Abstract
Background
The purpose of our research was to examine the relationship between male age and semen parameters in a range of ages (from 20s to 60s) in Egg Donation Program (EDP) cycles. EDP provides a pool of high quality oocytes, thus allowing better analysis of the sperm efficacy.
Discussion
The retrospective study population consisted of 484 male partners of patients undergoing EDP in in-vitro fertilization (IVF) treatments. A comparison was made of male age and sperm parameters within two groups: cycles resulting in a pregnancy (pregnant group) and cycles which failed to achieve a pregnancy (non-pregnant group). The men involved in the pregnant group were found to be significantly younger 43.2 ± 8.1 than those of the non-pregnant group 46.8 ± 7.8 (p = 0.003). Analysis of sperm morphology revealed a significant prevalence of teratozoospermia in males of the non-pregnant group, as compared to the males of the pregnant group (29% vs. 11%, respectively). The results also demonstrate that sperm parameters are apparently not diminished until men reach the age of 40. However, between 40–50 years old semen parameters deteriorate. Male age was found to be related to a reduction in sperm strict criteria: 44.8 in normozoospermia, 47.9 (p = 0.02), 48.4 (p = 0.04) and 51.9 (p = 0.001) years old in mild teratozoospermia, moderate teratozoospermia and severe teratozoospermia, respectively. Additionally, the results showed that the percentage of “healthy” embryos on day 3 of embryo culture was lower in the non-pregnant group (26%), as compared with the pregnant group (34%; p = 0.01).
Conclusion
Our study confirms that sperm parameters are reduced by age and suggests that this age-dependent effect could be a reason for failures in IVF cycles even in EDP couples.
Keywords: Egg donation, Infertility, Male age, Sperm
Introduction
The increase in male life expectancy has raised issues concerning the impact of aging on male fertility. Although the effect of maternal age on fertility is well known, it is disputable whether fertility is affected by paternal age as well. Maternal ageing is a significant contributor to human infertility [1] due, primarily, to the precipitous loss of functional oocytes in women [2]. However, human spermatogenesis continues well into advanced ages, allowing men to reproduce during senescence. Understanding the effect of male age on fertility has become increasingly important due to the fact that a growing number of men are choosing to father children at older ages [3].
Semen quality is generally considered to be a measure of male fertility. Changes in semen quality can occur from the effects of age [4], which is associated with diminished semen volume, sperm motility or sperm morphology [5–6]. Most studies are based on volunteers, sperm donors and infertility clinic groups. Some of these studies were prepared on statistically small groups with young volunteers and sperm donors making it difficult to identify an age effect. For this reason data was recently collected on approximately 2,000 men whose partners were totally sterile [7].
The purpose of our study was to examine the magnitude and shape of the relationship between age and semen parameters in men across a wide range of ages (20 to 60) in the Egg Donation Program (EDP) cycles. EDP provides a relatively homogenous pool of high quality oocytes, which allow us to make inferences about male factors throughout the in vitro fertilization process and early embryonic development.
Methods
Patients and semen preparation
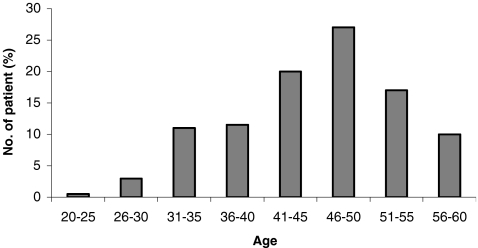
The study population consisted of 484 male IVF patients participating in the oocyte donor program, within the age range of 25 to 60 years old (Fig. 1). Each male is represented by only one data point.
Fig. 1.
Distribution of ages from 484 male partners intended for EDP
All freshly ejaculated semen samples were delivered to our IVF laboratory within 1 h of collection. The same laboratory technician performed all the examinations, with blind analysis of the age or identity of the patients. Semen analysis was performed according to published guidelines [8] and strict criteria [9]. Each specimen was tested for semen volume, spermatozoa concentration and motility (Mackler camera), and was stained by Spermac Stain kit (FeriPro, Belgium) for definition of strict criteria. Post-ejaculated liquefied semen was washed in 10 ml of Flushing Medium (MediCult, Denmark), supplemented with 3% of human serum albumin (HSA; Irvine Scientific, CA, USA) and then centrifuged at 600×g for 5 min. The pellet was resuspended in 0.5 ml of Flushing Medium 3% HSA, and the sperm cell suspension was cryopreserved in ampoules with 0.5 ml of Sperm Freezing Medium (MediCult), then incubated for 5 min at room temperature, followed by incubation in nitrogen vapors for 30 min and finally plunged into liquid nitrogen, until use.
Oocytes donation and fertilization
Donors The age of the donor is one of the most important factors influencing outcome [10]. Young women, of an average age of 24 (range 20–26), were accepted into the Egg Donation Program (EDP) cycles. All of these women were healthy, had normal menstrual cycles and no determined pathology. All these oocyte donors were recruited in accordance with acceptable ethical principles approved by the Israeli Health Ministry and HTFA (UK). Half of them had given birth in the past, none of them were under going infertility treatment, and all showed a normal ovarian reserve [11], which was assessed by determination of basal cycle day 3 serum FSH, LH and estradiol levels [12]. There is no general agreement on which is the best ovarian stimulation protocol to use in donors with the aim of recovering a cohort of fertilizable oocytes of highest developmental potential while minimizing the risk of ovarian hyperstimulation. Our stimulation regime included a long protocol for pituitary desensitization with a single administration of 3.75 mg I.M. of triptorelin acetate (Decapeptyl CR, Ferring, Germany) starting in the mid-luteal phase of the previous cycle. After the menstrual period gonadotropin stimulation with highly purified HMG (75 IU of FSH and 75 IU of LH; Menopure, Ferring, Germany) was initiated on day 2 after menses at the dose of 600 IU/l for 3 consecutive days and then continued in a step-down fashion until the lead follicles were ≥16 mm in diameter. Ultrasound-guided transvaginal oocyte retrieval was performed 35 h after hCG. Mature (M II) and morphologically normal oocytes from each donor were randomly allocated to two to three couples (six to seven oocytes/patient).
Fertilization The fertilization of the donated oocytes was performed by the ICSI method, after thawing and washing of the sperm. ICSI was performed in a dish with Flushing Medium 3% HSA droplets, covered with mineral oil (MediCult), equilibrated in an atmosphere of 5% CO2 at 37°C, followed by culturing in P1 Medium (Irvine Scientific) supplemented with 10% Serum Substitute Supplement (SSS; Irvine Scientific).
Fertilization was recorded if two pronuclei (2 pN) could be detected 12–18 h after microinjection. All embryos at the stage of 2 pN, obtained during these cycles, were prepared for cryopreservation in ampoules, utilizing Embryo Freezing Pack (MediCult), and then frozen by Planer (Planer Products Ltd, Sunbury, Middlesex, UK) cryo-apparatus. They were subsequently stored in liquid nitrogen until the recipient was ready for the embryo transfer. This allowed for easier orchestration of the recipient cycle for embryo transfer.
Recipients A total of 484 recipient couples were studied. There are controversies regarding the impact of the age of the recipient on implantation and pregnancy outcome. While some investigators have found a lower pregnancy rate in recipients of more advanced age [13], others have not observed this relationship [14]. The preparation of the recipient’s endometrium for embryo transfer is typically performed in estradiol-progesterone-supplemented cycles. Endometrial preparation, endometrial sonographic appearance and serum steroid hormonal levels have been signaled as important factors of success [15]. All women were screened for normalcy of the uterine cavity by means of hysterosalpingography, hydrosonography or hysteroscopy. The recipients’ ages ranged from 24 to 54 years with an average age of 44.3 ± 6.81 years old. They had been offered oocyte donation because of advanced reproductive age, previous poor ovarian response, repeated failure of IVF cycles or premature ovarian failure.
Endometrial preparation of the recipients was performed with oral estradiol valerate (Progynova, Schering AG, Berlin, Germany) 4 mg/day for 3 days followed by 6 mg/day for 3 days, and then 8 mg/day for 6 days. The administration of intravaginal progesterone 100 mg (Endometrin 100; Ferring, Germany) at a dose of 100 mg, three times a day, was begun on day 15 or later, but not before the endometrial thickness reached 7 mm. There was no need to synchronize the follicular phase with the donor’s cycle since all the embryo transfers were of thawed 2 pN embryos. Endometrial thickness and pattern were assessed on day 15 (prior to the initiation of progesterone supplementation) by transvaginal ultrasonography. Three zygotes were then thawed on day 3 of progesterone administration (day +1 of normal ovulatory cycle), using Embryo Thawing Pack (MediCult), and cultured in P1 Medium, supplemented with 10% SSS. Embryos which developed to the stage of six to eight blastomers, and contained less than 10% fragmentation on day 3 (48 h after thawing) were defined as “good” embryos. One or two embryos (average 1.8) were transferred on day 3 (day 18 of the supplemented cycle) after sequentional estrogen-progesterone preparation of the endometrium. There was no excess of post-thaw embryos which were not transferred due to the low morphology of the embryo or its arrested development. At 14 days after the embryo transfer, a blood test for β-hCG assessment was performed. Clinical pregnancy was confirmed at 6 weeks by an ultrasound examination to detect the presence of a gestational sac and assess its viability.
Statistical analysis
Male age was compared between the cycles resulting in a pregnancy (pregnant group; n = 110) and those which failed (non-pregnant group; n = 374). These two groups were then examined for the survival rate of zygotes, following thawing, and the embryo development rate on day 3 of the embryo culture. The main question was to define whether a decrease in sperm strict criteria is a function of male age that could lead to failed pregnancy. For this reason all spermatozoal samples were divided by strict criteria and male ages accepted in each strict criteria group. To find the critical age threshold with respect to sperm parameters all spermatozoa samples were divided into small male age groups of 5 years each and sperm volume, morphology, concentration and motility were compared between these age groups. For the statistical analysis we employed Wilcoxon Signed Rank test by Statistical Analysis System (SAS) for Windows V8. Paired results were compared by paired Student’s test (t test). Statistical significance of differences between individual parameters was examined by t test analysis of variance. Values of p < 0.05 were considered statistically significant. Data are presented as means ± standard deviation (SD).
Results
The average age of 484 men in the study was 45.9 ± 8.2 years old (range 25–60 years old; Fig. 1). The recipient women's average age was 44.3 ± 6.81 years old (range 24–54). The zygote survival rate (after freezing and thawing processes) was 83% and 90% in the non-pregnant and pregnant groups, respectively. The percentage of “good” embryos on day 3 of embryo culture was significantly lower in the non-pregnant group (26%), as compared with the pregnant group (34%; p = 0.01; Table 1). We found that men were significantly younger in the pregnancy group with a average age of 43.2 ± 8.1, as compared with an age of 46.8 ± 7.8 (p = 0.003) in the non-pregnant group (Table 1). Moreover, the average age of men in the pregnant group, with a pregnancy that progressed over 12 weeks, was 43.2 ± 7.8. The average age of men in the pregnant group, with missed abortion, was 46.4 ± 2.1 (Table 1).
Table 1.
Comparison of zygote survival rate, high quality embryo rate and male age between the pregnant (n = 110) and the non-pregnant (n = 374) groups
| Zygote survival rate (%) | High quality embryo rate on day 3 (%) | Male age (years ± SD) | |
|---|---|---|---|
| Non-pregnant | 90 | 26 | 46.8 ± 7.8a |
| Pregnant | 83 | 34 | 43.2 ± 8.1 |
| pregnancy (>12 weeks) | 43.2 ± 7.8 | ||
| missed abortion (<12 weeks) | 46.4 ± 2.1 |
aStatistically different from pregnant group
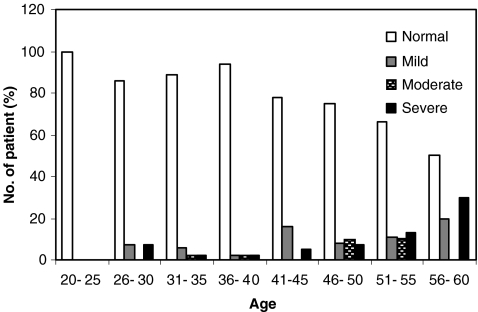
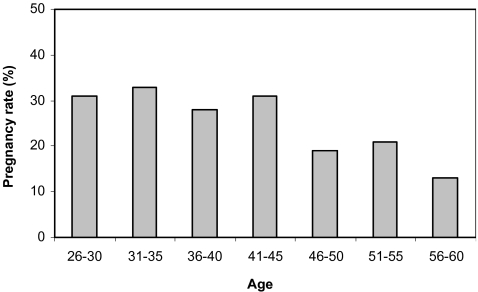
A relationship was found between the age and sperm morphology of the male partner (Table 2). Male age grew as the morphology declined: in normozoospermia 44.8 ± 8.3 years old, mild teratozoospermia 47.9 ± 7.7 years old (p = 0.02), moderate teratozoospermia 48.4 ± 7.0 years old (p = 0.04) and severe teratozoospermia 51.9 ± 7.3 years old (p = 0.001). Analysis of strict criteria revealed a significant prevalence (28%; p = 0.04) of teratozoospermia in males of the non-pregnant group, as compared to 11% in the males of the pregnant group (Table 2). The male age with normal strict criteria was significantly older in the non-pregnant group, as compared with the pregnant group, 45.2 ± 8.4 and 42.9 ± 8.0, respectively (p = 0.05). In the non-pregnant group the male age was older with a decline in morphology of spermatozoa (Table 2). Only one severe teratozoospermic sample, from a 56 years old male, was found in the pregnant group. As the results show, sperm morphology is not diminished until the age of 40 (Fig. 2). However, in older men the number of normal strict criteria cases is decreased concomitantly with higher incidences of mild- to severe- teratozoospermia. Other sperm parameters such as volume, concentration and motility also decrease with age (Table 3). The pregnancy rate of 30% of males under the age of 45 is subsequently decreased to 13% for those between 56–60 years (Fig. 3).
Table 2.
Comparison of male age between different sperm strict criteria groups and between the pregnant and the non-pregnant groups
| Parameters | Sperm strict criteria | ||||
|---|---|---|---|---|---|
| Normal (>14) | Mild (9–13) | Moderate (5–8) | Severe (0–4) | Total | |
| n | 370 | 49 | 25 | 40 | 484 |
| Male age | 44.8 ± 8.3 | 47.9 ± 7.7 | 48.4 ± 7.0 | 51.9 ± 7.3 | |
| p value | 0.02 | 0.04 | 0.001 | ||
| n | 272 | 43 | 20 | 39 | 374 |
| Non-pregnant (%) | 72 | 12 | 6 | 10 | 100 |
| Non-pregnant (years) | 45.2 ± 8.4 | 47.0 ± 7.7 | 48.7 ± 7.6 | 51.7 ± 7.4 | |
| p value | 0.01 | 0.04 | 0.001 | ||
| n | 98 | 6 | 5 | 1 | 110 |
| Pregnant (%) | 89 | 5 | 5 | 1 | 100 |
| Pregnant (years) | 42.9 ± 8.0 | 47.1 ± 5.0 | 45.4 ± 7.3 | 56.0 ± 0.0 | |
| p value | 0.05 | ||||
Fig. 2.
Diminishing of semen strict criteria according to the age of male partners. The histograms represent types of normo- or teratozoospermia
Table 3.
Comparison of semen volume, semen concentration and motility between different groups of male age
| Male age (years old) | |||||||
|---|---|---|---|---|---|---|---|
| 26–30 | 31–35 | 36–40 | 41–45 | 46–50 | 51–55 | 56–60 | |
| Volume (ml) | 4.8 ± 1.8 | 4.5 ± 2.7 | 4.1 ± 1.8 | 3.2 ± 1.5 | 2.9 ± 1.9a | 2.5 ± 1.0a | 2.3 ± 1.3a |
| Concentration (106/ml) | 114 ± 65 | 90 ± 58 | 72 ± 51 | 67 ± 37 | 58 ± 38 | 56 ± 37a | 16 ± 13a |
| Total count (106) | 547 ± 230 | 405 ± 265 | 280 ± 183 | 192 ± 179 | 168 ± 176 | 150 ± 138a | 37 ± 40a |
| Morphology (%) | 14.8 ± 1.8 | 13.7 ± 2.1 | 13.6 ± 1.7 | 12.9 ± 1.8 | 12.1 ± 1.6 | 11.2 ± 1.7a | 10.1 ± 1.9a |
| Motility (%) | 78 ± 28 | 70 ± 24 | 60 ± 25 | 60 ± 23 | 56 ± 20 | 53 ± 21a | 31 ± 19a |
| Progressive motility (%) | 39 ± 27 | 41 ± 26 | 44 ± 24 | 44 ± 23 | 39 ± 22 | 35 ± 18 | 12 ± 19a |
aStatistically different from groups until age of 40 years old
Fig. 3.
Distribution of pregnancy rate according to the age of male partners
Discussion
Most IVF patients referred to EDP are older women, after several years of unsuccessfully trying to resolve the female factor by means of conventional IVF procedures. Egg donation provides a relatively homogenous pool of high quality oocytes from young women, which allows variations in sperm quality with male age as a major dependent variable. Using EDP, as a novel model, we offer evidence of a male-age-dependent decline in morphology along with other sperm parameters and pregnancy rate.
In our study fertilization rates are not affected. Paulson and coauthors found no association between male age and the fertilization rate of donated oocytes in-vitro [16]. However, in contrast to our results, these authors also show a lack of association between male age and pregnancy rates. We observed a clear tendency to a reduction in early embryo development and pregnancy rates with increasing age, possibly as a result of changes in sperm morphology. It is very well known, that very severe sperm morphological defects (for example macrocephalic sperm or severely amorphous head morphology) may affect the quality of embryos, as they have a high rate of chromosomal abnormalities [17]. A significant majority of the successful in our pregnant group resulted from younger males, who also had better sperm parameters and speculatively gave a happier choice of higher quality embryos than those of the older males in the non-pregnant group. In support of the importance of spermatozoa quality in pregnancy achievement, Rosenbusch and Sterzik reported that men from couples with repeated spontaneous abortions had higher sperm chromosome abnormalities than men from fertile couples [18]. Therefore, we can speculate that the absence of pregnancy in the non-pregnant group, who had displayed low sperm parameters, could be associated with the deterioration in the sperm morphology, thus leading to genetic embryo abnormalities, lack of implantation and/or early miscarriage.
It has been documented in various publications that morphology in human spermatozoa, according to strict criteria, WHO criteria and the teratozoospermia index has no predictive value for the outcome after either IVF or ICSI [19–21]. In addition, there is no consensus on the direct relationship between spermatozoa morphology and genetic abnormalities. It has been postulated that there is no correlation between sperm morphology and genetic condition, which leads us to believe that there is no guarantee that spermatozoa with good morphology will not have any genetic abnormalities, and vice versa [22]. Other reports, such as Martin and co-authors, demonstrated that oligozoospermic samples have an increased probability of sperm chromosome abnormality, whether from mild to severe oligozoospermia [23], whereas chromosomal abnormalities of spermatozoa are not higher in aging males. It has been shown that low sperm morphology is associated with DNA fragmentation in men with an average age of 35 [24]. In addition, the same investigators reported that an increased DNA fragmentation rate decreases the pregnancy rate. A percentage of DNA damaged spermatozoa was demonstrated to be continuously on the rise within the age range of 20 to 60 [25].
The current study indicates a reduction in normozoospermia, with an increase in teratozoospermia, from 40 to 60 years old. Indeed, our results demonstrate that 89% of men in the pregnant group had normal sperm morphology and achieved 34% high quality embryos on day 3 of development, as opposed to in the non-pregnant group, where only 72% had normal sperm morphology and achieved 26% high quality embryos on day 3 of development. This indicates that younger men could have a higher probability of spermatozoa with better morphology, as well as a higher capacity to produce more viable embryos. There is a strong possibility that minor morphological anomalies, which are impossible to observe under conventional microscope magnification may allow a sperm with a lower fertility capacity to be chosen and included in the normal sperm strict criteria category. This point implies the need for the employment of a higher optical magnification for sperm selection, especially for ICSI. Such a new proposal is important and could be implemented not only in couples with previous ICSI failures, as performed by Bartoov’s methodology [26], but in general use for each routine ICSI procedure.
Average ages in both pregnant and non-pregnant groups were in the same range with the majority in the age range of 40 to 50. This age range falls into the “gray zone” of critical alterations in sperm morphology, which can explain the small, although significant differences between ages in these two groups. We have to admit that several pregnancies occurred from teratozoospermic men, with one case of pregnancy obtained from a 56 year old man with severe teratozoospermia. Pregnancies from teratozoospermic men, such as this one, show the power of ICSI method to facilitate “fishing out” of normal spermatozoon from a pool of severely teratozoospermic specimens.
In the current study, we focused on sperm morphology. We also demonstrated here the decrease in other sperm parameters such as volume, concentration and motility. This reduction of sperm parameters by male age is well established and described [27–30].
How much of recipient impact is in implantation and pregnancy rates? There are controversies regarding the impact of the age of the recipient on implantation and pregnancy outcome. While some investigators have found a lower pregnancy rate in recipients of more advanced age [13], others have not observed this relationship [14]. Natural fertility rates decline in women as they enter the fifth decade of life. By the time of the perimenopause pregnancy rarely occurs, whether or not assisted reproductive techniques are initiated. However, if oocytes are donated by young women to older women, both embryo implantation and pregnancy rates are restored to normal levels in recipients. These results strongly suggest the pregnancy wastage experienced by older women is largely a result of degenerative changes within the aging oocyte, rather than senescent changes in the uterus. The poor prognosis for fertility in older women can be reversed through oocyte donation from younger individuals [31.].
Unlike women, who are more fertile below the age of 40, men can conceive children well beyond their 40s; the critical age threshold with respect to sperm production in men is unknown. Nevertheless, it is important to know whether advanced paternal age is associated with diminished semen quality, therefore resulting in a higher risk of infertility. Our data imply that the alteration in semen quality is not dramatic and occurs gradually in men between the ages of 40 and 50. However, in some cases fertility may be preserved up to a very old age, especially if the ICSI method, that permits one “to fish out” normal spermatozoa in moderate and severe teratozoospermic samples, is successfully employed.
We have observed that sperm morphology and other sperm parameters decrease with age and that the age of male partners may affect the outcome of IVF cycles. We are therefore assuming that age is an important factor in male fertility. Sperm quality nevertheless remains the main male factor associated with the success of the IVF cycle.
References
- 1.Joffe M, Li Z. Male and female factors in fertility. Am J Epidemiol 1994;140:921–9. [DOI] [PubMed]
- 2.Lansac J. Delayed parenting. Is delayed childbearing a good thing? Hum Reprod 1995;10:1033–5. [DOI] [PubMed]
- 3.Ventura S, Martin J, Curtin S, Mathews T. Report of Final Natality Statistics, 1995. Monthly Vital Statistics Report. Vol. 45, no. 11, suppl. National enters for Health Statistics, Hyattsville, MD, 1997 [PubMed]
- 4.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 2001;75:237–48. [DOI] [PubMed]
- 5.Schwartz D, Mayaux M-J, Spira A, Moscato M-L, Jouannet P, Czyglik F, et al. Semen characteristics as a function of age in 833 fertile men. Fertil Steril 1983;39:530–5. [DOI] [PubMed]
- 6.Spandorfer SD, Avrech OM, Colombero LT, Palermo GD, Rosenwaks Z. Effect of paternal age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod 1998;13:334–8. [DOI] [PubMed]
- 7.de La Rochebrochard E, Mouzon J, Thepot F, Thonneau P; FIVNAT. Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril 2006;85:1420–4. [DOI] [PubMed]
- 8.World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. Cambridge, UK: Cambridge University Press; 1992.
- 9.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Veeck LL, Morshedi M, Brugo S. New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology 1987;30:248–51. [DOI] [PubMed]
- 10.Stolwijk AM, Zielhuis GA, Sauer MV, Hamilton CJ, Paulson RJ. The impact of the woman’s age on the success of standard and donor in vitro fertilization. Fertil Steril 1997;67:702–10. [DOI] [PubMed]
- 11.American Society for Reproductive Medicine (ASRM). Guidelines for gametes and embryo donation. A practice committee report. Guidelines and minimum standards, ASRM 1997 [DOI] [PubMed]
- 12.Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu HC, et al. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril 1988;50:298–307. [DOI] [PubMed]
- 13.Borini A, Violini F, Bianchi L, Bafalo MG, Trevisi MR, Flamigni C. Improvement of pregnancy and implantation rates in cyclic women undergoing oocyte donation after long-term down-regulation. Hum Reprod 1995;10:3018–21. [DOI] [PubMed]
- 14.Paulson RJ, Hatch IE, Lobo RA, Sauer MV. Cumulative conception and live birth rates after oocyte donation: implications regarding endometrial receptivity. Hum Reprod 1997;12:835–9. [DOI] [PubMed]
- 15.Yaunis JS, Simon A, Laufer N. Endometrial preparation: lessons from oocyte donation. Fertil Steril 1996;66:873–84. [DOI] [PubMed]
- 16.Paulson RJ, Milligan RC, Sokol RZ. The lack of influence of age on male fertility. Am J Obstet Gynecol 2001;184:818–22. [DOI] [PubMed]
- 17.Kahraman S, Findikli N, Biricik A, Oncu N, Ogur C, Sertyel S, et al. Preliminary FISH studies on spermatozoa and embryos in patients with variable degree of teratozoospermia and history of poor prognosis. Reprod Biomed Online 2006;12(6):752–71. [DOI] [PubMed]
- 18.Rosenbusch B, Sterzik K. Sperm chromosomes and habitual abortion. Fertil Steril 1991;56:370–2. [DOI] [PubMed]
- 19.Enginsu ME, Dumoulin JC, Pieters MH, Bras M, Evers JL, Geraedts JP. Evaluation of human sperm morphology using strict criteria after Diff-Quik staining: correlation of morphology with fertilization in vitro. Hum Reprod 1991;6:854–8. [DOI] [PubMed]
- 20.Host E, Ernst E, Lindenberg S, Smidt-Jensen S. Morphology of spermatozoa used in IVF and ICSI from oligozoospermic men. Reprod Biomed Online 2001;3:212–5. [DOI] [PubMed]
- 21.McKenzie LJ, Kovanci E, Amato P, Cisneros P, Lamb D, Carson SA. Pregnancy outcome of in vitro fertilization/intracytoplasmic sperm injection with profound teratospermia. Fertil Steril 2004;82:847–9. [DOI] [PubMed]
- 22.Bianchi PG, Manicardi GC, Urner F, Campana A, Sakkas D. Chromatin packaging and morphology in ejaculated human spermatozoa: evidence of hidden anomalies in normal spermatozoa. Mol Hum Reprod 1996;2:139–44. [DOI] [PubMed]
- 23.Martin H, Rademaker AW, Greene C, Ko E, Hoang T, Barclay L, Chernos J. A comparison of the frequency of sperm chromosome abnormalities in men with mild, moderate, and severe oligozoospermia. Biol Reprod 2003;69:535–9. [DOI] [PubMed]
- 24.Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, Guerin JF. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod 2003;18:1023–8. [DOI] [PubMed]
- 25.Singh NP, Muller CH, Berger RE. DNA damage and apoptosis in human sperm. Fertil Steril 2003;80:1420–30. [DOI] [PubMed]
- 26.Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, Bartoov B. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online 2006;5:634–8. [DOI] [PubMed]
- 27.Jung A, Schuppe HC, Schill WB. Comparison of semen quality in older and younger men attending an Andrology clinic. Andrology 2002;34:116–22. [DOI] [PubMed]
- 28.Dondero F, Mazzilli F, Giovenco P, Lenzi A, Cerasaro M. Fertility in older men. J Endocrinol Invest 1985;8:87–91. [PubMed]
- 29.Singer R, Sagiv M, Levinsky H, Allalouf D. Andrological parameters in men with high sperm counts and possible correlation with age. Arch Androl 1990;24:107–11. [DOI] [PubMed]
- 30.Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, et al. The association of age and semen quality in healthy men. Hum Reprod 2003;18:447–54. [DOI] [PubMed]
- 31.Sauer MV. The impact of age on reproductive potential: lessons learned from oocyte donation. Maturitas 1998;30:221–5. [DOI] [PubMed]