is the prototype of the first group and contains four RNA recognition motifs (RRMs) at its N terminus and a unique C-terminal PABP domain [15–17]. PABPN1 is the prototype of the second group of PABPs. It is smaller, with only one RRM, and is present in all cells of organisms, including oocytes and embryos [18–20]. An embryonic poly(A)-binding protein (ePAB) was identified in Xenopus and belongs to the first group of PABP [21]. As the predominant PABP during Xenopus early development, ePab most likely regulates unmasking/translation of maternal mRNAs and may influence poly(A) tail length as well. Therefore, it is suggested that ePab may play an important role during oocyte maturation and early embryonic development. Disregulation of ePab may cause problems during oocyte and early embryonic development. Recently, the mouse ePab gene was identified and its expression pattern was characterized during mammalian oogenesis as well as preimplantation embryo development in the mouse [22]. Subsequently, an embryonic poly(A)-binding protein 2 (ePABP2) was identified in Xenopus and mouse, and was similar to the second group of PABP in the structure [20]. The mouse ePabp2 expression pattern was also characterized in the early development and adult ovarian tissue. In this study, we isolated the human ePAB and ePABP2 cDNAs and analyzed the expression patterns in various adult human tissues, oocytes, and preimplantation embryos.
Materials and methods
Isolation of the human ePAB cDNA and analysis of its expression pattern in various tissues
The mouse ePab cDNA has been previously isolated [22]. Using the mouse ePAB amino acid sequence [22], we found its homologous region in the human genome sequences (XM_001131956 in Genbank). A pair of primers (PABF1 and PABR1) was made using the human homologous region encompassing the putative introns. PCR was carried out with a human ovary cDNA library (BD Biosciences Clontech, Tokyo, Japan) as a template. Nested PCR was performed with the primers, PABF2/PABR2 and a 10-fold dilution of the first PCR product as a template. The oligonucleotides that were employed were: PABF1; 5′-TTACGTGGGCGATCTGCACC-3′, PABR1; 5′-GGGTCCTGTGGTCTGAGTAC-3′, PABF2; 5′-CCGAGGCCATGCTCTATGAG-3′ and PABR2; 5′-CTACTCTCTGGGTATGAGGC-3′. The resultant nested PCR product was subcloned into a T-Easy vector (Promega, Madison, WI, USA), and several representative clones were sequenced in both directions. The 5′RACE and 3′RACE were done with the primers 5RAPAB1, 5RAPAB4, 3RAPAB1, 3RAPAB5, AP1 (Clontech), and AP2 (Clontech). Their sequences of oligonucleotides were as follows: 5RAPAB1; 5′-GACTTGCGAAGTCCTGGGTCTCGCTGGG-3′, 5RAPAB4; 5′-AGTTGATGTAGGCGTAGCCCAGCGAGCG-3′, 3RAPAB1; 5′-AGAGGCGACAAAGGCCGTGACAGAGATG-3′, 3RAPAB5; 5′-TGAGCAACCCCCTCCTGGGCTCCTTTC-3′.
Both RACE products were also subcloned, and several representative clones were sequenced in both directions. The isolated full-length cDNA sequences were compared with human genomic sequences. All of the first PCRs were carried out using an Advantage 2 PCR kit (Clontech) under the following conditions and according to the manufacturer’s instructions: initial denaturation at 95°C for 150 s; 32 denaturation cycles at 95°C for 15 s; and annealing and extension at 68°C for 180 s. All of the nested PCRs were performed under the same conditions except for the 20 cycles.
For the expression analysis of the human ePAB, PCR was done with the primers PABRTF2 and PABRTR2. The analyzed human cDNAs were: spleen, thymus, prostate, testis, ovary, small intestine, colon, leukocyte, brain, heart, kidney, liver, lung, skeletal muscle, pancreas, and placenta (Clontech). The sequences of the primers were: PABRTF2; 5′-ATCCTGTCCATCCGCGTGTG-3′ and PABRTR2; 5′-AGATGTTGGTGAACTCCAGG-3′. PCR conditions were initial denaturation at 95°C for 150 s, followed by 32 cycles of denaturation at 95°C for 15 s, annealing and extension at 68°C for 90 s using an Advantage 2 PCR Kit.
RT-PCR of oocytes and preimplantation embryos
Oocytes and embryos that were either unfertilized or were clinically not required for IVF were used. All the patients gave their written informed consent to undergo a molecular analysis of their oocytes and embryos, and the study was approved by ethics committee of Asahikawa Medical College. Total RNA from oocytes or embryos was obtained by using a RNAqueous Microkit (Ambion, Austin, TX, USA), according to the manufacturer’s instructions and kept at −80°C until use. First, reverse transcription (RT) reactions with oligo d(T) primers and the RETROscript kit (Ambion) were performed on the total RNA from 70 oocytes or embryos, according to the manufacturer’s instructions. Next, PCR was carried out on cDNA from the equivalent of five oocytes or embryos with the primers GF1 and GR2. Nested PCR was done with the primers GF2 and GR4. The sequences of the oligonucleotides used were: GF1; 5′-CAGCACATAGCACCTATCGG-3′, GR2; 5′-GGTAATAGGCTGGATGGGTG-3′, GF2; 5′-CTGTGCACATCCCAGGACAG-3′, GR4; 5′-AGAGTTTGCAGGGCCTTAGG-3′. PCR for G3PDH was performed using the same RT product as a positive control. PCR conditions were: initial denaturation at 95°C for 150 s; 32 cycles of denaturation at 95°C for 15 s; and annealing and extension at 68°C for 90 s, using an Advantage 2 PCR Kit. All of the nested PCRs were performed under the same conditions except for the 20 cycles.
Isolation of the human ePABP2 cDNA
A set of primers, PABP2NF1 and PABP2NR1, in the human homologous region encompassing the putative introns, and PCR was carried out with a human ovary cDNA library as a template. The sequences of the oligonucleotides used were: PABP2NF1; 5′-ATGTGGCCCTTCCCGAGCCG-3′ and PABP2NR1; 5′-TCACCGGTTCTGCCCTTGTGG-3′. Semi-nested PCR was done with the primers (PABP2NF2/PABP2NR1) and a tenfold dilution of the first PCR product as a template. The sequences of PABP2NF2 were: 5′-CGACTCAGGCCTGGCTCCAG-3′. The product from the semi-nested PCR was subcloned into a T-Easy vector, and several representative clones were sequenced in both directions. The 5′RACE and 3′RACE were performed with the primers 5RAPABN1, 5RAPABN2, 3RAPABN1, 3RAPABN2, AP1 (Clontech) and AP2 (Clontech). The sequences of the oligonucleotides were: 5RAPABN1; 5′-GGCCTGCTCCATGGCACACACCTTCATC-3′, 5RAPABN2; 5′-CTCCAGCTCCTGGTCAGGCAATGGGCAC-3′, 3RAPABN1; 5′-GGGAGGTCCACCGAGTCACGATCCTGTG-3′, 3RAPABN2; 5′-GTTCTCTGGACACCCCAAGGGTTATGCC-3′. Both RACE products were subcloned and sequenced in both directions. The isolated full-length cDNA sequences were compared with human genomic sequences. PCR conditions were initial denaturation at 95°C for 150 s, followed by 32 cycles of denaturation at 95°C for 15 s, annealing and extension at 68°C for 180 s. All of the nested PCRs were performed under the same conditions except for annealing and extension at 68°C for 120 s.
For the expression analysis of the ePABP2, PCR was carried out with the primers PAB2RTF1 and PAB2RTR1. The analyzed human cDNAs were following: spleen, thymus, prostate, testis, ovary, small intestine, colon, leukocyte, brain, heart, kidney, liver, lung, skeletal muscle, pancreas, and placenta (Clontech). Nested PCR was performed with the primers (PAB2RTF2/PAB2RTR2) and a 10-fold dilution of the first PCR product as a template. The sequences of the primers were: PAB2RTF1; 5′-GCTTTCTGCTGTCTCTGCTG-3′, PAB2RTR1; 5′-GAAGAGGCTCTGGTCCAGCT-3′, PAB2RTF2; 5′-CAGGAGCTGGAGGCCATCAA-3′ and PAB2RTR2; 5′-CCTTGGTGGCAAACTCTATG-3′. All conditions of the first PCR and nested PCR were the same as the conditions of the first and nested PCR to isolate ePABP2 cDNA except for annealing and extension at 68°C for 90 s of the nested PCR.
Results
Based on the amino acid sequence deduced from the mouse ePab cDNA, we identified the human ePAB cDNA. Its open reading frame (ORF) is from nt 21 to 1013, encoding the proteins of 330 amino acids. The comparison of the isolated cDNA and the corresponding genomic sequence revealed that the human ePAB gene is located on chromosome 20 (20q12-q13.1) and consists of 14 exons encompassing over 29.2 kb (annotated as EU190483 in GenBank). There is 72% homology between human and mouse cDNA at the amino acid level (Fig. 1). In addition, human ePAB has a 61% homology with the human PABP1 at the amino acid level.
Fig. 1.
Comparison of amino acid sequences between human (upper sequence) and mouse ePAB (lower sequence). An amino acid identity of 72% was observed between the two sequences. Vertical lines indicate identical amino acids and asterisks represent stop codons. The four RRMs are underlined
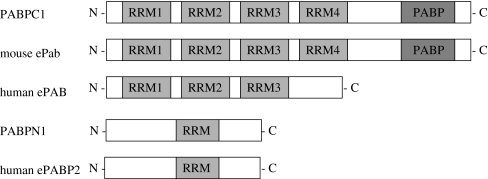
In general, the PABP gene family has four RRMs that bind RNA and a C-terminal PABP domain. Mouse ePAB also contains four RRMs and a C-terminal PABP domain; however, human ePAB has only three RRMs and lacks PABP domain (Fig. 2). The mouse ePab gene has two transcripts. It lacks exon11 by alternative splicing. Conversely, the human ePAB gene has only one transcript. The mouse ePab is expressed specifically to the mouse ovary; however, the human ePAB is not expressed specifically to the ovary but is present in various adult tissues (Fig. 3).
Fig. 2.
Schematic representation of the PABP family. RRMs are indicated by gray boxes and PABP domains are indicated by dark boxes
Fig. 3.
Expression patterns of ePAB in 16 human adult tissues. PCR analysis reveals a non-specific pattern in almost all tissues (upper panel). G3PDH was used as a positive control (lower panel)
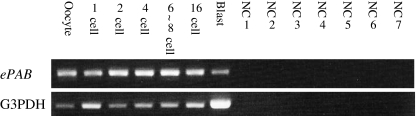
We evaluated the expression pattern of human ePAB mRNA in unfertilized oocytes and preimplantation embryos. Total RNA from 70 oocytes or embryos was used for RT reactions; amplification by PCR was then performed by using cDNA from the equivalent of five oocytes or embryos. Mouse ePab mRNA was expressed in oocytes, and in one-cell and two-cell embryos. However, it was not expressed in more advanced embryos. Human ePAB mRNA could be detected in at all stages, from oocytes to blastocysts (Fig. 4).
Fig. 4.
RT-PCR analysis of human ePAB in human oocytes and preimplantation embryos. NC (negative control) 1: oocyte; NC2: 1 cell embryo; NC3: 2 cell embryo; NC4: 4 cell embryo; NC5: 6–8 cell embryo; NC6: 16 cell embryo; NC7: blastocyst. G3PDH was used as a positive control (lower panel)
We identified the human ePABP2 cDNA. The ORF is from nt 13 to 861 and it encodes 282 amino acids (Fig. 5). There is 68% homology between human and mouse cDNA at the amino acid level and the cDNA in the coding region shares a 45% homology with the mouse ePabp2. The human ePABP2 gene is located on chromosome 16q24.3 and consists of seven exons encompassing over 3.2 kb (annotated as EU190484 in GenBank). This protein has a single RRM similar to that of type II nuclear PABPs. There is 43% homology between human ePABP2 and human type II nuclear PABP at the amino acid level; however, there is a 75% homology between the RRMs. In addition, expression analysis demonstrated that the human ePABP2 gene is expressed in various adult tissues (Fig. 6).
Fig. 5.
Comparison of amino acid sequences between human (upper sequence) and mouse ePABP2 (lower sequence). An amino acid identity of 68% was observed between the two sequences. Vertical lines indicate identical amino acids and asterisks indicate stop codons. The RRM is underlined
Fig. 6.
Expression patterns of ePABP2 in 16 human adult tissues. PCR analysis shows non-specific pattern in almost all tissues (upper panel). G3PDH was used as a positive control (lower panel). In expression patterns of human ePABP2, one transcript was observed in the ovary; however, two transcripts were observed in the other tissues
Discussion
In this study, we identified the human ortholog of Xenopus and mouse ePab. It encodes 330-aa protein with 72% identity compared to mouse ePab amino acids. Mouse ePab has a spliced form lacking exon11; however, human ePAB has no splicing variant and encodes only three RRMs and lacks PABP domain. Typically, PABP has four RRMs highly conserved in the N terminus, and PABP domain in the C terminus, which is a variable site [23]. The RRMs bind poly(A) and eIF4G as a translation initiation factor; the first two RRMs are specific to poly(A) binding, and the fourth RRM is critical to the nonspecific RNA binding. The C terminus is involved in the interaction between PABP and PABP; it is then necessary for cooperative binding to poly(A). These two parts are conserved in most PABPs but not seen completely in human ePAB ortholog. Lacking the C terminus may reduce the ability of the PABP domain to associate cooperatively to poly(A) and may have an influence because the C terminus is concerned with mRNA stability, as well as the nuclear export and translational repression of PABP mRNA itself [23].
Mouse embryo gene activation begins at the two-cell stage. Mouse ePab is expressed in unovulated oocytes and decreases gradually until the two-cell stage; subsequently, it is not present. In contrast, mouse Pabp1 (cytoplasmic PABP) expression exhibits a low baseline in oocytes through the four-cell stage; it is elevated at the eight-cell, when it becomes more pronounced in blastcysts [22]. It has been suggested that the shift of the expression pattern from ePab to Pabp1 corresponds to the transition from maternal to embryo gene activation; therefore, mouse ePab contributes to the control of maternal gene expression.
In the human, zygotic gene activation (ZGA) occurs at the four to eight-cell stage [24]. The human ePAB expression presents in oocytes up to the blastocyst stage; it is especially strong from the two- to eight-cell stage and then decays rapidly at the blastocyst stage. The human ePAB is expressed in a nonspecific pattern, which is unlike that of the mouse ePab; furthermore, human ePAB is not specific to gonadal tissue. Therefore, one should carefully consider the possibility that, in the human, ePAB may be involved in translation activation of maternal mRNA and cleavage division. The human ePAB gene is expressed in various adult human tissues. We currently do not know what possible role it plays in other tissues. Thus, further analysis of human ePAB is indicated, such as using human fetal tissues at the various stages.
Recently, ePabp2 was identified in Xenopus and the mouse; it is related to the known PABP2 [20, 25]. PABP2 (PABPN1 in the human) is a nuclear PABP that directs the polymerization and controls the size of the mRNA poly(A) tail during pre-mRNA processing. This nuclear PABP has both a single RRM and an arginine-rich C-terminal domain that is necessary for the recognition of poly(A). The cytoplasmic new RNA binding protein, ePabp2, is expressed in oocytes, early development, and in adult ovarian tissues. It has been suggested that ePABP2 may also contribute to the regulation of maternal translational activation during early development in a similar manner as ePAB. The identified human ePABP2 encodes 278-aa protein, and has a 68% identity with the mouse ePABP2, which encodes 273 amino acids. The sequence identity of single RRM of human ePAB is 74% with the PABP2 RRM domain greater than any other RRM of known PABPs.
Conclusions
This is the first report on human ePAB and ePABP2 that may relate to cleavage division; however, these substances were not expressed specifically to the ovary, and biological discrepancies exist between the human and the mouse. There is a possibility of the presence of a new human PABP family that is predominantly expressed during oocyte and early embryo development. Further analysis is strongly indicated in regard to the genes that play a role in human embryo cleavage.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (No. 19591887) from the Ministries of Education, Culture, Sports, Science and Technology and Health, Labour and Welfare of Japan.
Footnotes
Capsule
We successfully isolated the human ePAB and ePABP2 cDNAs and analyzed the expression patterns in humans.
References
- 1.Van Voorthis BJ. In vitro fertilization. N Engl J Med. 2007;356:379–86. [DOI] [PubMed]
- 2.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978;2:366. [DOI] [PubMed]
- 3.Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–46. [DOI] [PubMed]
- 4.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81:551–5. [DOI] [PubMed]
- 5.Cui SXS, Kim NH. Maternally derived transcripts: identification and characterisation during oocyte maturation and early cleravage. Reprod Fertil Dev. 2007;19:25–34. [DOI] [PubMed]
- 6.Stebbins-Boaz B, Hake LE, Richter JD. CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 1996;15:2582–92. [PMC free article] [PubMed]
- 7.Mendez R, Hake LE, Andresson T, Littlepage LE, Ruderman JV, Richter JD. Phosphorylation of CPE binding factor by Reg2 regulates translation of c-mos mRNA. Nature 2000;404:302–7. [DOI] [PubMed]
- 8.Gebauer F, Xu W, Cooper GM, Richter JD. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994;13:5712–20. [DOI] [PMC free article] [PubMed]
- 9.Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. Timely translation during the mouse oocyte-to-embryo transition. Development 2000;127:3795–803. [DOI] [PubMed]
- 10.Croisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter JD. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 2000;103:435–47. [DOI] [PubMed]
- 11.Uto K, Sagata N. Nek2B, a novel maternal form of Nek2 kinase, is essential for the assembly or maintenance of centrosomes in early Xenopus embryos. EMBO J. 2000;19:1816–26. [DOI] [PMC free article] [PubMed]
- 12.Stutz A, Conne B, Huarte J, Gubler P, Volkel V, Flandin P, et al. Masking, unmasking, and regulated polyadenylation cooperate in the translational control of a dormant mRNA in mouse oocytes. Genes Dev. 1998;12:2535–48. [DOI] [PMC free article] [PubMed]
- 13.Richter JD, Lorenz LJ. Selective translation of mRNAs at synapses. Curr Opin Neurobiol. 2002;12:300–4. [DOI] [PubMed]
- 14.Cao Q, Richter JD. Dissolution of the maskin-elF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 2002;21:3852–62. [DOI] [PMC free article] [PubMed]
- 15.Blobel G. A protein molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci USA. 1973;70:924–28. [DOI] [PMC free article] [PubMed]
- 16.Mangus DA, Evans MC Jacobson A. Poly(A)-binding proteins: multifunctional scaffolds for the posttranscriptional control of gene expression. Genome Biol. 2003;4:223. [DOI] [PMC free article] [PubMed]
- 17.Kuhn U, Eahle E. Structure and function of poly(A) binding proteins. Biochim Biophys Acta. 2004;1678:67–84. [DOI] [PubMed]
- 18.Wahle E. A novel poly(A)-binding protein acts as a specificity factor in the second phase of messenger RNA polyadenylation. Cell 1991;66:759–68. [DOI] [PubMed]
- 19.Wahle E, Lustig A, Jeno P, Maurer P. Mammalian poly(A)-binding protein II. Physical properties and binding to polynucleotides. J Biol Chem. 1993;268:2937–45. [PubMed]
- 20.Good PJ, Abler L, Herring D, Sheets MD. Xenopus embryonic poly(A) binding protein 2 (ePABP2) defines a new family of cytoplasmic poly(A) binding proteins expressed during the early stages of vertebrate development. Genesis 2004;38:166–75. [DOI] [PubMed]
- 21.Voeltz GK, Ongkasuwan J, Standart N, Steitz JA. A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev. 2001;15:774–88. [DOI] [PMC free article] [PubMed]
- 22.Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preomplantation embryos. Proc Natl Acad Sci USA. 2005;102:367–72. [DOI] [PMC free article] [PubMed]
- 23.Melo EO, Dhalia R, de Sa CM, Standart N, de Melo Neto OP. Identification of a C-terminal poly(A)-binding protein (PABP)–PABP interaction domain. J Biol Chem. 2003;278:46357–68. [DOI] [PubMed]
- 24.Hamatani T, Ko MSh, Yamada M, Kuji N, Mizusawa Y, Shoji M, et al. Global gene expression profiling of preimplantation embryos. Hum Cell. 2006;19:98–117. [DOI] [PubMed]
- 25.Cosson B, Braun F, Paillard L, Blackshear P, Osborne HB. Identification of a novel Xenopus laevis poly(A) binding protein. Biol Cell. 2004;96:519–27. [DOI] [PubMed]