Abstract
Purpose
While performing the mild ovarian stimulation protocol with a GnRH antagonist, the pregnancy rate was compared between the groups, which were divided by the degree that the luteinizing hormone (LH) level decreased.
Materials and methods
Patients aged 27 to 42years (36.1 ± 3.79) underwent 308 IVF cycles who opted for IVF via the mild ovarian stimulation protocol began clomiphene citrate on day 3 and recombinant FSH on day 5. A GnRH antagonist was administered when the dominant follicle reached 14mm. Serum LH was measured at the time of GnRH antagonist administration and at the time of hCG injection. The pregnancy rate and implantation rate were compared between 50 cycles in which the LH level dropped less than one-third and the control (LH level within 1/3).
Result(s)
The pregnancy rate for the group in which the LH level fell less than one third was 18%. Conversely, the pregnancy rate for the control group was 39%. The implantation rate was 18% for the less than one-third group and 26% for the control group. Both the pregnancy rate and the implantation rate for the group in which the LH level fell less than one-third were significantly lower than that of control (p < 0.02).
Conslusion(s)
When performing the mild ovarian stimulation protocol, serum LH should be followed. If the serum LH level is less than one-third at the time of hCG injection, both the pregnancy rate and implantation rate are significantly lower.
Keywords: Clomiphene citarate, Recombinant follicle-stimulation hormone, Gonadotropin-releasing hormone antagonist, In vitro fertilization
Introduction
Recently, to minimize the patient’s economic burden and physical discomfort, the mild ovarian stimulation protocol (also known as friendly in vitro fertilization [IVF]) is performed. Many ovarian stimulation protocols, such as clomiphene citrate (CC) alone and in combination with human menopausal gonadotoropin (HMG) and/or follicle stimulating hormone (FSH), have been reported [1–3]. In a natural cycle, ovulation before oocyte retrieval is one of the problems. Gonadotropin-releasing hormone (GnRH) antagonists are used to suppress natural ovulation [4–7].
A GnRH antagonist rapidly and efficaciously controls the LH surge. Williams et al. reported a cancellation rate of 0%; thus, proving the validity of a GnRH antagonist for IVF [3]. Several injection methods for a GnRH antagonist have been reported, such as a single 3-mg injection of a GnRH antagonist [8], or a daily injection of 0.25mg of a GnRH antagonist [4, 7, 9, 10]. Although the number of retrieved oocytes is lower than that of the long-protocol method, which uses a GnRH agonist, there was no significant difference in the clinical pregnancy rate [3, 11]. In this retrospective study, the pregnancy rate and implantation rate were compared in relation to the degree of LH suppression by a GnRH antagonist.
Materials and methods
From January 2006 through December 2006, 308 patients aged 27 to 42years (36.1 ± 3.79) underwent 308 IVF cycles. The indications for IVF were tubal infertility, mild male factor infertility, unexplained infertility, early stage endometriosis, and polycystic ovary syndrome. Patients who were unable to achieve an ovarian transfer and intracytoplasmic sperm injection (ICSI) cases were excluded. The average number of past IVF procedures was 2.34.
The couples opted for the mild stimulation protocol and full informed consent was obtained. Baseline serum FSH and LH level (<15mIU/ml) were checked and CC (100mg/day) was given from cycle day three through seven. Controlled ovarian hyperstimulation was started using a recombinant follicle stimulating hormone 150IU (rFSH; Follistim, Organon, Tokyo, Japan) or human menopausal gonadotropin 150IU (HMG; Humegon, Japan Organon, Osaka, Japan) beginning on cycle day5 and repeated every other day. Ultrasound was performed on cycle day nine and serum LH levels were determined (ultrasonographic follicular size and serum levels of LH and E2 were determined at every visit until the day of oocyte retrieval). A daily injection of a GnRH antagonist 0.25mg (Cetrotide; Nippon Kayaku, Tokyo, Japan) was initiated when the lead follicle size was >14mm.
Human chorionic gonadotropin 10,000U (hCG; Profasi, Serono, Tokyo, Japan) was administered 35 to 36h before IVF and oocytes were retrieved via ultrasound-guided transvaginal needle aspiration. The embryos were cultured in Universal IVF Medium (MediCult a/s, Jyllinge, Denmark) and BlastAssist System 1, 2 (MediCult a/s) in a 5% CO2, 5% O2, and 90% N2 environment. Embryos were transferred 3 to 5days after aspiration. Veeck’s classification and Gardner’s classification [12] were used for embryo scoring (good quality: day3, seven cell, G1, G2; and day5, over three AA). Luteal support was given for 2weeks (a 222 mg progesterone vaginal suppository was inserted every 12h and 125mg of hydroxyprogesterone caproate was injected intramuscularly once a week).
A clinical pregnancy was defined as an hCG level > 25IU/l and implantation rate was confirmed by the presence of a gestational sac on ultrasound. The chi square test and Student’s t test were used for statistical analysis, and p < 0.05 was defined as a statistically significant.
Results
Comparison examination of embryo quality, the pregnancy rate, and the implantation rate was conducted on 50 cycles in which the LH level fell less than one-third of the value at the time of the hCG injection (Group A) and control group (LH level within 1/3; 334 cycles). The LH level was determined at the time of the GnRH antagonist injection.
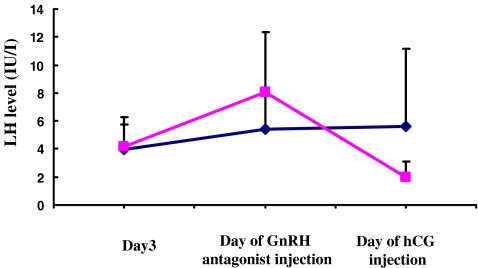
In comparison of Group A and control group, the ages of the women were 36.7 ± 3.63years for Group B and 36.1 ± 3.8years for the control group. The number of retrieved oocytes was 7.74 ± 4.47 in Group A and 6.35 ± 3.43in the control group. The number of embryo transfer (ETs) was 1.31 ± 0.47 in Group A and 1.45 ± 0.55 in the control group. The transition of the LH level from the day3 to the day of GnRH antagonist injection was 4.14 ± 2.12, 8.1 ± 4.27, and 2 ± 1.11 in Group A. In the control group, the LH value transitioned from 3.95 ± 1.76, 5.41 ± 2.94, and 5.633 ± 5.54IU/l (Fig. 1). There was no statistically significant difference in the above parameters between Group A and the control group.
Fig. 1.
The transition of the of serum LH level
The percentage of good embryo quality by Veeck’s and Gardner’s classification was 86% in Group A and 84% in the control (not statistically significant). The clinical pregnancy rate was 18% in Group A and 39% in the control group. The implantation rate was 18% in Group A and 26% in the control group. Both the clinical pregnancy and the implantation rate were found to have a statistically significant difference between Group A and the control group (p < 0.05; Table 1).
Table 1.
Clinical findings of group in which LH fell less than one third and control
| LH drop less than 1/3 | Control | |
|---|---|---|
| No. of cycles | 50 | 258 |
| Av. of age | 36.7 ± 3.63 | 36.1 ± 3.8 |
| Past IVF | 2.36 ± 1.75 | 1.92 ± 1.63 |
| Av. of oocyte retrievals | 7.74 ± 4.47 | 6.35 ± 3.43 |
| Embryo transfer (ET) | 1.31 ± 0.47 | 1.45 ± 0.55 |
| GnRH antagonist (days) | 2.72 ± 0.7 | 2.50 ± 0.67 |
| No. of MII | 6.36 ± 3.74 | 5.21 ± 3.32 |
| LH level on day 3 | 4.14 ± 2.12 | 3.95 ± 1.76 |
| LH level before GnRH antagonist (IU/l) | 8.1 ± 4.27 | 5.41 ± 2.94 |
| LH level on hCG injection (IU/l) | 2 ± 1.11 | 5.63 ± 5.54 |
| Embryo quality (%)a | 86 | 84.1 |
| Clinical Pregnancy (% per ET) | 18* | 39* |
| Implantation (% per ET) | 18* | 26* |
*p < 0.02
aGood quality: day 3, seven cell, G1, G2; and day 5, over three AA
Discussion
It has been mentioned that the mild ovarian stimulation protocol has the advantages of that low-cost and low-risk for IVF patients [1, 3, 13]. The development of GnRH antagonists has made mild ovarian stimulation protocol possible. GnRH antagonists promptly and effectively suppress LH levels. Two GnRH antagonist protocols have been reported [4, 7–10]. In our study, the daily injection protocol was performed because the GnRH antagonist can be tailored to suit the LH level. Pelinck et al. reported a good pregnancy rate using a GnRH antagonist with rFSH (without CC) and noted that pregnancy rates after IVF with minimal, late follicular phase stimulation are encouraging; furthermore, for all indications for IVF, the patients had better results [13]. A recent study found no difference in clinical results between the mild ovarian stimulation protocol and the conventional long protocol [3, 14]. Furthermore, there was no cancellation because of ovulation [3]. This is because the ovulation accompanying the LH surge is controlled by the GnRH antagonist; thus, oocyte retrieval can be ensured.
In this retrospective study, we compared pregnancy rates and implantation rates according to the degree of LH suppression grade by a GnRH antagonist. Hwang et al. reported serum hormone level fluctuations in detail when using the mild stimulation protocol with a GnRH antagonist [7]. The serum LH level dropped markedly one day after the injection of a GnRH antagonist; then returned to the original value four days after the injection. Our control LH levels followed a similar pattern. The question becomes, does a drop in the LH level reduce pregnancy rate?
Rapid LH suppression by a GnRH antagonist may prevent oocyte maturation. In this study, we did not observe any morphological findings of the oocyte consistent with maturation. A previous report also suggested that GnRH antagonist administration resulted in more mature oocytes and embryos of better quality compared with a GnRH agonist protocol [15]. Eldar-Geva et al. reported that a lower IVF embryo transfer success using a GnRH-antagonist/GnRH-agonist protocol does not appear to be related to an adverse effect on oocyte quality [16]. Lee et al. have suggested that GnRH antagonists do not have a detrimental effect on oocyte quality or embryo development [17].
Although was not statistically significant, the LH levels in the cases in which LH level fell to one third on the day of hCG injection were higher than those of the control group, which increased about two times the day 3 level. Studies by Olivennes et al. and Hwang et al. contained cases with an LH elevation on the day of GnRH antagonist administration; the pregnancy rate for these cases was 22% [7, 8]. Therefore, LH elevation is not the sole factor for a low pregnancy rate.
It has been suggested that LH may exert a direct effect on the uterus. Tesarik et al. reported that endometrial maturation is impacted in women with low endogenous LH [18]. LH receptors are localized in the endometrium and involved in the reproductive function [19, 20]. In the goat, treatment with a GnRH antagonist inhibits gene and protein expressions of LH receptors during the development of corpus luteum [21]. These reports suggest that rapid LH suppression effects endometrial receptivity. However, since the metabolism of a GnRH antagonist occurs at an early stage, it is difficult to confirm that the effect of a GnRH antagonist continues until the implantation window. As an adequate dose of HCG was injected before ovum aspiration, it is possible that compensation for insufficient LH is via hCG.
Currently, there is no way to detect LH suppression prior to oocyte aspiration. The reasons for a low pregnancy rate secondary to LH suppression cases are currently unknown. If there is no impact on embryo quality, frozen embryo transfer may resolve this problem. Further investigation is indicated.
Footnotes
Capsule If the serum LH level is less than one-third at the time of hCG injection, both the pregnancy rate and implantation rate are significantly lower following the Mild IVF stimulation protocol.
Contributor Information
Atsushi Yanaihara, Phone: +81-3-37848550, FAX: +81-3-37848355, Email: yanaihara@med.showa-u.ac.jp.
Takeshi Yorimitsu, Phone: +81-45-9881124, FAX: +81-45-9881125.
Hiroshi Motoyama, Phone: +81-45-9881124, FAX: +81-45-9881125.
Motohiro Ohara, Phone: +81-45-9881124, FAX: +81-45-9881125.
Toshihiro Kawamura, Phone: +81-45-9881124, FAX: +81-45-9881125.
References
- 1.Branigan EF, Estes MA. Minimal stimulation IVF using clomiphene citrate and oral contraceptive pill pretreatment for LH suppression. Fertil Steril 2000;73(3):587–90. [DOI] [PubMed]
- 2.Weigert M, Krischker U, Pohl M, Poschalko G, Kindermann C, Feichtinger W. Comparison of stimulation with clomiphene citrate in combination with recombinant follicle-stimulating hormone and recombinant luteinizing hormone to stimulation with a gonadotropin-releasing hormone agonist protocol: a prospective, randomized study. Fertil Steril 2002;78(1):34–9. [DOI] [PubMed]
- 3.Williams SC, Gibbons WE, Muasher SJ, Oehninger S. Minimal ovarian hyperstimulation for in vitro fertilization using sequential clomiphene citrate and gonadotropin with or without the addition of a gonadotropin-releasing hormone antagonist. Fertil Steril 2002;78(5):1068–72. [DOI] [PubMed]
- 4.Craft I, Gorgy A, Hill J, Menon D, Podsiadly B. Will GnRH antagonists provide new hope for patients considered ‘difficult responders’ to GnRH agonist protocols? Hum Reprod 1999;14(12):2959–62. [DOI] [PubMed]
- 5.Nikolettos N, Al-Hasani S, Felberbaum R, Demirel LC, Kupker W, Montzka P, et al. Gonadotropin-releasing hormone antagonist protocol: a novel method of ovarian stimulation in poor responders. Eur J Obstet Gynecol Reprod Biol 2001;97(2):202–7. [DOI] [PubMed]
- 6.Tavaniotou A, Albano C, Smitz J, Devroey P. Effect of clomiphene citrate on follicular and luteal phase luteinizing hormone concentrations in in vitro fertilization cycles stimulated with gonadotropins and gonadotropin-releasing hormone antagonist. Fertil Steril 2002;77(4):733–7. [DOI] [PubMed]
- 7.Hwang JL, Huang LW, Hsieh BC, Tsai YL, Huang SC, Chen CY, et al. Ovarian stimulation by clomiphene citrate and hMG in combination with cetrorelix acetate for ICSI cycles. Hum Reprod 2003;18(1):45–9. [DOI] [PubMed]
- 8.Olivennes F, Alvarez S, Bouchard P, Fanchin R, Salat-Baroux J, Frydman R. The use of a GnRH antagonist (Cetrorelix) in a single dose protocol in IVF-embryo transfer: a dose finding study of 3 versus 2 mg. Hum Reprod 1998;13(9):2411–4. [DOI] [PubMed]
- 9.Diedrich K, Diedrich C, Santos E, Bauer O, Zoll C, al-Hasani S, et al. [Suppression of endogenous LH increase in ovarian stimulation with the GnRH antagonist Cetrorelix]. Geburtshilfe Frauenheilkd 1994;54(4):237–40. [DOI] [PubMed]
- 10.Albano C, Felberbaum RE, Smitz J, Riethmuller-Winzen H, Engel J, Diedrich K, et al. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. European Cetrorelix Study Group. Hum Reprod 2000;15(3):526–31. [DOI] [PubMed]
- 11.Hohmann FP, Macklon NS, Fauser BC. A randomized comparison of two ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle-stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. J Clin Endocrinol Metab 2003;88(1):166–73. [DOI] [PubMed]
- 12.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod 1998;13(12):3434–40. [DOI] [PubMed]
- 13.Pelinck MJ, Vogel NE, Hoek A, Arts EG, Simons AH, Heineman MJ. Minimal stimulation IVF with late follicular phase administration of the GnRH antagonist cetrorelix and concomitant substitution with recombinant FSH: a pilot study. Hum Reprod 2005;20(3):642–8. [DOI] [PubMed]
- 14.Weghofer A, Margreiter M, Bassim S, Sevelda U, Beilhack E, Feichtinger W. Minimal stimulation using recombinant follicle-stimulating hormone and a gonadotropin-releasing hormone antagonist in women of advanced age. Fertil Steril 2004;81(4):1002–6. [DOI] [PubMed]
- 15.Minaretzis D, Alper MM, Oskowitz SP, Lobel SM, Mortola JF, Pavlou SN. Gonadotropin-releasing hormone antagonist versus agonist administration in women undergoing controlled ovarian hyperstimulation: cycle performance and in vitro steroidogenesis of granulosa-lutein cells. Am J Obstet Gynecol 1995;172(5):1518–25. [DOI] [PubMed]
- 16.Eldar-Geva T, Zylber-Haran E, Babayof R, Halevy-Shalem T, Ben-Chetrit A, Tsafrir A, et al. Similar outcome for cryopreserved embryo transfer following GnRH-antagonist/GnRH-agonist, GnRH-antagonist/HCG or long protocol ovarian stimulation. Reprod Biomed Online 2007;14(2):148–54. [DOI] [PubMed]
- 17.Lee JR, Choi YS, Jee BC, Ku SY, Suh CS, Kim KC, et al. Cryopreserved blastocyst transfer: impact of gonadotropin-releasing hormone agonist versus antagonist in the previous oocyte retrieval cycles. Fertil Steril 2007;88(5):1344–9. [DOI] [PubMed]
- 18.Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online 2003;7(1):59–64. [DOI] [PubMed]
- 19.Licht P, von Wolff M, Berkholz A, Wildt L. Evidence for cycle-dependent expression of full-length human chorionic gonadotropin/luteinizing hormone receptor mRNA in human endometrium and decidua. Fertil Steril 2003;79(Suppl 1):718–23. [DOI] [PubMed]
- 20.Ziecik AJ, Bodek G, Blitek A, Kaczmarek M, Waclawik A. Nongonadal LH receptors, their involvement in female reproductive function and a new applicable approach. Vet J 2005;169(1):75–84. [DOI] [PubMed]
- 21.Kawate N, Tsuji M, Tamada H, Inaba T, Sawada T. Inhibition of luteinizing hormone receptor expression during the development of caprine corpora lutea by administration of gonadotropin-releasing hormone antagonist. Mol Reprod Dev 2002;63(4):444–50. [DOI] [PubMed]