Abstract
Purpose
To evaluate the effect of the size of zona pellucida opening by laser assisted hatching for frozen cleaved embryo that were thawed after both fresh and frozen cleaved embryo transfer cycles failed and were cultured to blastocyst after thawing in patients with multiple implantation failures.
Materials and methods
Of 101 consecutive procedures (October 2003 to June 2006), 30 patients declined to perform assisted hatching and were selected as control group, 40 patients had 40 μm opening of the zona (October 2003 to January 2005), 31 patients had 50% of the zona opening (February 2005 to June 2006).
Results
The pregnancy, implantation and delivery rates were significantly higher in 50% opening group (74%, 52% and 65%) compared to control group (17%, 10% and 13%; P < 0.01) and 40 μm opening group (43%, 27% and 38%; P < 0.04).
Conclusions
The size of the zona pellucida opening may affect the outcome of frozen cleaved embryo transfer.
Keywords: Assisted hatching, Blastocyst, Frozen embryo, Laser, Multiple implantation failures
Introduction
Assisted reproductive technology generally produces surplus embryos that can be cryopreserved for later use. The success of a cryopreservation programme will undoubtedly increase the cumulative pregnancy rates attained in IVF/ICSI. Success associated with the transfer of frozen thawed embryos has been generally lower than that obtained with fresh embryo transfer [1]. One explanation may be due to zona pellucida (ZP) hardening during the freezing and thawing process. Therefore, an artificial opening in the ZP, i.e. assisted hatching (AH), may be useful for frozen thawed embryo transfer. However, very recent studies focused on the benefit of AH following frozen thawed embryo transfer reported controversial results with decreased [2], similar [3, 4] or increased [5, 6] implantation rates in the AH group compared to the control group. Different types of AH have been developed. Mechanical ZP dissection with glass pipettes [7] or chemical ZP opening using acidic Tyrode’s solution [8, 9] are in wide use. Recently, AH by 1.48 μm diode laser has been introduced and has enabled to reliably produce equally sized ZP opening [10–13]. However, studies evaluating the effect of the size of ZP opening by laser AH for frozen cleaved embryos are lacking. One reason for this may be due to the fear that opening the ZP at the cleavage stage may have adverse effect such as loss of blastomeres through the opened ZP [11, 12]. This problem can be overcome by performing AH at the blastocyst stage. The aim of this retrospective study was to evaluate the results of two different sizes (40 μm or 50% of ZP) of ZP opening by laser AH for frozen cleaved embryos that were thawed after both fresh and frozen cleaved embryos transfer cycles failed and were cultured to blastocyst after thawing in patients with multiple implantation failures.
Materials and methods
Study period and patients
The frozen cleaved embryos were thawed after both fresh and frozen cleaved embryo cycles failed and were cultured to blastocyst after thawing in patients had at least two previous failures of fresh and frozen day 2 or day 3 embryo transfers. Between October 2003 and June 2006, 101 consecutive patients entered this frozen cleaved embryo transfer program and were explained of AH before embryo transfer. Of these, 30 patients declined to perform AH and were selected as control group had an intact ZP. In all, 71 patients consented to perform AH. 40 μm opening of the ZP was performed for 40 patients between October 2003 and January 2005 (AH40M group). 50% of the ZP opening was performed for 31 patients between February 2005 and June 2006 (AH50% group). We do not have Institutional Review Board in our private clinic. Drs K. Kinutani and M. Kinutani are members of Japan Society of Obstetrics and Gynecology (JSOG) and Kinutani Women’s Clinic have been registered as certified fertility centres by JSOG. The experiment was conducted in patients following informed consent and according to the guidelines of JSOG. Over the study period, there were no changes in the laboratory procedures and the culture media utilized.
Patient treatment and embryo culture
Women were treated with gonadotrophin-releasing hormone analogue buserelin acetate (MOCHIDA, Tokyo, Japan) from either the preceding mid-luteal phase in a long treatment protocol or second day of the cycle in a short treatment protocol. Ovarian stimulation was carried out with human menopausal gonadotrophin (hMG; Nikken, Tokyo, Japan) or urinary FSH (Fertinorm; Serono, Japan). Follicular development was monitored with serial vaginal ultrasound examinations and serum E2 measurements. They were administered human chorionic gonadotrophin (hCG; TEIZO, Tokyo, Japan) when dominant follicles reached a diameter of 18 mm. Oocytes were collected 35 h after hCG administration using a vaginal ultrasound-guided procedure and were incubated in human tubal fluid (HTF) medium (Irvine Scientific, California, USA) containing 10% (v/v) serum substitute supplement (SSS; Irvine) at 37°C in an atmosphere of 6% CO2, 5% O2 and 89% N2. The day of oocyte retrieval was considered as day 0. Sperm preparation was carried out using discontinuous ISolate™ (Irvine) gradient. Mature oocytes were either inseminated with sperm 5–7 h after oocyte retrieval at the concentration of 100,000 to 200,000 motile sperm per ml for 5 to 10 oocytes or microinjected with a single spermatozoon. Fertilization was confirmed at 15–18 h after insemination by the presence of two pronuclei. Fertilized oocytes were washed well and cultured in P-1™ Medium (Irvine) until day 3.
Slow freezing method and survival assessment
After the transfer of fresh cleaved embryos, surplus day 2 or day 3 embryos were cryopreserved using a programmable freezer (FREEZE CONTROL® MODEL CL-863, CryoLogic Pty Ltd, Australia). The embryos were cryopreserved using Dulbecco’s phosphate buffered saline solution (PBS 1×; Irvine) supplemented with 20% (v/v) SSS (Irvine), 0.1 mol/l sucrose (Nacalai Tesque, Inc., Kyoto, Japan) and 1.4 mol/l ethylene glycol (EG; Sigma Chemical Co., Missouri). The freezing program for embryos in our clinic was as follows: starting temperature: 20°C; rate of cooling: 2°C/min from 20°C to −7°C; soak at −7°C for 5 min; manual seeding; hold the temperature at −7°C for 10 min; rate of cooling: 0.3°C/min from −7°C to −32°C; rate of cooling: 4°C/min from −32°C to −86°C. The frozen straw was quickly transferred from freezing chamber to a reservoir of liquid nitrogen.
For all the couples, after failure of fresh cleaved embryos, frozen embryos were thawed at room temperature for 40 s and then at 30°C in a water bath for 40 s. Subsequently, the cryoprotectant was removed by washing the embryos successively with a decreasing concentration of EG. After thawing, frozen embryos were examined the total number of visible blastomeres and the presence of lysed blastomeres, and the embryos with more than 75% of visible blastomeres were judged to have survived.
Thawed embryos culture and grading of blastocysts
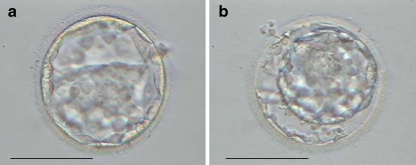
For all the couples, after failure of frozen-cleaved embryo transfer, the remaining frozen day 2 or day 3 embryos were thawed and cultured for better embryo selection before embryo transfer. Following thawing, thawed day 2 (the day of thawing was considered as day 2) embryos were placed in Blast Assist Medium 1 (Medicult, Denmark), in Blast Assist Medium 2 (Medicult) on day 3 and cultured until day 6; thawed day 3 (the day of thawing was considered as day 3) embryos were placed in Blast Assist Medium 2 (Medicult) and cultured until day 6 at 37°C in an atmosphere of 6% CO2, 5% O2 and 89% N2. On day 5, the inner cell mass (ICM) and trophectoderm (TE) of the blastocyst was examined. The ICM grading was as follows: (1) tightly packed, many cells; (2) loosely grouped, several cells; (3) very few cells. The TE grading was as follows: (1) many cells forming a tightly knit epithelium; (2) few cells; (3) very few cells forming a loose epithelium [14]. In all cases only day 5 expanded blastocysts which were not hatching or had not hatched from the ZP and scoring BB or higher were transferred (Fig. 1a). Cryopreservation of supernumerary blastocysts on day 5 or day 6 was performed using vitrification method. Only expanded blastocysts scoring BB or higher by day 6 were vitrified.
Fig. 1.
a A human blastocyst developed from frozen cleaved embryo b immersed into 0.2 mol/l sucrose solution for 2 min. Bar represents 100 μm
AH methods
AH was performed at the expanded blastocyst stage. Before AH, the blastocysts were placed into 1.0 ml of 25°C 0.2 mol/l sucrose in Sperm Washing Medium (Irvine) to shrink the blastocyst away from the ZP allowing the hole to be opened through the ZP without potential harm to the embryo itself (Fig. 1b).
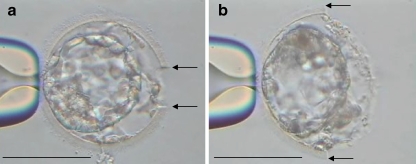
40 μm or 50% of the ZP opening AH using laser (Zilos-tk Laser, Hamilton Thorne Research, Beverly, MA, USA) was performed as follows. Embryos were stabilized with a holding pipette held at the 9 o’clock position (Fig. 2a,b), and positioned with the laser target located on the outer edge of the ZP. The power of laser was 100% and the pulse duration was 500 μs. By using this setting, a 10 μm hole was formed in the ZP by one laser shot. Multiple irradiations along the convex periphery of ZP from outward to inward were used to form a 10 μm opening. Similarly, another opening was formed next to the first one. This procedure was repeated until a 40 μm opening (Fig. 2a) or 50% of the ZP opening was formed. The opening of 50% of the ZP was initiated at one point and continued until half of the ZP was opened (e.g. laser opening was initiated at the 12 o’clock position, and consecutive shots were applied until the 6 o’clock position of the embryo was reached; Fig. 2b).
Fig. 2.
Human blastocyst developed from frozen cleaved embryo just after laser assisted hatching at the blastocyst stage. a The embryo created 40 μm opening and b 50% of the zona pellucida opening. Two arrows point the end of opening in the zona pellucida. Bar represents 100 μm
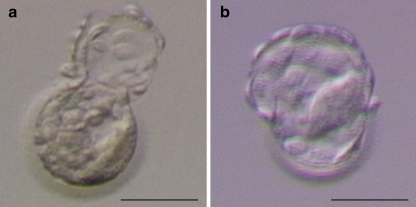
After the AH procedure was completed, the blastocysts were rinsed several times and were cultured in Blast Assist Medium 2 (Medicult) for 3 h until transfer (Fig. 3a,b).
Fig. 3.
Human blastocyst developed from frozen cleaved embryo 3 h after laser assisted hatching at the blastocyst stage. a The embryo created 40 μm opening and b 50% of the zona pellucida opening. Bar represents 100 μm
Endometrial preparation and assessment of pregnancy
Embryo transfer was performed in hormone replacement treatment cycles. All women received transdermal estradiol (Estraderm 1.4–5.8 mg/2 day, Kissei, Tokyo, Japan) with gonadotrophin-releasing hormone analogue for the preparation of the endometrium. The administration of progesterone (vaginal 400 mg daily) was initiated when endometrial thickness exceeded 10 mm. Embryo transfer was scheduled on day 5 after the initiation of progesterone treatment. One to two blastocysts were transferred into the patient’s uterus. Edwards-Wallace Embryo replacement catheter (SIMS Portex Ltd., UK) was used for embryo transfer. Pregnancy was first assessed by urinary hCG 9 days after blastocyst transfer, and then clinical pregnancy was confirmed by the presence of fetal heart activity 30 days after blastocyst transfer.
ZP digestion by pronase
In order to assess if the cryopreservation process induced hardening of the ZP, the time for ZP lysis was recorded after enzymatic treatment of fresh blastocysts (n = 10) or blastocysts developed from frozen cleaved embryos (n = 10), which had been donated by consenting patients. The ZP was enzymatically removed according to the method of Fong et al. [15]. The blastocysts were transferred into 1 ml of 37°C 10 IU/ml pronase solution (Sigma, St. Louis, MO) in Sperm Washing Medium (Irvine). After transferring the blastocysts into the pronase solution, the embryos were not moved until the ZP had been completely dissolved. The time for complete dissolution of the ZP was recorded at ×400 magnification under an inverted Hoffman modulation contrast microscope with a 37°C stage warmer.
Statistics
The Mann–Whitney test, unpaired Student’s t test, χ2 and Fisher’s exact test were used as appropriate to determine statistical differences among groups. A P value of <0.05 was considered significant.
Results
Table 1 summarizes some relevant clinical aspects and thawed embryo development of the patient groups. The age of women at thawing, duration of infertility, mean number of prior failures of embryo transfers, primary infertility, secondary infertility, cause of infertility, mean number of thawed embryos, survival rate after thawing and mean number of embryos developed to blastocysts (days 5 and 6) were similar among the three groups.
Table 1.
Comparison of demographic characteristics and thawed embryo development of the control, AH40M and AH50% groups
| Control | AH40M | AH50% | |
|---|---|---|---|
| No. of women | 30 | 40 | 31 |
| Mean age of women at thawing (years) ± SD | 31.2 ± 2.9 | 32.4 ± 3.8 | 33.1 ± 3.7 |
| Mean duration of infertility (years) ± SD | 6.5 ± 2.0 | 6.2 ± 3.3 | 6.8 ± 2.7 |
| Mean no. of prior failures of embryo transfer ± SD | 4.3 ± 1.4 | 4.2 ± 2.6 | 4.4 ± 2.8 |
| No. with primary infertility | 8 | 12 | 10 |
| No. with secondary infertility | 22 | 28 | 21 |
| Cause of infertility | |||
| Tuboperitoneal (%) | 5 (17) | 5 (12) | 5 (16) |
| Endometriosis (%) | 3 (10) | 3 (8) | 2 (7) |
| Male (%) | 7 (23) | 12 (30) | 6 (19) |
| Unexplained (%) | 15 (50) | 20 (50) | 18 (58) |
| No. of thawed embryos | 186 | 229 | 189 |
| Mean no. of thawed embryos ± SD | 6.2 ± 3.6 | 5.7 ± 4.2 | 6.1 ± 3.3 |
| No. of survival embryos after thawing (%) | 168 (90) | 211 (92) | 178 (94) |
| No. of embryos developed to blastocysts (days 5 and 6) (%) | 81 (48) | 105 (50) | 93 (52) |
| Mean no. of blastocysts (days 5 and 6) ± SD | 2.7 ± 1.3 | 2.6 ± 2.4 | 3.0 ± 2.3 |
There were no statistically significant differences among the three groups.
Control No assisted hatching, AH40M 40 μm opening of the zona pellucida by laser assisted hatching, AH50% 50% of the zona pellucida opening by laser assisted hatching
Table 2 summarizes the outcomes of embryo transfer. Morphology scores of blastocysts and mean number of blastocysts transferred were similar among the three groups. The pregnancy, implantation and delivery rates were significantly lower in control group (17%, 10% and 13%) compared to AH40M group (43%, 27% and 38%; P < 0.05) and AH50% group (74%, 52% and 65%; P < 0.01). In addition, significantly differences in pregnancy, implantation and delivery rates were observed between AH40M and AH50% groups (P < 0.04). No differences in the multiple pregnancy rate was found among the three groups. All multiple pregnancies were observed in AH50% group and were dizygotic twins. In all, 42 infants have been born, four from the control group, 15 from the AH40M group and 23 from the AH50% group. All delivered infants have had normal physical profiles up to the present.
Table 2.
Comparison of outcomes of frozen cleaved embryo transfer of the control, AH40M and AH50% groups
| Control | AH40M | AH50% | |
|---|---|---|---|
| No. of cycles for transfer | 30 | 40 | 31 |
| No. of blastocysts transferred | 52 | 62 | 52 |
| Morphology scores of blastocysts | |||
| AA (%) | 22 (42) | 25 (40) | 20 (38) |
| AB (%) | 10 (19) | 12 (20) | 10 (19) |
| BB (%) | 20 (39) | 25 (40) | 22 (42) |
| Mean no. of blastocysts transferred ± SD | 1.7 ± 0.5 | 1.6 ± 0.5 | 1.7 ± 0.5 |
| No. of clinical pregnancies (%) | 5 (17)a | 17 (43)b | 23 (74)c |
| No. of multiple pregnancies (%) | 0 (0) | 0 (0) | 4 (17) |
| No. of embryos implanted (%) | 5 (10)a | 17 (27)b | 27 (52)c |
| No. of deliveries (%) | 4 (13)a | 15 (38)b | 20 (65)c |
Morphology scores of blastocysts: e.g. AA = inner cell mass grading was A and trophectoderm grading was A, for details see “Materials and methods.”
Control No assisted hatching, AH40M 40 μm opening of the zona pellucida by laser assisted hatching at the blastocyst stage, AH50% 50% of the zona pellucida opening by laser assisted hatching at the blastocyst stage
a–cValues with different alphabet in the same column differ significantly (P < 0.05)
Enzymatic treatment of the ZP
The enzymatic digestion of the ZP with pronase was significantly faster for fresh blastocysts (90.3 ± 9.8 s; mean ± SD) compared to blastocysts developed from frozen cleaved embryos (104.9 ± 10.1 s; P < 0.01).
Discussion
This study suggests that 50% of the ZP opening group by laser assisted hatching significantly improves the pregnancy, implantation and delivery rates of frozen cleaved embryos that were cultured to blastocyst after thawing as compared with control and 40 μm opening groups in patients with multiple implantation failures. As the present study is retrospective and non-randomized, several biases may have been introduced that may cast doubt on the conclusions. However, there were no changes in the clinical and laboratory protocols during the study period. There was no change in the culture media utilized. Laboratory and clinical personnel were the same. Similar technique and same catheters were used for transferring embryos. There was no difference between all three treatment groups regarding patient characteristics such as mean female age at thawing, mean duration of infertility and mean number of prior failures of embryo transfer. Although the patients of AH40M and AH50% groups were selected sequentially, those of control group were selected was dependent upon their decision during the entire investigation period. Embryo characteristics were also similar such as the rate of thawed embryos developed to the blastocyst, the incidence of grade AA and AB blastocysts and the number of embryos transferred. Therefore, taking into consideration all of the above, we believe it is unlikely that significant bias was introduced.
The implantation rate of control group was as low as 10% (5 of 52). There may be two reasons for this. First, the embryos were thawed after both fresh and frozen cleaved embryo cycles failed. All patients who entered this frozen cleaved embryo transfer program had at least two previous failures of conventional day 2 or day 3 embryo transfers. Therefore, all patients had at least four implantation failures of embryo transfer and were diagnosed as poor prognosis. Second, the embryos were frozen as cleaved embryos followed by culture after thawing. A failure in hatching does prevent implantation. Hatching deficiencies can result from zona hardening, which might occur after cryopreservation [16, 17] or after in vitro culture of human embryos [18, 19]. Balaban et al. reported that implantation rate of cryopreserved day 3 embryos that were allowed to cleave one day in vitro after thawing and were transferred without AH was 9.9% (58 of 585) in a prospective randomized study [6]. This result is congruent with that of control group (10%; 5 of 52) of the present study, In addition, the resistance to enzymatic removal of ZP of blastocysts developed from frozen cleaved embryos by pronase significantly increased as compared with fresh blastocysts. These observations suggest that hatching deficiencies can result from hardening of the ZP, which is due to the cryopreservation process of slow freezing and continued post-thaw culture to the blastocyst. Consequently, we suggest that AH is a critical factor for helping frozen cleaved embryos that have developed to the blastocyst after thawing to divest themselves of their ZP, thus improving their potential for implantation.
The pregnancy and implantation rates of AH40M group were significantly lower as compared with the AH50% group. Lyu et al. reported that when the slit size after AH for fresh human blastocysts was 55–65 μm, significantly more of the larger ICM became trapped by the ZP opening during hatching than the smaller ICM [20]. Moreover, they also reported that all human blastocysts completely hatched after creating the slit with size beyond 100 μm. Therefore, it is suggested that 40 μm of ZP of frozen cleaved embryos that were cultured to blastocyst after thawing is inadequate for the completion of hatching process in some cases, however, it can be considerably improved by 50% of the ZP opening.
Laser AH for frozen cleaved embryos is normally performed at the cleavage stage [3, 6]. In this study, we performed laser AH for frozen cleaved embryos at the blastocyst stage. At this stage, the TE attaches to the ZP and there is no perivitelline space. Therefore, it is possible that TE is damaged as soon as the ZP is opened by laser. To avoid this drawback, before AH, the blastocysts were placed into 0.2 mol/l sucrose solution to shrink the blastocyst away from the ZP allowing the hole to be opened through the ZP without potential harm to the embryo itself. During AH procedure, the blastocysts were exposed to sucrose solution for a while, and so might be damaged as a result of chemical toxicity. However, sucrose is frequently added to cryopreservation solution for mammalian oocytes/embryos and the toxicity of this non-permeating agent is quite low [21]. Consequently, immersing the blastocysts to the sucrose solution may have little detrimental effect on the embryos and enables to perform AH at the blastocyst stage.
To make opening in the ZP, we used multiple shots of laser beam. This procedure may pose potential harm to embryos, especially in making 50% of the ZP opening. Hartshorn et al. demonstrated that embryos at the eight-cell stage are able to respond to thermal shock by activating heat shock protein (hsp) production, as shown by the sharp increase in hsp70i transcription that follows embryo exposure to elevated temperature [22]. They also reported that in eight-cell mouse embryos, laser ZP drilling did not stimulate hsp70i expression, even in the blastomeres closet to the laser beam [23]. Moreover, Kanyo and Konc reported that there was no evidence of an increase in chromosomal aberrations or congenital malformations for 134 children born after laser AH [24]. In addition, our results for 50% of the ZP opening by laser demonstrated high pregnancy, implantation and delivery rates. However, the fact that a pregnancy is established does not preclude that there are other underlying anomalies. Therefore, further long-term studies, such as looking at birth defects and other potential anomalies that can occur later, are needed to confirm and assess safety of 50% of the ZP opening using laser.
The preferred outcome of assisted reproductive technology is a singleton delivery. To achieve this it is evident that a single embryo transfer should be performed. Gardner et al. proposed that before considering single blastocyst transfers, it would appear prudent to transfer two blastocysts to ensure that the clinic can establish acceptable implantation and pregnancy rates [25]. Rates could be set arbitrarily at >45% and >60% for implantation and pregnancy rates, respectively. In this study it has been confirmed that high implantation and pregnancy rates (52% and 74% respectively) can be attained with the transfer of an average of 1.7 blastocysts developed from frozen cleaved embryos with 50% of the ZP opening. These results suggest that if the safety of 50% of the ZP opening AH using either laser or other method is confirmed the move to single embryo transfer will be possible for frozen cleaved embryo transfer.
Conclusions
This is the first report evaluating the effect of the size of ZP opening by laser AH on clinical outcome of frozen cleaved embryos that were cultured to blastocyst after thawing in patients with multiple implantation failures of embryo transfer. Our study number of treatment cycles was small and this study is retrospective. Further prospective randomized trials comparing the effectiveness of the size of ZP opening using laser may be needed. However, it should be noted that the amount of ablation used here was large and its safety is not yet proven. Although the immediate clinical data were not affected as this study shows, it is possible that it may have more long-term repercussions on the level of DNA damage. Full ZP ablation should not become standardized practice without appropriate pre-clinical studies in both animal and human spare material including assessment of DNA damage.
Footnotes
Capsule
The size of the zona pellucida opening by laser assisted hatching may affect the outcome of frozen cleaved embryo transfer in multiple implantation failures.
References
- 1.European IVF-Monitoring Programme (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2000. Results generated from European registers by ESHRE. Hum Reprod. 2004;19:490–503. [DOI] [PubMed]
- 2.Primi MP, Senn A, Montag M, Van der Ven H, Mandelbaum J, Veiga A, et al. A European multicentre prospective randomized study to assess the use of assisted hatching with a diode laser and the benefit of an immunosuppressive/antibiotic treatment in different patient populations. Hum Reprod. 2004;19:2325–33. [DOI] [PubMed]
- 3.Ng EH, Naveed F, Lau EY, Yeung WS, Chan CC, Tang OS, et al. A randomized double-blind controlled study of the efficacy of laser-assisted hatching on implantation and pregnancy rates of frozen-thawed embryo transfer at the cleavage stage. Hum Reprod. 2005;20:979–85. [DOI] [PubMed]
- 4.Sifer C, Sellami A, Poncelet C, Kulski P, Martin-Pont B, Bottero J, et al. A prospective randomized study to assess the benefit of partial zona pellucida digestion before frozen-thawed embryo transfers. Hum Reprod. 2006;21:2384–9. [DOI] [PubMed]
- 5.Gabrielsen A, Agerholm I, Toft B, Hald F, Petersen K, Aagaard J, et al. Assisted hatching improves implantation rates on cryopreserved-thawed embryos. A randomized prospective study. Hum Reprod. 2004;19:2258–62. [DOI] [PubMed]
- 6.Balaban B, Urman B, Yakin K, Isiklar A. Laser-assisted hatching increases pregnancy and implantation rates in cryopreserved embryos that were allowed to cleave in vitro after thawing: a prospective randomized study. Hum Reprod. 2006;21:2136–40. [DOI] [PubMed]
- 7.De Vos A, Van Steirteghem A. Zona hardening, zona drilling and assisted hatching: new achievements in assisted reproduction. Cells Tissues Organs. 2000;166:220–7. [DOI] [PubMed]
- 8.Cohen J, Elsner C, Kort H, Massey J, Mayer MP, Wiemer K. Impairment of the hatching process following IVF in the human and improvement of implantation by assisted hatching using micromanipulation. Hum Reprod. 1990;5:7–13. [DOI] [PubMed]
- 9.Cohen J, Alikani M, Trowbridge J, Rosenwaks Z. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Hum Reprod. 1992;7:685–91. [DOI] [PubMed]
- 10.Germond M, Rink K, Nocera D, Delacretaz G, Senn A, Fakan S. Microdissection of mouse and human zona pellucida using a 1.48-mm diode laser beam: efficacy and safety of the procedure. Fertil Steril. 1995;64:604–11. [DOI] [PubMed]
- 11.Mantoudis E, Podsiadly BT, Gorgy A, Venkat G, Craft IL. A comparison between quarter, partial and total laser assisted hatching in selected infertility patients. Hum Reprod. 2001;16:2182–6. [DOI] [PubMed]
- 12.Blake DA, Forsberg AS, Johansson BR, Wikland M. Laser zona pellucida thinning—an alternative approach to assisted hatching. Hum Reprod. 2001;16:1959–64. [DOI] [PubMed]
- 13.Hsieh YY, Huang CC, Cheng TC, Chang CC, Tsai HD, Lee MS. Laser-assisted hatching of embryos is better than the chemical method for enhancing the pregnancy rate in women with advanced age. Fertil Steril. 2002;78:179–82. [DOI] [PubMed]
- 14.Gardner DK, Schoolcraft WB. In-vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond 1999. London, UK: Parthenon; 1999. p. 378–88.
- 15.Fong CY, Bongso A, Ng SC, Kumar J, Trounson A, Ratnam S. Blastocyst transfer after enzymatic treatment of the zona pellucida: improving in-vitro fertilization and understanding implantation. Hum Reprod. 1998;13:2926–32. [DOI] [PubMed]
- 16.Tucker MJ, Cohen J, Massey JB, Mayer MP, Wiker SR, Wright G. Partial dissection of the zona pellucida of frozen-thawed human embryos may enhance blastocyst hatching, implantation, and pregnancy rates. Am J Obstet Gynecol. 1991;165:341–5. [DOI] [PubMed]
- 17.Check JH, Hoover L, Nazari A, O’Shaughnessy A, Summers D. The effect of assisted hatching on pregnancy rates after frozen embryo transfer. Fertil Steril. 1996;65:254–7. [DOI] [PubMed]
- 18.DeFelici M, Siracusa G. Spontaneous hardening of the zona pellucida of mouse oocytes during in vitro culture. Gamete Res. 1982;6:107–13. [DOI]
- 19.Downs SM, Schroeder AC, Eppig JJ. Serum maintains the fertilizability of mouse oocytes matured in vitro by preventing hardening of the zona pellucida. Gamete Res. 1986;15:115–22. [DOI]
- 20.Lyu QF, Wu LQ, Li YP, Pan Q, Liu DE, Xia K, et al. An improved mechanical technique for assisted hatching. Hum Reprod. 2005;20:1619–23. [DOI] [PubMed]
- 21.Kasai M. Advances in the cryopreservation of mammalian oocytes and embryos: development of ultrarapid vitrification. Reprod Med Biol. 2002;1:1–9. [DOI] [PMC free article] [PubMed]
- 22.Hartshorn C, Anshelevich A, Wangh LJ. Rapid, single-tube method for quantitative preparation and analysis of RNA and DNA in samples as small as one cell. BMC Biotechnol. 2005;13(5):2. [DOI] [PMC free article] [PubMed]
- 23.Hartshorn C, Anshelevich A, Wangh LJ. Laser zona drilling does not induce hsp70i transcription in blastomeres of eight-cell mouse embryos. Fertil Steril. 2005;84:1547–50. [DOI] [PubMed]
- 24.Kanyo K, Konc J. A follow-up study of children born after diode laser assisted hatching. Eur J Obstet Gynecol Reprod Biol. 2003;110:176–80. [DOI] [PubMed]
- 25.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81:551–5. [DOI] [PubMed]