Abstract
Purpose
The objective of this study was to use a nonhuman primate model to examine the effects of growth hormone (GH) on oocyte in vitro maturation (IVM).
Methods
Immunocytochemistry confirmed the presence of GH receptors in rhesus cumulus oocyte complexes and the cytoplasm of embryonic blastomeres. Recombinant human GH (r-hGH) was added to IVM medium and cumulus expansion, nuclear maturation, cytoplasmic maturation and embryo development were analyzed.
Results
Cumulus expansion was highest in the presence of 1 and 10 ng/ml r-hGH. The addition of r-hGH during IVM increased the percentage of embryos progressing to at least the 9–16 cell stage. In a separate study, 100 ng/ml r-hGH was supplemented to IVM and embryo culture medium and no effect was observed.
Conclusions
The presence of GH receptors along with increased cumulus expansion and embryos progressing to the 9–16 cell stage supports the hypothesis that r-hGH may be involved in oocyte maturation.
Keywords: Cumulus cells, Growth hormone, Non-human primate
Introduction
While in the follicle, the oocyte is surrounded by a network of cumulus and granulosa cells along with follicular fluid. This complex system enables the oocyte to receive nutrients and signals, which maintain and stimulate the oocyte during maturation [1, 2]. In vivo, follicles develop with increasing follicle stimulating hormone (FSH) levels [3]. A spontaneous luteinizing hormone (LH) surge then triggers maturation and ovulation of the oocyte from its follicle [3]. While it is known that FSH and LH are needed for maturation of the oocyte, the complex environment surrounding the oocyte makes it difficult to determine what nutrients, hormones and growth factors are beneficial for IVM.
For women undergoing superovulation in fertility clinics, follicular growth is stimulated by exogenous gonadotropins. The growth and support of multiple follicles is accomplished with FSH while the LH surge is mimicked with human chorionic gonadotropin (hCG) [4]. Oocytes can be aspirated without hCG, however these oocytes are compromised and have a decreased capacity to develop to the blastocyst stage in the non-human primate [5]. Optimizing the recovery and maturation of immature oocytes before stimulation with hCG is the first step in developing a successful IVM protocol. In vitro maturation is a desirable alternative as it reduces the risks for side effects (ovarian hyperstimulation syndrome), reduces cost and gives women who are sensitive to hormones a chance to recover some of their oocytes [4, 6]. In most primates, including macaques, apes and humans, only one oocyte matures and ovulates each menstrual cycle; accessing the remainder of the recruited cohort of oocytes before they degenerate would lead to an abundant supply of oocytes for use in research and clinical fertility treatments. In vitro maturation could be extremely beneficial for use in assisted reproductive technologies if the success rate increases.
The addition of GH to culture media has stimulatory effects on oocyte maturation in cattle, rats, horses, pigs and rabbits [7–10]. Growth hormone is produced by the pituitary, affecting growth, metabolism and reproduction [11, 12]. While GH increases production of estradiol in human granulosa cells cultured in vitro, little research regarding GH on IVM in the human or nonhuman primate has been done [13]. Human granulosa cells, cumulus cells and oocytes have been shown to express mRNA for the GH receptor (GHR) suggesting that GH may play a role in oocyte maturation [14, 15]. In vivo administration of GH enhanced the IVM and fertilization of human germinal vesicle (GV) oocytes supporting the hypothesis that GH may be essential for maturation [16]. Furthermore, higher concentrations of GH in human follicular fluid (4.1 ng/ml) appear to be indicative of follicles that yield developmentally competent oocytes compared to follicles with lower GH levels (2.1 ng/ml) [17].
Growth hormone improves the development of embryos when added to embryo culture media in mice, cattle and pigs [18–22]. In the mouse, GH added to embryo culture medium increased the proportion of 2-cell embryos forming blastocyst stage embryos and increased trophectoderm (TEM) cell counts in blastocyst stage embryos [18, 21]. Embryo culture medium supplemented with GH increased the percentage of bovine embryos reaching the blastocyst stage and increased inner cell mass (ICM) and TEM cell numbers [19, 22]. Growth hormone added to embryo culture medium increased the diameter of blastocyst stage porcine embryos [20].
In an effort to understand the influence growth factors have on the cumulus oocyte complex (COC) we investigated the effects of recombinant human GH (r-hGH) on maturation of rhesus macaque oocytes. This animal model was chosen for its similarity to ovarian function in the human and its accessibility in research. The in vitro effect of r-hGH in IVM medium on nuclear maturation, cumulus expansion, cytoplasmic maturation and developmental competence was evaluated. The effect of r-hGH on development was also investigated with r-hGH added to embryo culture media. The presence of GHRs on macaque COCs, developing embryos and blastocyst stage embryos was verified.
Materials and methods
Immunohistochemistry of ovarian tissue
Ovaries from four females were recovered at necropsy and placed in 4% paraformaldehyde for approximately 24 h and then moved into 70% ethanol. Ovaries were embedded in paraffin and sectioned at 2–5 μm by the UC Davis Mutant Mouse HistoPathology Lab. Tissue sections were placed on superfrost plus microscope slides (Fisher Scientific, Pittsburgh, USA) and stored at 4°C. Slides were deparaffinized and rehydrated in steps of xylene (2×), 100% ethanol (2×), 80% ethanol, 50% ethanol and distilled water in coplin jars. Slides were laid flat and a pappen (Daido Sangyo Co., Ltd., Tokyo, Japan) was used to create a hydrophobic barrier around the tissue section. For the remainder of the staining protocol solutions were added and removed from the tissue section with a pipetman. Sections were incubated with 0.2% Triton X-100, 0.1% Tween-20 in DPBS at 37°C in a humidified chamber for 30 minutes. Sections were then blocked with 1% bovine serum albumin, 30% normal goat serum in DPBS for 40 minutes in a humidified chamber at 37°C. The blocking solution was removed and sections were placed at 4°C overnight with mouse monoclonal anti-human GHR antibody in 0.2% Triton X-100, 0.1% Tween-20 in DPBS (R and D Systems, Minneapolis, USA). The primary antibody was substituted with 0.2% Triton X-100, 0.1% Tween-20 in DPBS overnight at 4°C for the negative controls. Tissue sections were washed (×3) with 0.2% Triton X-100, 0.1% Tween-20 in DPBS and incubated at 37°C for 1 h in a humidified environment with Alexa 555 goat anti-mouse IgG2b in 0.2% Triton X-100, 0.1% Tween-20 in DPBS (Molecular Probes, Carlsbad, USA). Slides were washed with in 0.2% Triton X-100, 0.1% Tween-20 in DPBS (×3), mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, USA) and stored at 4°C in the dark. Tissue sections were assessed using a Delta Vision microscope (Applied Precision, Issaquah, USA) with an Olympus UPlan Apo 60×/1.20w water emersion objective (Olympus Optical Co., Ltd., Tokyo, Japan).
Hormone injections and immature oocyte collection
Adult female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center (CNPRC). Animals were caged individually with a 0600–1800 hours light cycle and at a temperature maintained at 25–27°C. Animals were allowed to socialize by being housed in pairs during the day from approximately 0800–1400 hours. Animals were fed a diet of Purina Monkey Chow and water ad libitum. Seasonal produce, seeds and cereal were offered as supplements for environmental enrichment. Only females with a history of normal menstrual cycles were selected for this study. Females in this study were 4–12 years old. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis.
Females were observed daily for signs of vaginal bleeding and the first day of menses was assigned cycle day 1. Recombinant macaque FSH (r-mFSH; National Hormone and Peptide Program, AF Parlow, Los Angeles, USA) was administered (37.5 IU) twice daily, intramuscularly starting on cycle days 1–4 for 7 days total. The GnRH antagonist, Antide (Ares-Serono, Randolph, USA), was administered subcutaneously (0.5 mg/kg body wt.) once daily in the morning on days when r-mFSH was given to prevent endogenous gonadotropin secretion. Antide was given to half of the females used for the developmental study (n = 5) after COCs from a few females were observed to be mature upon retrieval (these females were not included in our study). Serum samples taken at time of oocyte aspiration were assayed for LH to confirm that an endogenous surge had not occurred. All serum samples were analyzed with a bioLH assay at the Endocrine Services Laboratory at Oregon NPRC (Beaverton, Oregon) as previously described [23]. All LH levels were below 25 ng/ml, not indicative of an endogenous LH surge, which ranges between 200 and 800 ng/ml [24, 25]. Collection of immature oocytes was performed on the morning following the last dose of r-mFSH. Animals were immobilized with ketamine hydrochloride (10 mg/kg) and oocytes were aspirated with a needle coated in 10,000 IU/ml heparin (Elkins-Sinn Inc., Cherry Hill, USA) by ultrasound-guided oocyte collection as previously described [26, 27]. Oocytes were collected into Tyrodes lactate (TL)-Hepes medium (37°C) containing 0.1 mg/ml polyvinyl alcohol (PVA) and 5 ng/ml recombinant human FSH (r-hFSH; Organon Inc., Roseland, USA) [28]. Aspirates were immediately placed in a heated isolette (37°C) where oocytes were retrieved from aspirates using an EM Con filter (Veterinary Concepts, Spring Valley, USA). To further eliminate the possibility of using oocytes that had been exposed to an LH surge, the morphology of COCs was observed and was found to be consistent with immature oocytes that were not exposed to an endogenous LH surge. Only oocytes completely surrounded by cumulus cells were used for IVM experiments. A total of 24 females were superovulated in which the entire cohort of COCs were used for the purpose of this study. Details regarding COC numbers retrieved per female and distribution between treatment groups is described thoroughly in the results section.
Hormone injections and mature oocyte collection
For collection of mature COCs, females were given recombinant hCG (1000 IU Ovidrel; Serono, Rockland, USA) on treatment day 8 in addition to the hormonal treatment outlined above for immature COC collection. COCs were aspirated 27–32 h following administration of hCG. Oocytes were aspirated and retrieved as outlined for immature oocyte collection.
Immunocytochemistry for GH receptor on COCs
Immature and mature COCs were collected for immunocytochemistry of GHR. All COCs used for immunocytochemistry were supernumerary COCs obtained from control cohorts of unrelated studies. A total of fourteen immature COCs (germinal vesicle stage) from five different females, superovulated with only r-mFSH (two to three immature COCs per female) were stained for GHR. Five additional immature COCs from three of the females mentioned above (one to two COCs per female) were stained as negative controls. Eight mature COCs from four different females, superovulated with r-mFSH and hCG (two mature COCs per female) were stained for GHR. Four additional COCs of varying nuclear status from three females mentioned above were stained as negative controls. Immature COCs were fixed immediately after aspiration in a microtubule stabilizing buffer for 1 h [29]. COCs were placed in blocking solution of phosphate buffered saline consisting of 2% powdered milk, 2% normal goat serum, 2% BSA, 0.1M glycine and 0.01% Triton X-100 overnight at 4°C [29]. After collection of mature COCs, the COCs were transferred into 70 µl drops of chemically defined, protein-free hamster embryo culture medium 9 (HECM-9) under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air [30]. Mature COCs were fixed 8 to16 h later in a microtubule stabilizing buffer for 1 h and then placed in blocking solution overnight at 4°C. Mature COCs were fixed within this time frame to ensure that oocyte nuclear status would be at metaphase II.
Immature and mature COCs were transferred from blocking solution into 30 μl drops of DPBS on separate ten well slides (PolySciences Inc., Warrington, USA). Washes were performed by the removal and subsequent addition of 30 μl of new media for 5 minutes. A pulled glass pipet was used for extraction of the media, leaving the COC on the slide at all times. All COCs were washed (×3) with DPBS and placed at 4°C overnight with mouse monoclonal anti-human GHR antibody. COCs were washed (×3) with DPBS and incubated at 37°C for 1 h in a humidified environment with Alexa 555 goat anti-mouse IgG2b. Immature COCs were washed in DPBS (×3), mounted in Vectashield with DAPI and stored at 4°C in the dark. Mature COCs were washed in DPBS (×3) and incubated in a 37°C, humid environment for 2 h with monoclonal rat anti-alpha-tubulin antibody and monoclonal mouse anti-beta-tubulin antibody (Accurate Scientific, Westbury, USA). After washing in DPBS (×3), COCs were incubated as described above for 1 h with Alexa 488 goat anti-mouse IgG conjugate and Alexa 488 goat anti-rat IgG conjugate (Molecular Probes, Carlsbad, USA). Mature COCs were washed in DPBS (×3), mounted in Vectashield with DAPI and stored at 4°C in the dark. The primary antibody was substituted with DPBS overnight at 4°C for the negative controls. COCs were imaged with a Zeiss LSM 510 confocal laser scanning microscope with a Zeiss Plan-NeoFluar, 40×/1.3 oil emersion objective for fluorescence (Carl Zeiss, Jena, Germany). Specimens were imaged with the same settings and threshold was set in Adobe Photoshop (Adobe Systems Inc., San Jose, USA) such that no fluorescence was observed with negative controls. Nomarski images of COCs were taken with an Olympus BX61 microscope with a UPlanApo 40×/0.85 lens (Olympus Optical Co., Ltd., Tokyo, Japan).
In vitro oocyte maturation with various r-hGH concentrations
Retrieved immature COCs were randomly placed into treatment drops of 70 μl Connaught Medical Research Laboratories Medium-1066 (37°C) containing 10% bovine calf serum (Gem Cell, Woodland, USA), human FSH and human LH (0.03 IU/ml Pergonal; Ares-Serono, Randolph, USA), 1 μg/ml androstenedione (Steraloids, Newport, USA) and either 0, 1, 10 or 100 ng/ml of r-hGH (R and D Systems, Minneapolis, USA) and incubated in a humidified atmosphere of 5% CO2 in air for 24 h at 37°C [28]. Images of the COCs were taken before and after 24 h of IVM for cumulus expansion (see below). Following incubation, COCs were either fixed for assessment of nuclear maturation or prepared for in vitro fertilization (IVF). COCs were fixed in a microtubule stabilizing buffer for 1 h and placed in a blocking solution overnight. It should be noted that the primate GHR is species specific, interacting with only human or rhesus GH [31, 32]. This is beneficial to our study as it eliminates the potential for any bovine GH present in the serum used for IVM culture media to have a substantial effect. Bovine GH has about a 3,000-fold lower affinity for the primate GHR than human GH, which was used in our experiments [31].
Cumulus expansion
Cumulus expansion was measured with images of the COCs taken before and after the 24-h incubation. Dishes with COCs were placed on a heated 37.5°C Nikon Eclipse TE300 microscope (Nikon Corporation, Kawasaki, Japan) for less than 1 minute and imaged with a MicroPublisher camera (QImaging, Burnaby, Canada). A Nikon Plan 4×/0.10 objective was used (Nikon Corporation, Kawasaki, Japan). A hemocytometer was imaged for scaling purposes. The area of each COC was measured three times by the same person with Image J (National Institutes of Health, Bethesda, USA) and an average was taken to reduce human error. Replicate COCs within each treatment group were averaged for each female. Percent increase in cumulus expansion was found by dividing the difference between the average COC area before and after incubation by the COC area before incubation.
Nuclear maturation
Nuclear status was assessed by immunostaining oocytes after 24 h of IVM for tubulin, actin and nuclear material. Cumulus oocyte complexes assessed for nuclear status were also measured for cumulus expansion. COCs were transferred from blocking solution to 30 μl DPBS drops on ten well slides and incubated in a 37°C, humid environment for 2 h with monoclonal rat anti-alpha-tubulin antibody and monoclonal mouse anti-beta-tubulin antibody. After washing in DPBS (×3), COCs were incubated again for 1 h with Alexa 555 goat anti-mouse IgG conjugate and Alexa 555 goat anti-rat IgG conjugate (Molecular Probes, Carlsbad, USA). COCs were washed with DPBS (×3) and incubated for 1 h with Alexa 488 Phalloidin (Molecular Probes, Carlsbad, USA). After washing with DPBS (×3), COCs were mounted in Vectashield with DAPI and slides were stored in the dark at 4°C. Nuclear maturation was assessed using a Delta Vision microscope with an Olympus 40×, UApo 340/1.35 oil emersion objective (Olympus Optical Co., Ltd., Tokyo, Japan). Based on the arrangement of tubulin and nuclear material, COCs were classified into one of the following stages. The germinal vesicle (GV) stage refers to the immature oocyte arrested at the diplotene stage of prophase I while metaphase I (MI) is characterized by the alignment of chromosomes at the equatorial plate of the metaphase spindle [33]. The metaphase I to metaphase II transition (MI to MII transition) is defined here as including anaphase I and telophase I (nuclear material has been pulled apart by the metaphase I spindle and is at opposite ends of the tubulin spindle). Metaphase II (MII) is characterized by the presence of a polar body and the MII spindle [33].
Cytoplasmic maturation and development after IVF
After 28–32 h of incubation in IVM medium, COCs were placed in 10 mg/ml hyaluronidase (MP Biomedicals, Solon, USA) in TL-Hepes medium containing 0.1 mg/ml PVA (TL-Hepes PVA) that was pre-equilibrated to 37°C [28]. Some of the cumulus cells were removed with gentle pipetting in the hyaluronidase solution. Oocytes were rinsed and transferred into TL medium containing 0.1 mg/ml PVA (TL-PVA) under oil (37°C) and inseminated according to standard procedure for IVF of rhesus macaque oocytes [34]. Semen was collected from male macaques that had been trained for this procedure as previously reported [35]. Semen was washed and resuspended in TL-BSA medium [28]. The next morning, oocytes were transferred into 70 μl drops of HECM-9 medium under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2, 10% O2 and 85% N2 for 48 h. After the 48-h incubation, oocytes/zygotes that did not cleave were fixed for assessment of nuclear status (above). The remaining embryos were transferred into 70 μl drops of HECM-9 medium with 5% bovine calf serum under mineral oil and incubated as described above. Embryos were transferred to fresh medium every other day until no further development was observed. When development ceased, embryos were classified as: 2–4 cell, 5–8 cell, 9–16 cell, 17–32 cell, morula or blastocyst stage embryos. Blastocyst stage embryos were briefly put in an acid Tyrodes solution (TL-PVA, pH 2.0) to remove the zona pellucida and fixed in 4% paraformaldehyde in DPBS for 20 minutes. Fixed blastocyst stage embryos were transferred into a 30 μl drop of DPBS under mineral oil and stored at 4°C until immunocytochemistry for differential cell counting could be performed.
Differential staining of blastocyst stage embryos
Fixed blastocyst stage embryos were transferred into 30 μl drops of DPBS on a ten well slide. Embryos were washed as described above for COCs with 0.25% Triton X-100 in DPBS (×3) and incubated in 0.25% Triton X-100 in DPBS at 37°C in a humidified atmosphere for 1 h. Embryos were incubated again for 1 h in filtered blocking solution and then placed in Oct ¾ mouse monoclonal IgG2b (Santa Cruz Biotechnology, Inc., Santa Cruz, USA) in filtered blocking solution overnight at 4°C. Washes with 0.1% Tween20 in DPBS (x3) were performed and embryos were incubated with Alexa 488 goat anti-mouse IgG (Molecular Probes, Carlsbad, USA) in filtered blocking solution for 2 h. Washes were performed with 0.1% Tween20 DPBS (×3) followed by washes with filtered DPBS (×3). Blastocyst stage embryos were mounted in Vectashield with DAPI and slides were stored in the dark at 4°C. Optical sectioning was performed with a Delta Vision microscope using a 20×, Olympus UApo 340/0.70 water emersion objective (Olympus Optical Co., Ltd., Tokyo, Japan). Images were coded and read blindly using softWoRx (Applied Precision, Issaquah, USA). Manual cell counts were taken of the inner cell mass (ICM) and trophectoderm (TEM) cells.
Developmental study with r-hGH in IVM and embryo culture
Hormone injections and oocyte collection was performed as described above for immature COCs. All females in this study were administered Antide to prevent endogenous gonadotropin secretion. Retrieved COCs were randomly placed into treatment drops of 70 μl (37°C) Connaught Medical Research Laboratories Medium-1066 [28] containing 10% bovine calf serum, human FSH and human LH, 1 μg/ml androstenedione and 100 ng/ml of r-hGH and incubated in a humidified atmosphere of 5% CO2 in air for 24 h at 37°C. Following incubation, COCs were prepared for IVF as described above. Oocytes were transferred into TL-PVA medium with either 0 (treatment A) or 100 ng/ml r-hGH (treatment B) under oil (37°C) and inseminated as outlined previously. The next morning, oocytes were transferred into 70 μl drops of HECM-9 with either 0 (treatment A) or 100 ng/ml r-hGH (treatment B) under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2, 10% O2 and 85% N2 for 48 h. After the 48-h incubation, oocytes/zygotes that had not cleaved were fixed for assessment of nuclear status. The remaining embryos were transferred into 70 μl drops of HECM-9 medium with 5% bovine calf serum and either 0 (treatment A) or 100 ng/ml r-hGH (treatment B) under mineral oil and incubated as described above. Embryos were transferred to fresh medium every other day until no further development was observed. Embryo development was assessed and blastocyst stage embryos were fixed and stained as described above for differential cell counting.
Immunocytochemistry of GHR on embryos
All embryos used for immunocytochemistry were supernumerary embryos obtained from control cohorts of unrelated studies. Three different females were superovulated after receiving only r-mFSH for the collection of immature COCs. Four different females were superovulated with r-mFSH and hCG as described above for the collection of mature COCs. Cumulus oocyte complexes were collected as described above for immature and mature oocytes. Immature oocytes were placed into IVM medium and inseminated 28–32 h later as described previously. Mature oocytes were transferred into 70 μl drops of HECM-9 under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air. COCs were inseminated approximately 6–8 h later as described for IVF and media changes were performed as described above. Before fixation, blastocyst stage embryos and embryos from two of the females listed above were briefly put in an acid Tyrodes solution to remove the zona pellucida. All embryos were fixed in a microtubule stabilizing buffer. A total of 15 embryos, from the 2-cell stage to morula, from five different females (two to five embryos each) were stained for GHR. Four additional embryos, ranging from the 4-cell stage to morula, from three of the above females (one to two embryos each) were stained as negative controls. Five blastocyst stage embryos from three females, including one female listed above, (one to two embryos each) were stained for GHR with an additional two blastocyst stage embryos stained as negative controls. Embryos were stained for GHR as described above for immature COCs. Instead of incubation with the primary antibody, negative controls were incubated with DPBS. Specimens were imaged with a Zeiss LSM 510 confocal laser scanning microscope with a Zeiss Plan-NeoFluar 40×/1.3 oil emersion objective for fluorescence. Nomarski images of embryos were taken with an Olympus BX61 microscope with a UPlanApo 40×/0.85 lens. Specimens were imaged with the same settings and threshold was set in Adobe Photoshop such that no fluorescence was observed with negative controls.
Statistical analysis
All data are expressed as the mean ± SEM. Cumulus expansion was assessed with a blocked one-way ANOVA and Dunnetts Multiple Comparison. Each stage of nuclear maturation was assessed with one-way ANOVA blocked for female. Developmental status was assessed with paired student’s t tests for each developmental state. Total cell count, ICM cell count, trophectoderm cell count and percentage of ICM to total cell count in blastocyst stage embryos were assessed with student’s t tests. Data was analyzed with Prism software (GraphPad Software, Inc., San Diego, USA) and was considered to be statistically significant if P < 0.05.
Results
Growth hormone receptor
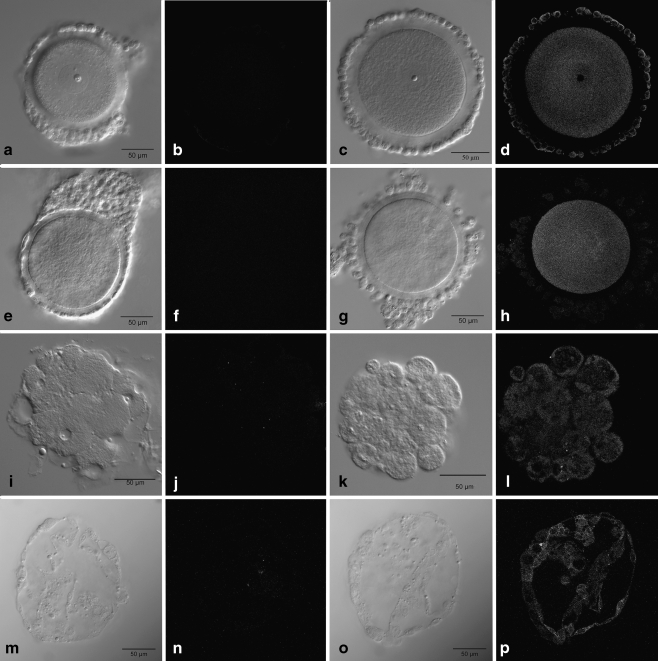
Immature COCs stained positive for GHR on the surface of cumulus cells and dispersed throughout the ooplasm and on the oocyte surface (Fig. 1d). Positive staining for GHR was not detected in the nucleolus. All immature COCs had a similar staining pattern. Negative controls showed a negligible amount of secondary background (Fig. 1b). Mature COCs (Fig. 1h) showed a similar staining pattern with respect to the oocyte while cumulus cells presented little to no staining on cumulus cell surfaces compared to the immature COCs. Again negative controls showed a negligible amount of secondary background (Fig. 1f). Nuclear status of mature COCs was confirmed based on spindle arrangement and nuclear material. All mature COCs were at MII. Nomarski images of the COCs are shown next to their respective fluorescence images for reference (Fig. 1a, c, e and g).
Fig. 1.
Laser scanning confocal images of rhesus macaque COCs, an 8- to 16-cell embryo and a blastocyst stage embryo stained for GHR along with negative controls. Nomarski images of germinal vesicle stage COCs (a, c), MII COCs (e, g), 8- to 16-cell embryos (i, k) and blastocyst stage embryos (m, o) are shown to the immediate left of their respective laser scanning confocal images. Negative controls for GHR can be seen for the germinal vesicle stage COC (b), MII COC (f), 8- to 16-cell embryo (j) and blastocyst stage embryo (n). Positive GHR immunostaining can be seen in the germinal vesicle COC (d), MII COC (h), 8- to 16-cell embryo (l) and blastocyst stage embryo (p)
Embryos stained for GHR all exhibited a similar staining pattern with GHRs distributed throughout the cytoplasm as seen in the 8–16 cell embryo and blastocyst stage embryo (Fig. 1l and p respectively). The distribution and localization of GHRs did not change throughout development. Negative controls showed a negligible amount of secondary background (Fig. 1j and n respectively). Nomarski images of the embryos are shown next to their respective fluorescence images for reference (Fig. 1i, k, m and o).
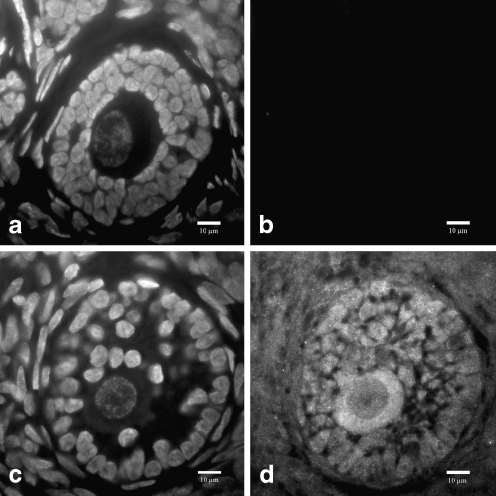
Ovarian tissue stained for GHR showed positive staining within the oocyte and surrounding granulosa cells (Fig. 2d). The surrounding stromal tissue (Fig. 2d) showed a weaker signal compared to the oocyte and granulosa cells but higher than the negative control (Fig. 2b). Ovarian tissue sections from four different females all exhibited a similar staining pattern. Nuclear material of the negative control tissue section and GHR stained tissue section was imaged for reference (Fig. 2a and c).
Fig. 2.
Ovarian tissue section immunostained for GHR and nuclear material (DAPI). A negative control for GHR immunostaining stains positive for nuclear material and negative for GHR (a and b, respectively). A growing preantral follicle stains positive for nuclear material and positive for GHR (c and d, respectively)
Effects of growth hormone on IVM
Cumulus expansion
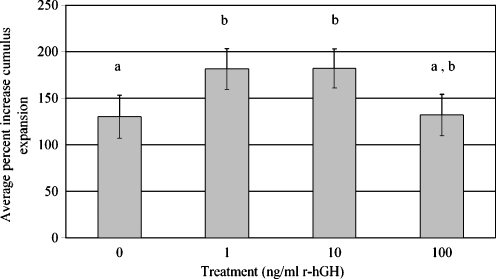
Cumulus oocyte complexes from nine females (n = 9) were used for cumulus expansion experiments (Fig. 3). An average of 22 ± 4 COCs from each female were evenly distributed between four treatment groups (average of 6 ± 1 COCs per treatment, 199 COCs total). Average percent increase in cumulus expansion was found to be higher in the 1 and 10 ng/ml r-hGH treatment groups and significantly greater than the control group. The percent increase in cumulus expansion was not significantly different in the 100 ng/ml r-hGH group compared to the control group (Fig. 3).
Fig. 3.
Average percent increase in cumulus expansion after 24 h in IVM medium supplemented with various concentrations of r-hGH. Each bar represents mean ± SEM. Significant differences between cumulus expansion are indicated by different letters (P < 0.05)
Nuclear maturation
Cumulus oocyte complexes from eight females (n = 8) were used for nuclear maturation experiments (Table 1). These COCs were also measured for cumulus expansion (above). An average of 18 ± 3 COCs from each female were randomly distributed between four treatment groups (average of 5 ± 1 COCs per treatment, 146 COCs total). After 24 h of incubation in maturation medium, less than 25% of oocytes on average matured to MII in each treatment group. The difference in the percentages of oocytes maturing to at least MI, MI to MII transition and MII was not found to be statistically significant between treatments (Table 1). Percentages were found based on the initial number of oocytes used in each treatment per female.
Table 1.
Nuclear maturation data
| Treatment during IVM (r-hGH) | Total number of oocytes used | Total number of oocytes maturing to or beyond each stage indicated (percentage of oocytes per female maturing to or beyond each stage indicated) | ||
|---|---|---|---|---|
| MI | MI to MII transition | MII | ||
| 0 ng/ml | 37 | 32 (86.4 ± 5.4) | 12 (34.6 ± 10.5) | 8 (22.9 ± 9.1) |
| 1 ng/ml | 36 | 28 (77.7 ± 9.2) | 12 (41.5 ± 8.2) | 4 (14.0 ± 5.5) |
| 10 ng/ml | 37 | 29 (81.7 ± 5.9) | 13 (40.8 ± 11.7) | 7 (20.8 ± 8.4) |
| 100 ng/ml | 36 | 34 (95.3 ± 3.3) | 17 (52.9 ± 11.8) | 8 (23.5 ± 7.3) |
Percentages are means ± SEM, n = 8 females
Cytoplasmic maturation and development
Initially COCs from all four treatment groups were observed after fertilization for developmental competence (n = 5 females). Treatment groups of 1, 10 and 100 ng/ml r-hGH were not statistically different. To increase the sample size, COCs from five more females were subjected to IVM/IVF with 0 or 100 ng/ml r-hGH only, for a total of ten females (Table 2). Each female had an average of 12 ± 1 COCs that were evenly distributed between the treatment groups (6 ± 1 COCs per treatment, 124 COCs total). The 100 ng/ml dose of r-hGH was chosen based on preliminary nuclear maturation data (data not shown). Data is only presented for the two treatment groups: 0 and 100 ng/ml r-hGH (n = 10 females).
Table 2.
Developmental assessment of embryos from various IVM treatment groups
| Experiment | Treatment during IVM (r-hGH) (ng/ml) | Treatment during Embryo Culture (r-hGH) (ng/ml) | Total number of oocytes | Total number of cleaved zygotes progressing to or beyond each developmental stage (Percentage of cleaved zygotes per female progressing to or beyond each developmental stage) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–4 cells | 5–8 cells | 9–16 cells | 17–32 cells | Morula | Blastocyst | |||||
| GH during IVM | Control | 0 | 0 | 58 | 41 (100 ± 0) | 38 (92.7 ± 4.9) | 30 (73.8 ± 5.3)a | 18 (44.3 ± 7.1) | 15 (37.0 ± 6.0) | 12 (29.2 ± 7.2) |
| + GH | 100 | 0 | 66 | 45 (100 ± 0) | 45 (100 ± 0) | 41 (91.5 ± 6.6)b | 24 (49.2 ± 7.5) | 19 (39.0 ± 7.3) | 13 (28.0 ± 6.9) | |
Percentages are means ± SEM
a, bSignificant differences are indicated with different letters (P < 0.05)
No difference was seen in the percentage of total oocytes per female that matured to MII between the control (75.8 ± 5.8) and 100 ng/ml r-hGH treatment group (75.1 ± 7.9) in the developmental study. This data was obtained from analyzing the nuclear status of oocytes/zygotes that did not cleave and classifying cleaved oocytes as having matured to MII. Additionally, there was no significant difference between the percentage of oocytes that cleaved out of those that matured to MII, in the control (93.8 ± 3.2) and 100 ng/ml r-hGH group (93.2 ± 3.9). The increase in percentage of embryos that progressed to at least the 9–16 cell stage out of total cleaved oocytes in the r-hGH treatment group was statistically significant when compared to the control group (Table 2). No other differences were seen in embryo development between treatment groups (Table 2).
Differential cell count of blastocyst stage embryos from IVM
A total of 24 blastocyst stage embryos were analyzed, 12 from the control group and 12 from the r-hGH treatment group (0 and 100 ng/ml r-hGH, Table 3). The average embryo age was 8 days for both the control and r-hGH treatment group. No significant difference was seen in ICM, TEM and total cell counts or ICM to total cell count ratio of blastocyst stage embryos between the control and r-hGH treatment groups (Table 3).
Table 3.
Blastocyst cell counts from various IVM treatment groups
| Experiment | Treatment during IVM (r-hGH) (ng/ml) | Treatment during Embryo Culture (r-hGH) (ng/ml) | No. of blastocysts | Embryo age (days) | ICM cell number | Trophectoderm cell number | Total cell number | % ICM/Total cell count | |
|---|---|---|---|---|---|---|---|---|---|
| GH during IVM | Control | 0 | 0 | 12 | 8 | 17.4 ± 3.5 | 107.3 ± 16.0 | 124.8 ± 17.6 | 14.8 ± 3.2 |
| +GH | 100 | 0 | 12 | 8 | 23.1 ± 2.7 | 110 ± 17.5 | 133.1 ± 18.8 | 19.7 ± 2.9 |
Values are means ± SEM
Effect of extending GH treatment in embryo culture
Embryo development
Cumulus oocyte complexes from six different females were used for embryo development experiments with r-hGH added to either the IVM medium or IVM and embryo culture medium. Each female had an average of 13 ± 1 COCs that were evenly distributed between the treatment groups (6 ± 1 COCs per treatment, 76 COCs total). No difference was seen in the percentage of COCs that matured to MII between treatment A (66.6 ± 6.9) and B (76.2 ± 7.0). The percentage of cleaved embryos out of those oocytes that matured to MII was not different between treatment A (90.3 ± 6.2) and B (94.8 ± 3.3). No differences were seen in embryo development between treatment groups (Table 4).
Table 4.
Development assessment of embryos from various embryo culture treatment groups
| Experiment | Treatment during IVM (r-hGH) (ng/ml) | Treatment during Embryo Culture (r-hGH) (ng/ml) | Total number of oocytes | Total number of cleaved zygotes progressing to or beyond each developmental stage (Percentage of cleaved zygotes per female progressing to or beyond each developmental stage) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–4 cells | 5–8 cells | 9–16 cells | 17–32 cells | Morula | Blastocyst | |||||
| GH during IVM and Development | Control (treatment A) | 100 | 0 | 37 | 22 (100 ± 0) | 20 (91.1 ± 5.9) | 17 (73.9 ± 12.2) | 13 (55.8 ± 13.6) | 10 (44.2 ± 10.0) | 9 (40.0 ± 10.4) |
| + GH (treatment B) | 100 | 100 | 39 | 28 (100 ± 0) | 28 (100 ± 0) | 25 (90.3 ± 6.2) | 17 (59.2 ± 11.7) | 14 (48.1 ± 8.8) | 12 (41.1 ± 8.6) | |
Percentages are means ± SEM
Differential cell count of blastocyst stage embryos from extended GH treatment
A total of 21 blastocyst stage embryos were analyzed, 9 from treatment A and 12 from treatment B (Table 5). The embryo age was 8 days for both treatment groups. No significant difference was seen in total cell counts, ICM and TEM cell counts of blastocyst stage embryos between treatment groups (Table 5).
Table 5.
Blastocyst cell counts from various embryo culture treatment groups
| Experiment | Treatment during IVM (r-hGH) (ng/ml) | Treatment during Embryo Culture (r-hGH) (ng/ml) | No. of blastocysts | Embryo age (days) | ICM cell number | Trophectoderm cell number | Total cell number | % ICM/Total cell count | |
|---|---|---|---|---|---|---|---|---|---|
| GH during IVM and Development | Control (treatment A) | 100 | 0 | 9 | 8 | 21.9 ± 3.6 | 118.0 ± 8.8 | 139.9 ± 12.1 | 14.9 ± 1.6 |
| + GH (treatment B) | 100 | 100 | 12 | 8 | 16.6 ± 2.9 | 104.1 ± 16.1 | 120.7 ± 18.2 | 13.6 ± 1.5 |
Values are means ± SEM
Discussion
This study is the first to report the presence of GHRs on cumulus and oocyte cell surfaces and in the ooplasm of rhesus macaque oocytes. The presence of GHRs on cumulus cell surfaces in immature COCs supports our data showing increased cumulus expansion in the presence of 1 and 10 ng/ml r-hGH. In humans, granulosa and cumulus cells express mRNA for the GHR while one study has reported positive GHR immunoreactivity in the granulosa cells of a minority of antral follicles studied [14, 15, 36]. There have been conflicting reports regarding the presence of GHR mRNA in human oocytes [14, 15]. To our knowledge, the localization and distribution of GHRs using immunocytochemistry in the rhesus macaque COC has not been reported. GHRs located on the oocyte and within the ooplasm in the rhesus macaque support the view that GH may have a direct effect on the oocyte as shown in the mouse [37]. The localization of GHRs on cumulus cell surfaces in immature macaque COCs also indicate that GH action on the oocyte may be mediated through cumulus cells similar to what is observed in the rat and bovine [7, 8, 38]. To our knowledge the mechanism of GH action in human or primate COCs has not been investigated. Given the biology of the GHR, the loss of cumulus cell surface receptors in mature COCs could be due to proteolytic cleavage of membrane receptors resulting in soluble GH binding proteins or internalization and degradation of receptors as a result of GH binding and negative regulation [32, 39]. The mechanism and function of GHR loss from cumulus cell surfaces during oocyte maturation in the primate is unknown but this downregulation occurs at the same time as the remodeling of cumulus cell processes during oocyte maturation in the rhesus macaque (VandeVoort unpublished). Embryos also stained positive for GHR supporting the hypothesis that GH may influence embryo development.
The addition of r-hGH to maturation medium increases cumulus expansion. The lower concentrations of r-hGH had a statistically significant effect compared to the control. As part of the microenvironment surrounding the oocyte, cumulus cells may affect nuclear and cytoplasmic maturation of the oocyte through signaling mechanisms and the production of growth factors [29, 40, 41]. Cumulus expansion has been reported as necessary for ovulation, and fertilization rates of oocytes with expanded cumulus are higher than oocytes with poorly expanded cumulus masses [40, 42, 43]. Cumulus expansion was found to be correlated with oocyte developmental capacity in cattle, mice and pigs [40, 44, 45]. In many IVF protocols the degree of expansion is often used as a measure of oocyte quality [40, 46, 47]. Growth hormone increases cumulus expansion in the bovine and equine as well, demonstrating that GH affects the COC in a variety of species [9, 48].
The percentage of oocytes that matured to MII in our 24 h maturation experiments (control group, 22.9 ± 9.1) was lower than in our studies that analyzed nuclear status 48 h after insemination (control group, 75.8 ± 5.8). The increased percentage of MII oocytes in the later group is likely due to the effect of time on progression of nuclear maturation [4]. Although our experiments show no effect of GH on maturation rates or percentage of oocytes maturing to MII, this is not the case for all species. An increase in rate of maturation has been seen in the bovine and rat [7, 49]. A higher percentage of oocytes maturing at a faster pace could increase the probability of successful fertilization and potentially increase the developmental capacity of the oocyte. Our nuclear maturation data shows no difference with the addition of r-hGH to the IVM medium, indicating that either the effects of r-hGH are too subtle to detect with our small sample size or that r-hGH offers no benefit to nuclear maturation in the rhesus macaque.
The percentage of embryos that developed to the 9–16 cell stage in the 100 ng/ml r-hGH treatment group was significantly higher than the control. This increase in developmental potential may indicate improved cytoplasmic maturation, which is necessary for the oocyte to undergo fertilization and development [50, 51]. Since cumulus expansion was not increased in the 100 ng/ml r-hGH treatment group compared to the control, we speculate that increased embryo development to the 9–16 cell stage may be a result of r-hGH acting directly on the oocyte. The absence of increased cumulus expansion in the 100 ng/ml treatment group may be a result of excess r-hGH, causing dissociation of GH(GHR)2 complexes into monomeric complexes at the surface of cumulus cells or from the formation of incompetent GH2(GHR)2 complexes [52–55]. The quantity of GHRs at the oocyte surface may be greater than the quantity present on cumulus cell surfaces and thus the high dose of GH at this location may still be stimulatory as the receptors are not overloaded. It could also be that the concentration of GH close to the oocyte surface is less that the concentration at the periphery of the COC, causing a maximal effect on the oocyte and an antagonistic effect on the cumulus cells. It should be noted that in preliminary developmental studies the effect of GH from the 1 and 10 ng/ml r-hGH treatment groups was similar to the 100 ng/ml treatment group, further indicating that GH actions on the cumulus cells and oocyte may be separate. Further studies are needed to determine the role of GHR downregulation during maturation and the mechanism of GH action on the oocyte and cumulus cells.
In the bovine, oocytes exposed to GH had enhanced migration of cortical granules and sperm aster formation supporting the hypothesis that GH improves cytoplasmic maturation [56]. The increase in percentage of embryos developing to the 9–16 cell stage is of interest as genome activation occurs at the six to eight cell stage in macaque and human embryos [57–63]. The incidence of developmental failure after the time of genome activation is higher for in vitro matured oocytes compared to in vivo matured oocytes and is thought to be due to improper cytoplasmic maturation [64]. The increased percentage of embryos progressing to at least the 9–16 cell stage, after exposure to r-hGH during IVM, indicates that r-hGH may improve cytoplasmic maturation allowing the embryo to transition from maternal to embryonic control of development. The time that embryos reach the 9–16 cell stage approximately coincides with a media change in which bovine calf serum is added into the culture medium. The addition of serum at this time may mask any effect of r-hGH on subsequent embryo development by supplying a wide array of growth factors and nutrients. It should be noted that if the addition of serum at this time does not mask subsequent effects on development then the similar progression of embryos to the morula or blastocyst stage in both treatment groups may indicate that r-hGH has little to no effect on embryo development. Small sample sizes within groups can be difficult to statistically evaluate and there is always the possibility that the significance of effects or lack of effects could be altered with larger group sizes. The addition of GH alone during IVM of rhesus macaque oocytes does not increase the developmental capacity (% blastocysts) to levels seen for in vivo matured oocytes (60.8%)[64].
The total cell count, TEM count, ICM count and percentage of ICM to total cell count did not differ between treatment groups. In the mouse and human, quantitative measurements of the ICM have been shown to be correlated with implantation potential while in the rhesus macaque inadequate development of the ICM has been observed with in vitro embryos compared to flushed in vivo embryos [65–68]. The data presented here show no difference in ICM counts and percentage of ICM to total cell counts between treatment groups, suggesting that r-hGH would not be beneficial in increasing implantation rates compared to the control group.
In our first set of experiments COCs were only exposed to r-hGH for 24 h during IVM. While r-hGH improves the percentage of embryos that develop to the 9–16 cell stage, this may be the only effect observed with such a limited time exposure. In an effort to determine if r-hGH is beneficial during embryo development we first confirmed the presence of GHRs on embryos produced from IVF of mature COCs.
To investigate the effects of r-hGH on embryo development we performed a study in which r-hGH was added to the IVM and embryo culture medium. No difference was seen in maturation, cleavage, development or cell counts of blastocyst stage embryos between treatment groups. Although the GHR is present in rhesus macaque embryos, r-hGH does not appear beneficial to embryo development or quality of blastocyst stage embryos. It should be noted that embryo development in this study may be impaired by IVM to the point where the embryos cannot benefit from the addition of GH to culture medium. The beneficial effects of GH on embryo development in mice and pigs were seen in studies in which in embryos produced in vivo were exposed to GH in vitro, making comparisons with our findings difficult [18, 20, 21]. The effects of GH on embryo development in the bovine were seen in studies in which COCs collected from slaughterhouse ovaries were matured and fertilized [19, 22]. While GH had a beneficial effect on embryo development in the bovine, comparing the quality of oocytes and IVM culture system of this species with that of primates is difficult.
Conclusions
Cytoplasmic maturation is challenging for researchers to study as there are numerous nutrients, growth factors, hormones and signaling factors that may be necessary for successful maturation. At present, culture conditions are not optimized and further research is needed in order to understand the environment surrounding the oocyte and to reproduce those conditions in culture. The presence of GH receptors in the COC along with increased cumulus expansion and development to the 9–16 cell stage supports the hypothesis that r-hGH may be beneficial to oocytes matured in vitro. While no difference was seen in the percentage of embryos developing to the blastocyst stage, it is possible that the measured endpoints of percent blastocyst stage embryos and ICM/total cell ratio may overlook subtle changes that have occurred. Embryo transfer experiments would provide more information on the developmental competence of GH treated oocytes and embryos, however the cost of this procedure in monkeys is prohibitive and precludes its use as a routine endpoint in embryo development. In lieu of embryo transfer experiments, subtle changes in cytoplasmic maturation may be detected with gene expression analysis after IVM. The presence of the GH receptor on the COC along with increased cumulus expansion demonstrates the involvement of GH in oocyte maturation and warrants further investigation.
Acknowledgments
The authors thank Dana Hill and Pei-Hsuan Hung for technical assistance. Part of this study was conducted in a facility constructed with support from Research Facilities Improvement Program Grants Numbers C06 RR17348-01 and C06 RR12088-01 from the National Center for Research Resources, National Institutes of Health.
Funding National Institutes of Health (RR00169 and RR13439 to C.A.V.).
Footnotes
Capsule
GH receptors are present on macaque COCs and r-hGH in IVM medium increased cumulus expansion and percentage of embryos progressing to the 9–16 cell stage.
References
- 1.Dekel N, Lawrence TS, Gilula NB, Beers WH. Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev Biol 1981;86:356–62. [DOI] [PubMed]
- 2.Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev 2002;61:414–24. [DOI] [PubMed]
- 3.Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Curr Opin Obstet Gynecol 2004;16:211–9. [DOI] [PubMed]
- 4.Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Updat 1998;4:103–20. [DOI] [PubMed]
- 5.Schramm RD, Bavister BD. A macaque model for studying mechanisms controlling oocyte development and maturation in human and non-human primates. Hum Reprod 1999;14:2544–55. [DOI] [PubMed]
- 6.Bergh PA, Navot D. Ovarian hyperstimulation syndrome: a review of pathophysiology. J Assist Reprod Genet 1992;9:429–38. [DOI] [PubMed]
- 7.Apa R, Lanzone A, Miceli F, Mastrandrea M, Caruso A, Mancuso S, et al. Growth hormone induces in vitro maturation of follicle- and cumulus-enclosed rat oocytes. Mol Cell Endocrinol 1994;106:207–12. [DOI] [PubMed]
- 8.Izadyar F, Van Tol HT, Colenbrander B, Bevers MM. Stimulatory effect of growth hormone on in vitro maturation of bovine oocytes is exerted through cumulus cells and not mediated by IGF-I. Mol Reprod Dev 1997;47:175–80. [DOI] [PubMed]
- 9.Marchal R, Caillaud M, Martoriati A, Gerard N, Mermillod P, Goudet G. Effect of growth hormone (GH) on in vitro nuclear and cytoplasmic oocyte maturation, cumulus expansion, hyaluronan synthases, and connexins 32 and 43 expression, and GH receptor messenger RNA expression in equine and porcine species. Biol Reprod 2003;69:1013–22. [DOI] [PubMed]
- 10.Yoshimura Y, Nakamura Y, Koyama N, Iwashita M, Adachi T, Takeda Y. Effects of growth hormone on follicle growth, oocyte maturation, and ovarian steroidogenesis. Fertil Steril 1993;59:917–23. [DOI] [PubMed]
- 11.Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab 2001;12:252–7. [DOI] [PubMed]
- 12.Hull KL, Harvey S. Growth hormone: roles in female reproduction. J Endocrinol 2001;168:1–23. [DOI] [PubMed]
- 13.Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J Endocrinol 1990;126:R1–4. [DOI] [PubMed]
- 14.Menezo YJ, el Mouatassim S, Chavrier M, Servy EJ, Nicolet B. Human oocytes and preimplantation embryos express mRNA for growth hormone receptor. Zygote 2003;11:293–7. [DOI] [PubMed]
- 15.Sharara FI, Nieman LK. Identification and cellular localization of growth hormone receptor gene expression in the human ovary. J Clin Endocrinol Metab 1994;79:670–2. [DOI] [PubMed]
- 16.Hassan HA, Azab H, Rahman AA, Nafee TM. Effects of growth hormone on in vitro maturation of germinal vesicle of human oocytes retrieved from small antral follicles. J Assist Reprod Genet 2001;18:417–20. [DOI] [PMC free article] [PubMed]
- 17.Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R, et al. Follicular fluid markers of oocyte developmental potential. Hum Reprod 2002;17:1017–22. [DOI] [PubMed]
- 18.Fukaya T, Yamanaka T, Terada Y, Murakami T, Yajima A. Growth hormone improves mouse embryo development in vitro, and the effect is neutralized by growth hormone receptor antibody. Tohoku J Exp Med 1998;184:113–22. [DOI] [PubMed]
- 19.Izadyar F, Van Tol HT, Hage WG, Bevers MM. Preimplantation bovine embryos express mRNA of growth hormone receptor and respond to growth hormone addition during in vitro development. Mol Reprod Dev 2000;57:247–55. [DOI] [PubMed]
- 20.Kidson A, Rubio-Pomar FJ, Van Knegsel A, Van Tol HT, Hazeleger W, Ducro-Steverink DW, et al. Quality of porcine blastocysts produced in vitro in the presence or absence of GH. Reproduction 2004;127:165–77. [DOI] [PubMed]
- 21.Markham KE, Kaye PL. Growth hormone, insulin-like growth factor I and cell proliferation in the mouse blastocyst. Reproduction 2003;125:327–36. [DOI] [PubMed]
- 22.Moreira F, Paula-Lopes FF, Hansen PJ, Badinga L, Thatcher WW. Effects of growth hormone and insulin-like growth factor-I on development of in vitro derived bovine embryos. Theriogenology 2002;57:895–907. [DOI] [PubMed]
- 23.Ellinwood WE, Resko JA. Sex differences in biologically active and immunoreactive gonadotropins in the fetal circulation of rhesus monkeys. Endocrinology 1980;107:902–7. [DOI] [PubMed]
- 24.Molskness TA, Woodruff TK, Hess DL, Dahl KD, Stouffer RL. Recombinant human inhibin-A administered early in the menstrual cycle alters concurrent pituitary and follicular, plus subsequent luteal, function in rhesus monkeys. J Clin Endocrinol Metab 1996;81:4002–6. [DOI] [PubMed]
- 25.Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod 2003;18:2257–63. [DOI] [PubMed]
- 26.Vandevoort CA, Baughman WL, Stouffer RL. Comparison of different regimens of human gonadotropins for superovulation of rhesus monkeys: ovulatory response and subsequent luteal function. J In Vitro Fert Embryo Transf 1989;6:85–91. [DOI] [PubMed]
- 27.VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology 2003;59:699–707. [DOI] [PubMed]
- 28.Boatman DE. In vitro growth of non-human primate pre- and peri- implantation embryos. In: Bavister BD, editor. The mammalian preimplantation embryo: regulation of growth and differentiation in vitro. New York: Plenum Press; 1987. p. 273–308.
- 29.Allworth AE, Albertini DF. Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Dev Biol 1993;158:101–12. [DOI] [PubMed]
- 30.McKiernan SH, Bavister BD. Culture of one-cell hamster embryos with water soluble vitamins: pantothenate stimulates blastocyst production. Hum Reprod 2000;15:157–64. [DOI] [PubMed]
- 31.Liu JC, Makova KD, Adkins RM, Gibson S, Li WH. Episodic evolution of growth hormone in primates and emergence of the species specificity of human growth hormone receptor. Mol Biol Evol 2001;18:945–53. [DOI] [PubMed]
- 32.Martini JF, Pezet A, Guezennec CY, Edery M, Postel-Vinay MC, Kelly PA. Monkey growth hormone (GH) receptor gene expression. Evidence for two mechanisms for the generation of the GH binding protein. J Biol Chem 1997;272:18951–8. [DOI] [PubMed]
- 33.Briggs D, Miller D, Gosden R. Molecular biology of female gametogenesis. In: Fauser B, Rutherford A, Strauss J, editors. Molecular biology in reproductive medicine. New York: The Parthenon Publishing Group; 1999. p. 254–6.
- 34.Schramm RD, Bavister BD. Follicle-stimulating hormone priming of rhesus monkeys enhances meiotic and developmental competence of oocytes matured in vitro. Biol Reprod 1994;51:904–12. [DOI] [PubMed]
- 35.Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol 1991;20:122–5. [PubMed]
- 36.Tamura M, Sasano H, Suzuki T, Fukaya T, Watanabe T, Aoki H, et al. Immunohistochemical localization of growth hormone receptor in cyclic human ovaries. Hum Reprod 1994;9:2259–62. [DOI] [PubMed]
- 37.Kiapekou E, Loutradis D, Drakakis P, Zapanti E, Mastorakos G, Antsaklis A. Effects of GH and IGF-I on the in vitro maturation of mouse oocytes. Hormones (Athens) 2005;4:155–60. [DOI] [PubMed]
- 38.Kolle S, Sinowatz F, Boie G, Lincoln D. Developmental changes in the expression of the growth hormone receptor messenger ribonucleic acid and protein in the bovine ovary. Biol Reprod 1998;59:836–42. [DOI] [PubMed]
- 39.Zhu T, Goh EL, Graichen R, Ling L, Lobie PE. Signal transduction via the growth hormone receptor. Cell Signal 2001;13:599–616. [DOI] [PubMed]
- 40.Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev 1993;34:87–93. [DOI] [PubMed]
- 41.Moor R, Osborn J, Crosby I. Cell interactions and ooctye regulation in mammals. In: Rolland R, Van Hall E, Hillier S, McNatty K, editors. Follicular maturation and ovulation. Oxford: Excerpta Medica; 1981. p. 249–64.
- 42.Ball GD, Leibfried ML, Lenz RW, Ax RL, Bavister BD, First NL. Factors affecting successful in vitro fertilization of bovine follicular oocytes. Biol Reprod 1983;28:717–25. [DOI] [PubMed]
- 43.Zhuo L, Kimata K. Cumulus oophorus extracellular matrix: its construction and regulation. Cell Struct Funct 2001;26:189–96. [DOI] [PubMed]
- 44.Qian Y, Shi WQ, Ding JT, Sha JH, Fan BQ. Predictive value of the area of expanded cumulus mass on development of porcine oocytes matured and fertilized in vitro. J Reprod Dev 2003;49:167–74. [DOI] [PubMed]
- 45.Rose-Hellekant TA, Libersky-Williamson EA, Bavister BD. Energy substrates and amino acids provided during in vitro maturation of bovine oocytes alter acquisition of developmental competence. Zygote 1998;6:285–94. [DOI] [PubMed]
- 46.Foote RH. In vitro fertilization and embryo transfer in domestic animals: applications in animals and implications for humans. J In Vitro Fert Embryo Transf 1987;4:73–88. [DOI] [PubMed]
- 47.Laufer N, Tarlatzis BC, Naftolin F. In vitro fertilization: state of the art. Semin Reprod Endocrinol 1984;2:197–219.
- 48.Kolle S, Stojkovic M, Boie G, Wolf E, Sinowatz F. Growth hormone-related effects on apoptosis, mitosis, and expression of connexin 43 in bovine in vitro maturation cumulus-oocyte complexes. Biol Reprod 2003;68:1584–9. [DOI] [PubMed]
- 49.Izadyar F, Colenbrander B, Bevers MM. In vitro maturation of bovine oocytes in the presence of growth hormone accelerates nuclear maturation and promotes subsequent embryonic development. Mol Reprod Dev 1996;45:372–7. [DOI] [PubMed]
- 50.Thibault C. Hammond memorial lecture. Are follicular maturation and oocyte maturation independent processes? J Reprod Fertil 1977;51:1–15. [DOI] [PubMed]
- 51.Thibault C, Gerard M, Menezo Y. Preovulatory and ovulatory mechanisms in oocyte maturation. J Reprod Fertil 1975;45:605–10. [DOI] [PubMed]
- 52.Cunningham BC, Ultsch M, De Vos AM, Mulkerrin MG, Clauser KR, Wells JA. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 1991;254:821–5. [DOI] [PubMed]
- 53.Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, Wells JA. Rational design of potent antagonists to the human growth hormone receptor. Science 1992;256:1677–80. [DOI] [PubMed]
- 54.Gent J, Van Den Eijnden M, Van Kerkhof P, Strous GJ. Dimerization and signal transduction of the growth hormone receptor. Mol Endocrinol 2003;17:967–75. [DOI] [PubMed]
- 55.Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci U S A 2002;99:9858–63. [DOI] [PMC free article] [PubMed]
- 56.Izadyar F, Hage WJ, Colenbrander B, Bevers MM. The promotory effect of growth hormone on the developmental competence of in vitro matured bovine oocytes is due to improved cytoplasmic maturation. Mol Reprod Dev 1998;49:444–53. [DOI] [PubMed]
- 57.Artley JK, Braude PR, Johnson MH. Gene activity and cleavage arrest in human pre-embryos. Hum Reprod 1992;7:1014–21. [DOI] [PubMed]
- 58.Schramm RD, Bavister BD. Onset of nucleolar and extranucleolar transcription and expression of fibrillarin in macaque embryos developing in vitro. Biol Reprod 1999;60:721–8. [DOI] [PubMed]
- 59.Tesarik J. Gene activation in the human embryo developing in vitro. In: Feichtinger W, Kemeter P, editors. Future aspects in human in vitro fertilization. Berlin: Springer-Verlag; 1987. p. 251–61.
- 60.Tesarik J, Kopecny V, Plachot M, Mandelbaum J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J Reprod Fertil 1986;78:463–70. [DOI] [PubMed]
- 61.Tesarik J, Kopecny V, Plachot M, Mandelbaum J. Early morphological signs of embryonic genome expression in human preimplantation development as revealed by quantitative electron microscopy. Dev Biol 1988;128:15–20. [DOI] [PubMed]
- 62.Tesarik J, Kopecny V, Plachot M, Mandelbaum J, Da Lage C, Flechon JE. Nucleologenesis in the human embryo developing in vitro: ultrastructural and autoradiographic analysis. Dev Biol 1986;115:193–203. [DOI] [PubMed]
- 63.Weston AM, Wolf DP. Timing of the maternal to embryonic transition in the rhesus monkey embryos. In Program of 27th annual meeting of the Society for the Study of Reproduction, Ann Arbor, MI, 1994, Abstract P297.
- 64.Schramm RD, Paprocki AM, VandeVoort CA. Causes of developmental failure of in-vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Hum Reprod 2003;18:826–33. [DOI] [PubMed]
- 65.Enders AC, Boatman D, Morgan P, Bavister BD. Differentiation of blastocysts derived from in vitro-fertilized rhesus monkey ova. Biol Reprod 1989;41:715–27. [DOI] [PubMed]
- 66.Enders AC, Schlafke S. Differentiation of the blastocyst of the rhesus monkey. Am J Anat 1981;162:1–21. [DOI] [PubMed]
- 67.Lane M, Gardner DK. Differential regulation of mouse embryo development and viability by amino acids. J Reprod Fertil 1997;109:153–64. [DOI] [PubMed]
- 68.Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril 2001;76:1157–67. [DOI] [PubMed]